Abstract
Collagen exposure in tissue activates platelets, initiates wound healing, and modulates adaptive immunity. In this report, data are presented to demonstrate a requirement for platelet-derived CD154 for both collagen-induced augmentation of T-cell immunity and induction of pro-tective immunity to Listeria challenge. Specifically, we demonstrate that Ad5 encoding the membrane-bound form of ovalbumin (Ad5-mOVA) delivered in collagen induces higher ovalbumin-specific cytotoxic T lymphocyte (CTL) activity in a dose-dependent manner compared with Ad5-mOVA delivered in PBS. Increased CTL activity was dependent on the ability of platelets to respond to collagen and to express CD154. Furthermore, mice immunized with low-dose Ad5-mOVA in collagen were able to control a challenge of Listeria monocytogenes recombinant for ovalbumin expression (Lm-OVA), whereas mice immunized with low-dose Ad5-mOVA in PBS were not. These data indicate that in a physiologic setting that mimics wounding, platelets perform a sentinel function when antigen dose is too low to provoke an efficient immune response, and can enhance the generation of antigen-specific CD8 T cells that are functionally relevant to the host.
Introduction
Because pathogens are capable of logarithmic expansion upon host infection, it is critical that both innate and adaptive immunity are initiated without delay to ensure survival. An effective adaptive response is dependent upon efficient activation of innate immunity since antigen presenting cells (APCs) that are not fully activated are poor stimulators of T- or B-cell responses.1-3 However, the initial infection usually involves entry of only a few microbes resulting in a very low antigen dose unlikely to provoke a prompt immune response. To compensate, the immune system has been designed with important activating early signaling components such as the Toll-like receptor (TLR) family, and costimulatory molecules such as CD40 ligand (CD40L, CD154), which help lower the threshold for cellular activation.4,5 CD154 is a molecule critical to adaptive immunity. It is required for T-dependent humoral responses including affinity maturation, somatic hypermutation, germinal center (GC) formation, and B-cell memory.6 In addition, there is a role in some models for CD154 in generation of normal CD8 T-cell memory and cross-presentation of class I–restricted antigen.7-13 Until recently, functionally relevant expression of CD154 was thought to be restricted to CD4 T cells where its purpose in cellular immunity is to stimulate APCs that in turn potently activate T cells2 ; however, functional CD154, which was able to generate an inflammatory response from endothelial cells, has also been reported on platelets.14
The role platelets can play in antimicrobial immunity is well documented. They are activated by and able to engulf bacteria and viruses, release antimicrobial and antifungal proteins, release reactive oxygen species, express TLR, protect against helminth infections, and play a role in modulating production of inflammatory cytokines in response to bacterial infections.15-17 Because of their ubiquitous presence in the circulation, platelets are the first cell type to specifically and intentionally accumulate at sites of injury where they are activated by many factors, including collagen. While releasing compounds necessary for wound repair, platelets also release/express molecules that exert potent influence on innate and adaptive immunity such as TGF-β, RANTES, P-selectin, CD154, and several chemoattractants.15-17 Because of these attributes, platelets are well poised to constitute another early signaling component in the immune response. Consistent with an early signaling function of platelets, we reported the ability of platelet CD154 to stimulate dendritic cells in vitro, a function corroborated by several other groups.18-21 We also reported the ability of WT platelets to enhance IgG production, and in concert with WT CD4 T cells, to enhance GC formation in CD154 knockout mice.18,22 Importantly, we showed that in response to low doses of antigen, WT mice cannot produce normal levels of IgG in the absence of platelets. Accordingly, we have proposed that platelets perform a sentinel function for adaptive and innate immunity, an observation that has been supported by others.23
To further investigate the role of platelets in a physiologic setting, we used a previously described wound model24 by subcutaneous injection of low doses of Ad5 encoding the membrane-bound form of chicken OVA (Ad5-mOVA) in collagen. This mimics a wound in that collagen is exposed, and the initial dose of pathogen is low. Providing further support for our premise that platelets provide early activating immune signals, we report here that platelets enhance OVA-specific CD8 T-cell responses to low doses of Ad5-mOVA that would otherwise fail to stimulate a detectable immune response. This observation depended on the ability of platelets to express CD154 and respond to collagen. Moreover, mice whose OVA-specific CD8 T-cell response was enhanced by platelet-derived CD154 showed increased clearance of Listeria monocytogenes recombinant for chicken ovalbumin (Lm-OVA) compared with mice whose platelets lacked CD154. These data support the hypothesis that platelet-associated modulation of the initial CD8 antiviral response results in a biologically significant advantage to the host in a setting where, without platelet involvement, the viral dose is too low to prompt effective activation of the adaptive CD8 T-cell compartment.
Methods
Mice
Normal C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). CD154 gene knockout mice (CD154−/−) (B6 background) were purchased from The Jackson Laboratories (Bar Harbor, ME) and maintained as a breeding colony. All infected mice were housed in accordance with biosafety regulations, and all animal experiments followed approved Institutional Animal Care and Use Committee and Institutional Review Board protocols.
