Abstract
Glycogen synthase kinase (GSK)3β is a ser-thr kinase that is phosphorylated by the kinase Akt. Although Akt has been shown to regulate platelet function and arterial thrombosis, its effectors in platelets remain unknown. We show here that agonist-dependent phosphorylation of GSK3β in platelets is Akt dependent. To determine whether GSK3β regulates platelet function, platelets from mice lacking a single allele of GSK3β were compared with those of wild-type (WT) controls. GSK3β+/− platelets demonstrated enhanced agonist-dependent aggregation, dense granule secretion, and fibrinogen binding, compared with WT platelets. Treatment of human platelets with GSK3 inhibitors renders them more sensitive to agonist-induced aggregation, suggesting that GSK3 suppresses platelet function in vitro. Finally, the effect of GSK3β on platelet function in vivo was evaluated using 2 thrombosis models in mice. In the first, 80% of GSK3β+/− mice (n = 10) formed stable occlusive thrombi after ferric chloride carotid artery injury, whereas the majority of wild-type mice (67%) formed no thrombi (n = 15). In a disseminated thrombosis model, deletion of a single allele of GSK3β in mice conferred enhanced sensitivity to thrombotic insult. Taken together, these results suggest that GSK3β acts as a negative regulator of platelet function in vitro and in vivo.
Introduction
Platelet activation is critical for hemostasis, as is evident from the identification of patients with bleeding disorders attributed to defects in platelet surface receptors or intracellular signaling molecules.1-11 The activation of platelets is also a central factor contributing to arterial thrombosis. Inhibitors of platelet agonists such as thrombin or adenosine diphosphate (ADP), or antagonists for their cell surface receptors, have been shown to inhibit platelet aggregation and reduce arterial thrombosis in both mouse models and humans.4,12,13 Thus, the signaling pathways by which these agonists activate platelets are under intense scrutiny, as they may suggest potential new risk factors for thrombosis or therapeutic targets. One signaling pathway of recent interest is the activation of the ser-thr kinases PI3K and Akt. Both thrombin and ADP activate G protein-coupled receptors on the platelet surface, which in turn have been shown to activate multiple isoforms of PI3K14 and Akt.13,15 Deletion of PI3Kγ in mice,16,17 inhibition of PI3Kβ in human platelets,18 and deletion of either Akt119 or Akt213 have all been shown to result in defective platelet aggregation and reduced sensitivity to thrombosis in various models. Therefore, the effectors of Akt are likely to play important roles in regulating platelet activation and thrombosis. However, of the dozens of Akt substrates identified to date, it is unclear which are present and functional in platelets
As in other cells, there are likely to be several Akt effectors in platelets. NOS3 is one candidate effector of Akt in platelets that has been shown to positively regulate platelet activity,20 although it has also been reported that NOS3-derived nitric oxide can negatively regulate platelet secretion.21 Phosphodiesterase 3A (PDE3A) has been shown to become phosphorylated in an Akt-dependent fashion and to regulate cyclic adenosine monophosphate (cAMP) levels in platelets,22 but its role in vivo has not been evaluated. The presence of glycogen synthase kinase (GSK)3β (GSK3β) in platelets has also been reported previously.23 In the reported study, short-term treatment of platelets with several GSK3β inhibitors (including LiCl) was shown to inhibit platelet activity in vitro. Yet, the role of GSK3β in platelet signaling and thrombosis was not evaluated. The goal of the present study is to more thoroughly investigate the role of GSK3β as a potential Akt effector in platelet function and thrombosis.