Reagents
The intracellular cytokine staining kit was purchased from PharMingen (San Diego, CA). All flow cytometry antibodies were purchased from eBioscience (San Diego, CA). Apyrase and PGE1 were purchased from Sigma-Aldrich (St Louis, MO). The platelet-depleting antibody (a mixture of clones p0p3 and p0p4 [p0p3/4]) was purchased from emfret Analytics (Eibelstadt, Germany). 5,6-Carboxyfluorescein diacetate, succinimidyl ester (CFSE) was purchased from Molecular Probes (Eugene, OR). Replication-deficient adenovirus type5-mOVA (Ad5-mOVA) was produced by the Gene Transfer Vector Core at the University of Iowa. The membrane-bound form of OVA (mOVA) consists of a fusion protein made up of the first 118 residues of the human transferring receptor, which includes the cytoplasmic tail and signal/anchor domain and is linked to residues 139-385 from mature OVA. This construct was placed in a shuttle plasmid that was recombined into Ad5 backbone DNA for packaging into viral particles.25 The collagen used is cross-linked, partially insoluble porcine skin type I collagen (powdered Surgifoam; Johnson & Johnson, New Brunswick, NJ) and was suspended at 30 mg/mL in PBS containing appropriate concentrations of virus for immunization.
Isolation of murine platelets
Murine platelets were isolated essentially as previously described.26 Briefly, mice were anesthetized and bled by cardiac puncture. Blood was collected into syringes containing 0.5 mL ACD (12.5 g/L Na citrate, 10.0 g/L d-glucose, 6.85 g/L citric acid), added to 5 mL PIPES (150 mM NaCl, 20 mM PIPES, pH 6.5), and spun at 100g for 15 minutes. The platelet-rich supernatant was collected, and 1 U/mL apyrase and 1 μM PGE1 (final concentrations) were added and spun at 1000g for 10 minutes. The platelet pellet was resuspended in Tyrode buffer (134 mM NaCl, 2.9 mM KCL 0.34 mM Na2PO4, 12 mM NaHCO3, 20 mM HEPES, 1 mM MgCl2, 5 mM glucose, and 0.5 mg/mL BSA [pH to 6.5]), and counted using a Coulter Particle Counter (Coulter, Miami, FL). All platelet manipulations were performed at room temperature (RT).
Flow cytometry
Cells were incubated with pretitrated amounts of appropriate antibodies for 30 minutes at 4°C in the dark, washed 2 × in flow cytometry buffer (2% FCS, 0.02% NaN3 in PBS), and resuspended in 2% paraformaldehyde. Events were collected on a FacScan or FacsCanto (Beckman, San Jose, CA), and analyzed using Cellquest (Becton Dickinson, San Jose, CA) or FlowJo software (TreeStar, Ashland, OR).
Bioassay for active murine soluble CD154
Quantitative real time reverse-transcription–polymerase chain reaction (RT-PCR) was performed to assess induction of MCP-1 mRNA via sCD154 in MS-1 cells, an immortalized pancreatic endothelial cell line that was obtained from ATCC (Manassas, VA) and routinely cultured in DMEM with 5% FBS. After 6-hour exposure to platelet-poor plasma or sCD154, total cellular RNA was isolated using the RNAeasy kit (Qiagen, Valencia, CA). First-strand synthesis was performed using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) in a reaction using 2 μg total RNA primed with random hexamers following the manufacturer's instructions. Of each reverse transcription reaction, 2 μL was subjected to real-time quantitative PCR using proprietary TaqMan primer and probe sets for mouse MCP-1, and 18s rRNA (Applied Biosystems, Foster City, CA). For each sample, 3 PCRs were performed. The resulting relative increase in reporter fluorescent dye emission was monitored by the TaqMan system (GeneAmp 5700 sequence detection system and software; PerkinElmer, Waltham, MA). The level of MCP-1 mRNA, relative to 18s rRNA, was calculated using the formula: relative mRNA expression = 2 − (Ct of MCP-1 − Ct of 18s rRNA), where Ct is the threshold cycle value.
In vivo CTLs
Splenocytes were harvested from syngeneic naive mice for use as targets. Half of the targets were loaded with 50 μg/mL of the CD8-recognized SIINFEKL OVA peptide for 1.5 hours at 37°C in RPMI/5% fetal calf serum. These targets were washed 2 ×, and then labeled for 10 minutes at 37°C in 10 μM CFSE at 2 × 106 cells/mL in RPMI/5% fetal calf serum. Splenocytes not SIINFEKL loaded were labeled with 1 μM CFSE. All cells were then washed 2 ×, combined, and injected retro-orbitally into mice immunized subcutaneously on the flank 13 days earlier with indicated doses of Ad5-mOVA in PBS or PBS with 30 mg collagen/mL. Eighteen hours later, spleens were harvested and flow cytometry was performed to assess the percentage of OVA-specific lysis of the high CFSE SIINFEKL-loaded targets compared with low CFSE unloaded.