GSK3β is a ser-thr kinase that is regulated by its phosphorylation on ser9.24 It is one of 2 mammalian isoforms (α and β), that have been shown to have diverse but overlapping roles in glucose metabolism,25 Alzheimer disease,26 and more recently, inflammation.27 The kinase activity of GSK3 is generally thought to be constitutive and is negatively regulated by phosphorylation on ser9 of the β isoform (or 21 on GSK3a).24,25 Phosphorylation of this residue by the ser-thr kinase, Akt, is associated with decreased GSK3 activity,28 which in many cases releases a tonic inhibition of the GSK3 substrate.29-32 We and others have previously shown that either of 2 isoforms of Akt (Akt1 or Akt2) can regulate platelet aggregation and thrombosis in mouse models.19,33 As our studies suggest that GSK3β is the more highly expressed GSK3 isoform in platelets, we sought to determine whether GSK3β acts as an effector of Akt in platelet signaling and function and whether GSK3β may regulate thrombosis in vivo.
The GSK3β−/− mouse dies in utero at day 14.5, presumably due to tumor necrosis factor (TNF)–mediated liver degeneration.34 Nevertheless, adult GSK3β+/− mice have been shown to exhibit effects (particularly, behavioral defects) similar in nature to mice treated with known inhibitors of GSK3.35 Our data suggest that haploinsufficiency of GSK3β renders mouse platelets hypersensitive to agonist and mice hypersensitive to thrombosis in vivo. Human platelets treated for 2 hours with GSK3 inhibitors also are hyper-responsive to agonist in vitro. Our results suggest that the inhibition of GSK3β in platelets may yield potential prothrombotic effects.
Methods
Materials
Unless otherwise specified, reagents were from Sigma-Aldrich (St Louis, MO). ARL66096 was a kind gift of Astra Zeneca (Wilmington, DE). ADP was from Chronolog (Havertown, PA). LY294002 was from Calbiochem-Novabiochem (San Diego, CA). The thrombin receptor agonist peptides (TRAPs), SFLLRN and AYPGKF, were synthesized by New England Peptide (Gardner, MA). Antibodies were from Cell Signaling (Boston, MA) (for GSK3β, Akt, Phospho-Akt-ser473, Phospho-GSK3β-ser9, actin), Santa Cruz (Santa Cruz, CA) (for GSK3α, tau), and Calbiochem (San Diego, CA) for phospho-tau-ser212.
Animals
GSK3β knock-out mice were generated as described,34 kindly provided by the laboratory of Dr James Woodgett, and were bred as heterozygotes. Wild-type C57BL/6 mice were bred from the same stock bred with GSK3β heterozygotes: age-matched controls of mice from 8 to 12 weeks of age were used for all experiments. Akt2−/−Akt1+/− mice were generated by crossing Akt2−/− and Akt1−/− mice, both obtained from Morris Birnbaum36,37 at the University of Pennsylvania. All animal procedures were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University.
Human blood
Blood for biochemical studies of human platelets was collected by venipuncture from adult human volunteers after written informed consent was obtained in accordance with the Declaration of Helsinki, as approved by the Institutional Review Board at Thomas Jefferson University.
Platelet isolation and immunoblotting
Blood was collected from the inferior vena cava of anesthetized mice (100 mg/kg pentobarbital) into a 1-cc syringe containing ACD (trisodium citrate, 65 mM; citric acid, 70 mM; dextrose, 100 mM; pH 4.4) at a ratio of 1:6 parts ACD/blood. Anticoagulated blood was diluted 50% with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)–Tyrode buffer (137 mM NaCl, 20 mM HEPES, 5.6 mM glucose, 1 g/L bovine serum albumin [BSA], 1 mM MgCl2, 2.7 mM KCl, 3.3 mM NaH2PO4) before centrifugation at 250g to remove red cells. Platelets from the resulting platelet rich plasma (PRP) were pelleted at 750g (10 minutes), washed once in HEN buffer (10 mM HEPES, pH 6.5, 1 mM EDTA [ethylenediaminetetraacetic acid], 150 mM NaCl) containing 0.05 U/mL apyrase and resuspended at a concentration of 4 × 108 platelets/mL in HEPES-Tyrode buffer containing 0.05 U/mL apyrase. Samples were treated with LY294002 for 15 minutes in the dark at 37°C if indicated. Agonist was added in a 2-μL volume to 100 μL platelets per sample; platelets were incubated for 0 to 10 minutes and were lysed by addition of 5 × Laemmli buffer containing a cocktail of protease inhibitors (Sigma-Aldrich). Lysates were resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an antibody to GSK3α, GSK3β, Akt phospho-ser 473, GSK3β phospho-ser9 (Cell Signaling Technology, Beverly, MA), or microtubule-associated protein (MAP)–tau phospho-thr231 (Calbiochem) at a 1:1000 dilution, then anti–rabbit alexafluor680 (LiCor) in 50% blotting buffer (LiCor) in Tris-buffered saline (TBS) and exposed on a LiCor fluorescence imager.