Detection of OVA-specific CD8 T cells
OVA-specific T cells were detected by surface staining for CD8 together with intracellular staining for IFN-γ as previously described27 after a 5-hour stimulation with 200 nM OVA 257-264 peptide in the presence of brefeldin A (Biolegend, San Diego, CA) diluted 1:1000. The frequency of OVA-specific CD8 T cells was determined by gating on total CD8+ cells, which were then analyzed for IFN-γ expression.
In vitro lytic assays
Mice were immunized with Ad5-mOVA and after 10 to 14 days, spleens were harvested, ground between 2 frosted microscope slides, treated with ACK lysis buffer, and washed 2 × to isolate splenocytes. To expand and detect OVA-specific CTLs generated by Ad5-mOVA immunization, 5 × 106 splenocytes/well were incubated with 2 × 105 mitomycin C–treated E.G7-OVA lymphoma cells/well in 24-well plates in a humidified 5% CO2 37°C incubator. E.G7-OVA lymphoma cells (E.G7) are the EL4 lymphoma cell line transfected with ovalbumin cDNA and express the OVA SIINFEKL peptide recognized by CD8 T cells.28 Thus, they are useful stimulators and targets of OVA-specific CTLs. Five days later, viable splenocytes were recovered and incubated with 51Cr-labeled E.G7 or control EL4 targets in 96-well round-bottom plates. Four hours later, the supernatants were harvested and counted. Percentage lysis for each sample was calculated using the formula (sample CPM − spontaneous release CPM) / (max release CPM − spontaneous CPM) × 100. In experiments using adoptive transfer of platelets, mice were immunized 4 hours after 3 × 108 platelets were injected.
Listeria challenge
Listeria monocytogenes expressing ovalbumin was a gift from Leo Lefrancois.29 An attenuated version of this strain was generated by inducing an in-frame deletion in the actA gene as previously described (actA− Lm-OVA).30,31 Bacteria were grown and cultured as previously described.32,33 All mice were infected via the tail vein with 105actA− Lm-OVA or 2 × 105 virulent Lm-OVA grown in tryptic soy broth to an OD600 of approximately 0.1, diluted in pyrogen-free 0.9% sodium chloride (Abbott Laboratories, North Chicago, IL), and injected intravenously in 0.2 mL per animal. Aliquots were plated onto tryptic soy agar containing 50 μg/mL streptomycin to verify the number of colony-forming units (CFUs) injected and verified by plate counts. Spleens were collected from mice 24 hours after infection with actA− Lm-OVA or 72 hours after infection with virulent Lm-OVA, and total CFUs per spleen were determined by homogenizing the spleens in 0.2% IGEPAL (Sigma-Aldrich), plating 10-fold serial dilutions onto tryptic soy agar containing 50 μg/mL streptomycin, and calculating colony count averages after overnight incubation at 37°C as previously described.33,34
Results
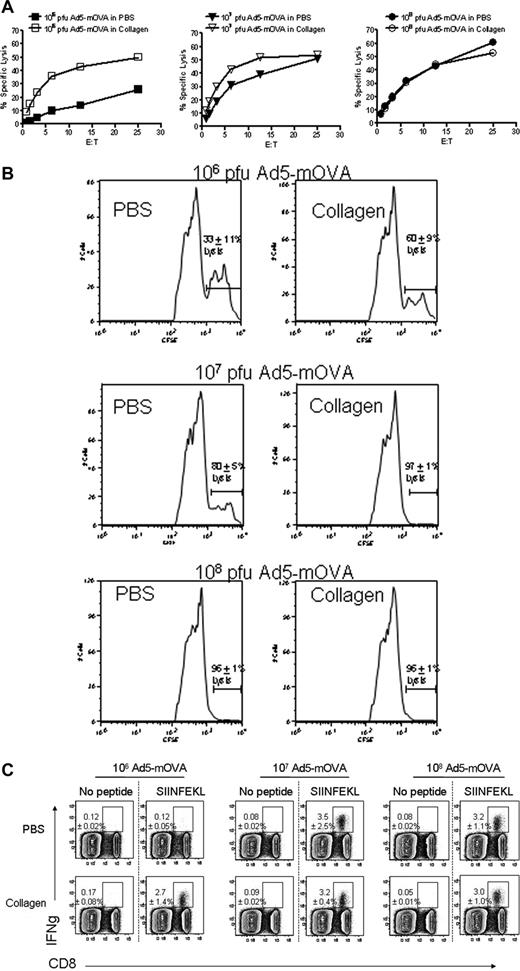
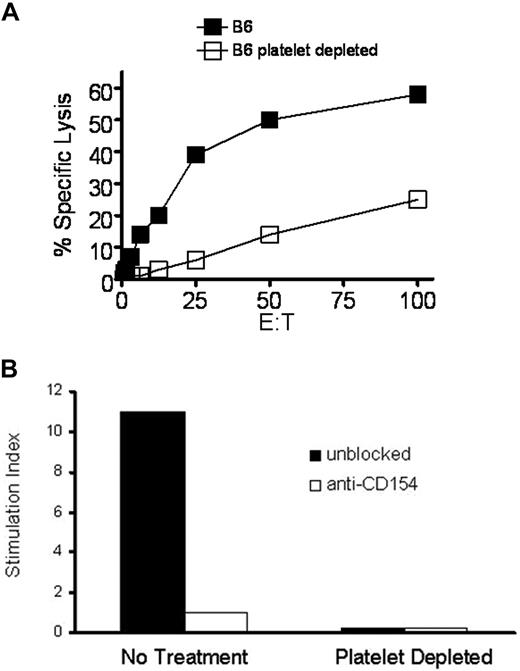
Previously, we demonstrated that mice injected intratumorally with canarypox recombinant for IL-2, IL-12, and TNF delivered in a collagen matrix were able to reject established tumors in a T cell–dependent manner.35 We also showed that mice immunized with Ad5-mOVA delivered in collagen have enhanced OVA-specific cytotoxic T lymphocyte (CTL) activity.25 Initially, we hypothesized that collagen-induced T-cell augmentation occurred because of a significant improvement in viral transgene expression, but further studies demonstrated that other noncollagen matrices, which also improved gene expression, did not augment T-cell responses (Jennifer M. Sowa, T.P.K., B.D.E., S.A.C., T.L.R., manuscript in preparation). Since platelets are highly responsive to collagen, and wild-type (WT) platelets enhance T-cell activity in CD154 gene knockout mice (CD154−/−),18 we hypothesized that platelets mediated the observed enhancement of T-cell responses in WT mice. Previous studies demonstrated that platelets contribute to the T-dependent B-cell response when antigen dose is low, but the contribution of platelets is circumvented as antigen concentration increases.22 To determine whether this observation is also true of the collagen-enhanced CD8 T-cell response, Ad5-mOVA dose titrations in collagen were performed. Figure 1 demonstrates that collagen-enhanced in vitro (Figure 1A) and in vivo (Figure 1B) CTL lytic activities are most apparent at low doses of virus. The enhancement is most likely due to the increased generation of OVA-specific CD8 T cells (Figure 1C). Importantly, the low dose effect is consistent with platelets performing an initial role for the immune response, where they serve to augment immunity under conditions of low antigen dose, but are dispensable at higher doses where their contribution can be overridden. To formally demonstrate that platelets have the ability to modulate CD8 T cells, C57BL/6 (B6) mice were depleted of platelets and immunized with Ad5-mOVA 24 hours later. The antiplatelet antibody results in more than 99% removal of circulating platelets, which begin to return 4 to 5 days later.36 After 14 days, CD8 T-cell activity was assessed.35 Figure 2A demonstrates that platelet depletion reduces in vitro CTL activity. Importantly, immunodepletion of platelets does not result in the release of active soluble CD154 (sCD154) as assessed by bioassay using platelet-poor plasma (Figure 2B). Since platelets appear to be necessary for optimal CD8 T-cell responses to Ad5-mOVA, further studies were performed in the collagen model to determine whether collagen-induced enhancement of CD8 T-cell function was mediated by platelets. Mice whose platelets cannot be activated by collagen were immunized with Ad5-mOVA in PBS or collagen. The activating platelet collagen receptor GPVI noncovalently associates with the signal-transducing Fc receptor common gamma chain (FcRγ), which is essential for its expression and function. Consequently, FcRγ−/− mice fail to express GPVI in their platelets and the cells are unresponsive to collagen.37 In addition in normal mice, intravenous injection of soluble collagen induces a rapid and fatal thromboembolism that is entirely dependent upon the ability of platelets to be activated by collagen through GPVI. JAQ1, a monoclonal ab against GPVI, has been shown to induce the complete and irreversible down-regulation of GPVI in circulating platelets in vivo resulting in a “GPVI knockout-like” phenotype and long-term protection from lethal collagen-induced thromboembolism.38 Figure 3A demonstrates that in FcRγ−/− mice, collagen delivery of virus does not result in enhanced CTL activity. Furthermore, normal B6 mice whose platelets are rendered nonresponsive to collagen by the blocking antibody JAQ1 also do not have increased CTL lysis after viral delivery in collagen (Figure 3B), demonstrating that collagen-mediated modulation of CTL activity is platelet-dependent. It is interesting to note that JAQ1 reduces lytic activity of the collagen group in Figure 3B below that of the PBS group. We have observed that JAQ1 even reduces lytic activity in mice immunized via PBS, indicating that in routine subcutaneous immunization the small amount of resultant tissue disruption/collagen exposure is enough to involve platelets in the immune response.