Platelet aggregation and secretion studies
Blood was isolated from the inferior vena cava of anesthetized mice (100 mg/kg pentobarbital) using a syringe containing 150 U/mL heparin (1:9 dilution with blood), diluted 50% with HEPES-Tyrode buffer, and spun at 250g for 4 minutes to remove red cells. Generally, blood from 2 mice of each genotype was used for aggregation experiments. Platelets were counted on a Coulter counter (Beckman-Coulter Z1), and the final platelet count was adjusted to 2.5 × 108/mL with platelet-poor plasma from mice of the same genotype. Aggregation was initiated with 2.5 μL of agonist applied to a 250-μL aliquot of PRP and measured in a dual channel ChronoLog lumi-aggregometer. To measure secretion of dense granule contents, luciferase (Chrono-lume; Chronolog) was added to PRP (final concentration of 100 nM) 2 minutes prior to aggregometry, and the ATP-dependent increase in luminescence was recorded concurrent with aggregation using AGGROLINK software (Chronolog). The ATP-dependent luminescence is directly proportional to the amount of ATP secreted from the dense granule and was calibrated to an ATP standard.
Fibrinogen binding
To measure fibrinogen binding to the platelet surface, washed mouse platelets (108/mL) were incubated simultaneously with 25 μg/mL AlexaFluor 488–labeled fibrinogen (Molecular Probes, Eugene, OR) and indicated concentrations of agonist peptide AYPGKF at 37°C for 10 minutes, then fixed in 1% paraformaldehyde containing Tyrode buffer for 10 minutes at 37°C, diluted 5 times with Tyrode buffer, and analyzed by flow cytometry.
Disseminated thrombosis model
Mice sedated with pentobarbital (100 mg/kg) were injected with a combination of collagen (170 mg/kg; Chrono-Log) and epinephrine (350 μM/kg) in 50 μL volume via tail vein. Mice were observed for 10 minutes and the time of breathing stasis recorded. Surviving mice were killed, and the lungs from mice receiving injections were dissected immediately after death, fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned. The sections were stained with hematoxylin and eosin.
FeCl3-induced carotid artery thrombosis
The right carotid artery of an anesthetized adult mouse (6-10 weeks of age, 18-30 kg treated with 100 mg/kg pentobarbital) was exposed to a strip of filter paper saturated with 10% FeCl3 for 2 minutes, then rinsed with phosphate-buffered saline (PBS), essentially as described.21 Arterial flow rate was recorded for 30 minutes with a Doppler flow probe. Stable occlusive thrombi were scored as complete cessation of blood flow, which remained for the 30-minute duration of the assay. Thrombi were scored as unstable if flow resumed before the end of the 30-minute time period or decreased by at least 80% from the initial flow rate, but remained incomplete. The animal was scored as having no occlusive thrombus if the flow rate never decreased by 80% of the initial flow rate during the term of the assay. The mice were killed at the end of the procedure. Statistical significance was calculated using Fisher test of exact probability using the website http://faculty.vassar.edu/lowry/fisher.html.