Delivery of Ad5-mOVA in collagen enhances recognition and lysis of OVA-expressing targets in a dose-dependent manner. (A) Mice were immunized subcutaneously with the indicated doses of Ad5-mOVA in PBS or 30 mg/mL collagen in the flank. After 14 days, spleens were harvested for lytic assays as described in “Methods.” Data are representative of 2 experiments with 3 mice per group. (B) Mice were immunized as in panel A, and in vivo CTLs were initiated 13 days later as described in “Methods.” SIINFEKL-loaded splenocyte targets were CFSE-high, and control targets were CFSE-low. Data are representative of 2 experiments of 3 to 5 mice per group. Percentage lysis is the average of both experiments plus or minus SEM. (C) Mice were immunized as in panels A and B, and spleens were harvested after 13 days for 5-hour stimulation with SIINFEKL peptide to determine levels of IFNγ-expressing OVA-specific CD8 T cells as assessed by flow cytometry. Each panel is representative of 3 mice. Lysis is the average plus or minus standard deviation.
Delivery of Ad5-mOVA in collagen enhances recognition and lysis of OVA-expressing targets in a dose-dependent manner. (A) Mice were immunized subcutaneously with the indicated doses of Ad5-mOVA in PBS or 30 mg/mL collagen in the flank. After 14 days, spleens were harvested for lytic assays as described in “Methods.” Data are representative of 2 experiments with 3 mice per group. (B) Mice were immunized as in panel A, and in vivo CTLs were initiated 13 days later as described in “Methods.” SIINFEKL-loaded splenocyte targets were CFSE-high, and control targets were CFSE-low. Data are representative of 2 experiments of 3 to 5 mice per group. Percentage lysis is the average of both experiments plus or minus SEM. (C) Mice were immunized as in panels A and B, and spleens were harvested after 13 days for 5-hour stimulation with SIINFEKL peptide to determine levels of IFNγ-expressing OVA-specific CD8 T cells as assessed by flow cytometry. Each panel is representative of 3 mice. Lysis is the average plus or minus standard deviation.
Platelet depletion lowers CTL response. (A) Mice were more than 99% depleted of platelets by intravenous injection of platelet-depleting monoclonal antibody and immunized with 106 pfu Ad5-mOVA intravenously 24 hours later. After 14 days, spleens were harvested for lytic assays. Data are representative of 4 experiments of 3 mice per group. (B) Immunodepletion of platelets does not release active sCD154. Mice were depleted of platelets as in panel A and platelet-poor plasma was harvested 24 hour later, the time at which adenovirus is injected for initiation of the immune response. Since commercial reagents with sufficient sensitivity are not available for the detection of murine sCD154 protein, plasma was bioassayed for activity as described in “Methods.” MS-1 cells were incubated for 6 hours with plasma from normal or depleted mice in the presence of MR1, a potent blocking monoclonal antibody against full-length or soluble murine CD154, and were assessed for MCP-1 mRNA content. Values were normalized and are expressed as stimulation index.
Platelet depletion lowers CTL response. (A) Mice were more than 99% depleted of platelets by intravenous injection of platelet-depleting monoclonal antibody and immunized with 106 pfu Ad5-mOVA intravenously 24 hours later. After 14 days, spleens were harvested for lytic assays. Data are representative of 4 experiments of 3 mice per group. (B) Immunodepletion of platelets does not release active sCD154. Mice were depleted of platelets as in panel A and platelet-poor plasma was harvested 24 hour later, the time at which adenovirus is injected for initiation of the immune response. Since commercial reagents with sufficient sensitivity are not available for the detection of murine sCD154 protein, plasma was bioassayed for activity as described in “Methods.” MS-1 cells were incubated for 6 hours with plasma from normal or depleted mice in the presence of MR1, a potent blocking monoclonal antibody against full-length or soluble murine CD154, and were assessed for MCP-1 mRNA content. Values were normalized and are expressed as stimulation index.
Platelets must be responsive to collagen for collagen-mediated CTL augmentation. (A) FcRγ−/− mice (platelets cannot be activated by collagen) were immunized subcutaneously on the flank with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen. After 14 days, spleens were harvested for lytic assay. (B) B6 mice were injected intravenously with 50 μg of the GPVI (platelet collagen receptor)–blocking monoclonal antibody JAQ1. After 24 hours, mice were immunized subcutaneously on the flank with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen. Fourteen days later, spleens were harvested for lytic assays. Each panel is representative of 2 experiments of 3 mice per group.
Platelets must be responsive to collagen for collagen-mediated CTL augmentation. (A) FcRγ−/− mice (platelets cannot be activated by collagen) were immunized subcutaneously on the flank with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen. After 14 days, spleens were harvested for lytic assay. (B) B6 mice were injected intravenously with 50 μg of the GPVI (platelet collagen receptor)–blocking monoclonal antibody JAQ1. After 24 hours, mice were immunized subcutaneously on the flank with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen. Fourteen days later, spleens were harvested for lytic assays. Each panel is representative of 2 experiments of 3 mice per group.
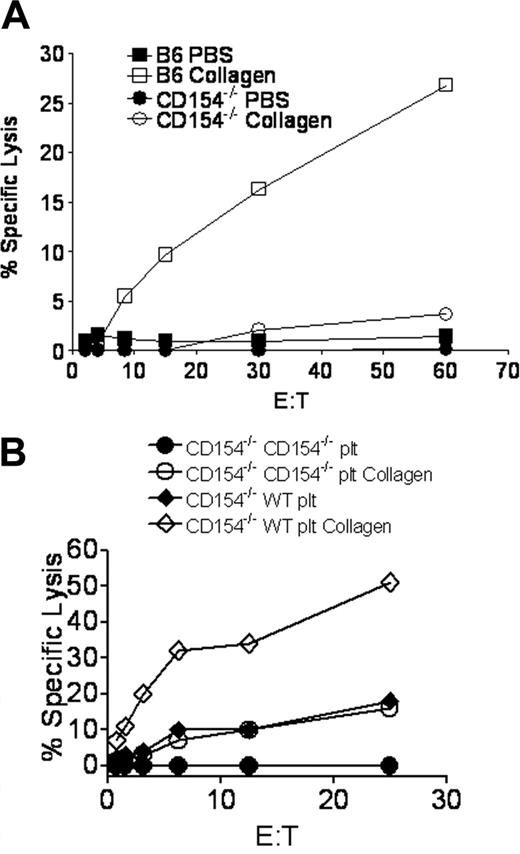
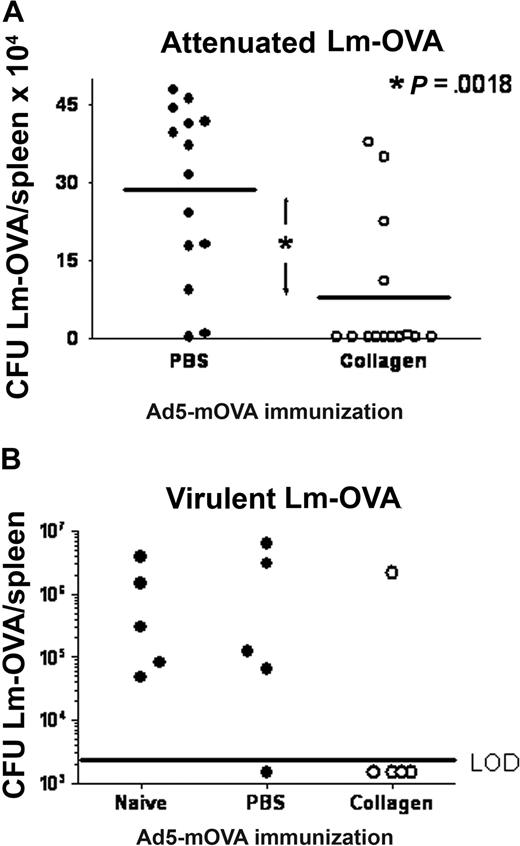
Since platelet CD154 was demonstrated to modulate T-cell responses,18 we investigated the ability of platelet CD154 to participate in collagen-mediated CTL enhancement. Figure 4A shows that collagen delivery of Ad5-mOVA in CD154−/− mice does not enhance in vitro CTL activity. To directly demonstrate the necessity of platelet CD154 in collagen-mediated CTL enhancement, WT B6 platelets were adoptively transferred into CD154−/− mice, which was previously demonstrated to provide functional circulating platelets for up to 4 days.18 Subsequent to platelet transfer, mice were immunized subcutaneously with Ad5-mOVA delivered in PBS or collagen. Figure 4B demonstrates that WT platelets are sufficient to permit collagen-mediated CTL enhancement in CD154−/− mice. Consistent with previous studies, immunization with Ad5-mOVA in PBS was also augmented by platelet transfer,18 but to a lesser degree versus immunization in collagen. This function can be directly attributed to platelet CD154 since the WT platelets are the only source of CD154 in this model, although collagen delivery of Ad5-mOVA in the absence of CD154 also results in a minor increase in CTL activity. This could be due to better transgene expression and antigen presentation or other CD154-independent components. To show that the collagen-mediated increase in antigen-specific CTLs has a beneficial biologic consequence, B6 mice immunized with Ad5-mOVA in PBS or collagen were challenged intravenously 14 days later with attenuated or virulent Listeria monocytogenes recombinant for ovalbumin (Lm-OVA). Twenty-four or 72 hours later, respectively, mice were killed and CFUs/spleen were assessed. Figure 5 demonstrates that mice immunized with Ad5-mOVA in collagen were better able to control Lm-OVA compared with mice immunized with Ad5-mOVA in PBS.