Results
Agonist-stimulated GSK3β phosphorylation in platelets is Akt dependent
To determine whether the presence of GSK3 may affect platelet function, first the expression of GSK3 isoforms in platelets was evaluated by immunoblotting platelet lysates with GSK3α- and GSK3β-selective antibodies. Immunoblots of human and mouse platelets with antibodies to GSK3β show strong bands at 48 kDa, while little if any immunoreactivity is seen in blots of the same platelet samples with GSK3α-directed antibody (Figure 1A,B; additional data not shown). While these are not quantitative, GSK3β is clearly highly expressed in mouse and human platelets, while expression of GSK3α is barely detectable. In addition, platelets from mice heterozygous for the GSK3β gene have 49.4% (± 7.5%) of the GSK3β detected in wild-type (WT) platelets when immunoblots for GSK3β are compared by densitometry, whereas Akt levels were normal (100% ± 3.6%, mean ± SD of 3 experiments, see representative immunoblot in Figure 1A). Therefore, GSK3β+/− mice were used in future parts of this study to determine the effects of reduced expression of GSK3β on platelet function and thrombosis.
GSK3β is present and phosphorylated after agonist treatment in platelets. (A) Lysates of washed human or WT mouse platelets (108) and mouse liver (20 μg/mL) were immunoblotted with an antibody to GSK3β. The second immunoblot shows a comparison of 108 platelets from WT mice and GSK3β+/− mice immunoblotted with an antibody to GSK3β. The blot was reprobed with an antibody to Akt. (B) Washed human and mouse platelets and HeLa cells (20 μg/mL) were immunoblotted with an antibody to GSK3α. (C) Washed human platelets were stimulated with ADP (10 μM) or thrombin (0.1 u/mL) for 10 minutes with or without ARL66096 (300 nM), A3P5PS (300 μM), or PP2 (50 μM). Lysates were then immunoblotted for Akt phospho-(ser473), GSK3β phospho-(ser9), and total Akt. The mean (± SD) of the fold increase in phosphorylation compared with untreated control for 3 experiments is shown in the graph above a representative experiment. (** indicates a significant difference compared with control with P ≤ .01 according to an unpaired Student t test).
GSK3β is present and phosphorylated after agonist treatment in platelets. (A) Lysates of washed human or WT mouse platelets (108) and mouse liver (20 μg/mL) were immunoblotted with an antibody to GSK3β. The second immunoblot shows a comparison of 108 platelets from WT mice and GSK3β+/− mice immunoblotted with an antibody to GSK3β. The blot was reprobed with an antibody to Akt. (B) Washed human and mouse platelets and HeLa cells (20 μg/mL) were immunoblotted with an antibody to GSK3α. (C) Washed human platelets were stimulated with ADP (10 μM) or thrombin (0.1 u/mL) for 10 minutes with or without ARL66096 (300 nM), A3P5PS (300 μM), or PP2 (50 μM). Lysates were then immunoblotted for Akt phospho-(ser473), GSK3β phospho-(ser9), and total Akt. The mean (± SD) of the fold increase in phosphorylation compared with untreated control for 3 experiments is shown in the graph above a representative experiment. (** indicates a significant difference compared with control with P ≤ .01 according to an unpaired Student t test).
Prior to investigating the functional role of GSK3β, we first sought to verify that GSK3β phosphorylation in platelets is regulated by platelet agonists in an Akt-dependent fashion. We have previously shown that phosphorylation of GSK3β ser9 is stimulated by thrombin in human and mouse platelets.33 We show here that, as with Akt phosphorylation of ser473, phosphorylation of GSK3βser 9 is stimulated by ADP as well as thrombin (Figure 1C). ADP-stimulated GSK3 phosphorylation is inhibited by an antagonist for the P2Y12 receptor for ADP (ARL66096), but not the P2Y1 receptor (A3P5PS). Thrombin-dependent GSK3 phosphorylation is also dependent on P2Y12 and is inhibited by an inhibitor of src family tyrosine kinases (PP2), as has been reported for Akt phosphorylation (Figure 1B). These results suggest that the P2Y12 ADP receptor and possibly PAR1 and/or PAR4 receptors for thrombin stimulate Akt phosphorylation via a src (or src family)-dependent pathway, which in turn stimulates phosphorylation of GSK3β.