Platelets must have CD154 for collagen-mediated CTL augmentation. (A) CD154−/− mice were immunized subcutaneously on the flank with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen. After 14 days, spleens were harvested for lytic assays. (B) CD154−/− mice were adoptively transferred with 3 × 108 B6 platelets 4 hours before subcutaneous immunization with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen on the flank. After 14 days, spleens were harvested for lytic assays. Each panel is representative of 2 experiments of 3 mice per group.
Platelets must have CD154 for collagen-mediated CTL augmentation. (A) CD154−/− mice were immunized subcutaneously on the flank with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen. After 14 days, spleens were harvested for lytic assays. (B) CD154−/− mice were adoptively transferred with 3 × 108 B6 platelets 4 hours before subcutaneous immunization with 106 pfu Ad5-mOVA in PBS or 30 mg/mL collagen on the flank. After 14 days, spleens were harvested for lytic assays. Each panel is representative of 2 experiments of 3 mice per group.
Mice immunized with Ad5-mOva delivered in collagen versus PBS have increased protection against Lm-OVA. (A) B6 mice were immunized subcutaneously on the flank with 106 pfu Ad5-mOva in PBS or 30 mg/mL collagen. Fourteen days later, mice were challenged intravenously with 105actA− Lm-OVA, an attenuated strain. Twenty-four hours later, spleens were harvested and CFUs/spleen were measured as described in “Methods.” Data are from 3 experiments of 4 to 5 mice per group. Wilcoxon sign-rank 2-tailed P = .002. (B) Mice were treated as in panel A, but were challenged with virulent Lm-OVA. Splenic CFU was assessed 72 hours later. LOD indicates limit of detection.
Mice immunized with Ad5-mOva delivered in collagen versus PBS have increased protection against Lm-OVA. (A) B6 mice were immunized subcutaneously on the flank with 106 pfu Ad5-mOva in PBS or 30 mg/mL collagen. Fourteen days later, mice were challenged intravenously with 105actA− Lm-OVA, an attenuated strain. Twenty-four hours later, spleens were harvested and CFUs/spleen were measured as described in “Methods.” Data are from 3 experiments of 4 to 5 mice per group. Wilcoxon sign-rank 2-tailed P = .002. (B) Mice were treated as in panel A, but were challenged with virulent Lm-OVA. Splenic CFU was assessed 72 hours later. LOD indicates limit of detection.
Discussion
Naturally acquired infections usually involve small initial doses of replication-competent pathogens, whereas experimental examination of immune responses frequently use high doses of replication-limited or -incompetent microbes for safety reasons or to stimulate a high number of effector cells that facilitate their study. T-cell receptor transgenic mice are also used to aid in studying adaptive immunity; however, the unusually high antigen-specific precursor numbers can significantly change the kinetics of the response.39 Investigations aimed at studying the biology of infections and immunity should use antigen doses close to the limit of immune detection as well as physiologic levels of antigen-specific precursors to more accurately reflect early immune initiation events. Dose titrations of replication-incompetent adenovirus demonstrate that subcutaneous immunization of 106 pfu is a threshold level for induction of detectable antigen-specific T- and B-cell responses (data not shown). It is at this dose that we tested the ability of platelets to contribute to T-cell activation with the objective of better approximating the biologic setting of initial low antigen dose near the limit of immune detection. The data reported herein using collagen to mimic wound healing demonstrate a role for platelets in modulating early low-dose antigen responses. Platelet-derived CD154 augmented CD8 T-cell responses against an adenovirus transgene, which was necessary for induction of a protective immune response against a subsequent Listeria challenge. We hypothesize that platelets function to release CD154 early in infection, modulating APC function. Early maturation of APCs provides the activation signals to T cells that are required to induce effective effector cell expansion early in the infection. Due to a lack of sufficiently sensitive commercial reagents, it is not possible to distinguish sCD154 activity from membrane bound.
T-cell responses are dependent on antigen presentation by dendritic cells (DCs) in draining lymph nodes where the quality of activation is dependent on the maturation status of the antigen-presenting DCs.1,3 The literature supports a dichotomy of T-cell activation, one from immature DCs and the other from mature DCs. In a setting where DCs are quiescent, T cells do not receive requisite second signals, which results in ignorance or peripheral tolerance. However, if antigen presenting DCs are licensed by TLR stimulation and receive maturation signals in the form of CD154, T cells receive all necessary signals for clonal expansion and full activation.5 Although DCs are exquisitely sensitive to activation through TLR, TLR signals do have a threshold that can be lowered.40 Since TLR9 signaling up-regulates CD40, platelets could enhance TLR9 signaling resulting in higher DC sensitivity to CD154, or it may be that the platelets simply serve as a source of early CD154. Regardless, our data support the hypothesis that platelets send early signals to DCs before foreign antigen/TLR agonists are present in sufficient concentration to mature DCs, which leads to increased T-cell activation. Consistent with this hypothesis, several reports demonstrate platelet-induced maturation of DCs.18-21 This would be expected to increase activation of not only CD8 T cells as presented here, but also of CD4 T cells. Indeed, we have already demonstrated that platelets cooperate with CD4 T cells to enhance the B-cell GC response. Although the platelet-CD4 T-cell–B-cell relationship is under current investigation, it seems likely that this would be accomplished via the follicular DCs.
One of the classic functions of platelets is activation by collagen resulting in release of procoagulants for the purpose of hemostasis and wound repair. However, many released components such as CD62P, IL-1β, and histamine also possess immunomodulatory function, while others including thrombocidins, TGF-β, CD154, RANTES, MIP-1α, MCP-3, and several other chemokines have primarily innate/adaptive immune function.17 That platelets can release/produce such a diverse array of biologic mediators indicates that their function is much broader than simply facilitating coagulation. Since platelets are the first cell to respond to injury compromising a vessel wall, they are uniquely poised to initiate innate and adaptive immunity. We have recently demonstrated that platelets are able to modulate adaptive T- and B-cell responses via CD154 expression.18 Others have shown that human platelets from immune thrombocytopenic purpura patients can directly stimulate autologous B cells to produce antiplatelet antibodies in vitro.41 In addition to direct stimulation of B cells, platelets, through direct contact with T cells, release RANTES, creating a feedback loop for T-cell response amplification.42 Furthermore, adoptively transferred human platelets via CD154 have been shown to cause immune-mediated rejection of allografts in mice.43 Consistent with an early signaling function in modulation of adaptive immunity, human platelets could support graft rejection only if they were activated at the site of surgery at the time of graft implantation.43 In our collagen immunization model, we hypothesize that platelets activated at the site of injury express CD154, which is able to stimulate DC maturation and enhance T-cell activation. Although we are currently pursuing this question, it is consistent with our initial report that platelets through CD154 can stimulate DCs in vitro, an observation corroborated by several others.19,20 Platelets, under conditions of injury, are also able to mature DCs in vitro.21 Conversely, other reports indicate that platelets can have inhibitory effects on/through DCs.44-46 The reason for these differences is unclear, although it may be related to effective platelet/DCs ratios or the mechanism of platelet activation in each model.
As previously published, the subcutaneous injection of collagen is a model for studying immune responses in injury.24 Collagen injection mimics a wound with exposed basement membrane collagen, but importantly allows critical control of antigen delivery and the amount of collagen that is exposed, circumstances that would not be possible with an actual wound. The implantation of collagen for studying immune responses has previously been thought to be an inert phenomenon. However, we now demonstrate that collagen exposure can affect the immune response, an event that is dependent on platelets and their ability to recognize collagen. Because the Ad5-mOVA infection is self-limiting, there are no ramifications for the mice that do not respond to the virus. However, in the normal physiologic setting of injury, invading pathogens will be able to replicate and pose a threat to the host. It may be that in this situation, prompt arousal of innate and adaptive immunity via platelets serves to limit microbial expansion and damage compared with what would occur in the absence of platelet involvement. In our model, Figure 4 demonstrates that platelet CD154 is required for collagen-mediated augmentation of the T-cell response. Because of this, we expect that the primary mechanism of platelet contribution to adaptive immunity is at the level of antigen presentation that is currently under investigation. It is well established that T-cell interaction with collagen via VLA-1 and VLA-2 is important for effector T-cell trafficking and maintenance of tissue-specific memory T cells.47,48 However, it has also been demonstrated that primary immune responses or effector T-cell presence in the spleen do not require these interactions.49 Since our studies probe primary and splenic secondary T-cell responses, it is unlikely that VLA interactions with collagen modulate our observations reported herein.
The clearance of Lm-OVA in Figure 5 demonstrates the effectiveness of collagen-elicited immunity to a low virus dose that otherwise would not generate a protective response. This could have clinical significance regarding vaccination of immunocompromised patients. Live vaccines such as oral polio or measles/mumps/rubella present special problems to HIV patients, or to patients with certain congenital defects who may not be able to control viral replication. However, if collagen-delivered vaccines containing orders of magnitude less virus resulted in effective immunization, it may be possible to protect these patients from threatening diseases without exposing them to substantial risk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Department of Defense (DAMD17-02-1-0079 [B.D.E.]) and the National Institutes of Health (DK071712 [B.D.E.], T32 A1007511 [N.W.S.], AI60924 and CA096691 [T.L.R.], others [J.T.H.]).
National Institutes of Health
Authorship
Contribution: B.D.E. designed, directed, and performed research and drafted the paper; N.W.S., S.A.C., and T.P.K. performed research; J.T.H. contributed to the integrity and analysis of data; B.N. contributed vital new reagents and experimental design; T.L.R. directed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bennett D. Elzey, Wishard Memorial Hospital, Room OPW 320, 1001 W 10th St, Indianapolis, IN 46202; e-mail: belzey@iupui.edu.