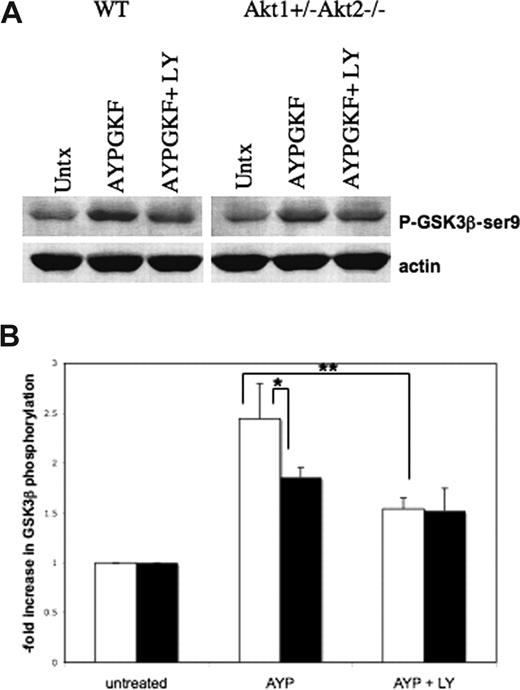
To determine whether phosphorylation of GSK3β ser9 is in fact dependent on Akt as predicted, PAR4-stimulated GSK3β phosphorylation was compared in platelets from WT mice and mice lacking 3 of 4 isoforms of Akt (Akt1+/−Akt2−/− mice), which have previously been shown to have defects in platelet aggregation and thrombosis.33 (Akt1−/−Akt2−/− mice die at birth38 ). PAR4-dependent GSK3β phosphorylation was reduced by 40.3% in platelets from Akt1+/−Akt2−/− mice compared with WT control platelets (statistically different, P ≤ .05, Student t test) and was reduced by 62.5% in WT platelets treated with PI3K inhibitor LY294002 (statistically different, P ≤ .01, Student t test; Figure 2). These results suggest that phosphorylation of GSK3β ser9 is partially dependent on Akt (isoforms 1 and 2) as well as PI3K. The inhibition of GSK3β phosphorylation achieved by treatment with PI3K inhibitors is slightly more than that evident from the loss of 3 alleles of Akt: this small discrepancy may be due to the remaining allele of Akt expressed in these platelets. There is also a degree of GSK3β phosphorylation that does not appear to be PI3K dependent, since the phosphorylation is only partially sensitive to LY294002.
Phosphorylation of GSK3βser9 is reduced by an inhibitor of PI3K and by deletion of 3 alleles of Akt in mouse platelets. (A) Washed mouse platelets from WT mice (□) or Akt1+/− Akt2−/− mice (■) in the presence or absence of LY294002 (100 μM) were stimulated with mouse TRAP (PAR4 agonist) AYPGKF (1 mM) for 10 minutes, then lysates were immunoblotted with an antibody to phospho-GSK3β-ser9. (B) The fold increase in GSK3β phosphorylation after AYPGKF treatment (± LY294002) was analyzed by densitometry. The means (± SD) are shown. (*P ≤ .05 by unpaired t test, **P ≤ .01by unpaired t test, n = 3.)
Phosphorylation of GSK3βser9 is reduced by an inhibitor of PI3K and by deletion of 3 alleles of Akt in mouse platelets. (A) Washed mouse platelets from WT mice (□) or Akt1+/− Akt2−/− mice (■) in the presence or absence of LY294002 (100 μM) were stimulated with mouse TRAP (PAR4 agonist) AYPGKF (1 mM) for 10 minutes, then lysates were immunoblotted with an antibody to phospho-GSK3β-ser9. (B) The fold increase in GSK3β phosphorylation after AYPGKF treatment (± LY294002) was analyzed by densitometry. The means (± SD) are shown. (*P ≤ .05 by unpaired t test, **P ≤ .01by unpaired t test, n = 3.)
Haploinsufficiency of GSK3β enhances aggregation, secretion, and fibrinogen binding in mouse platelets
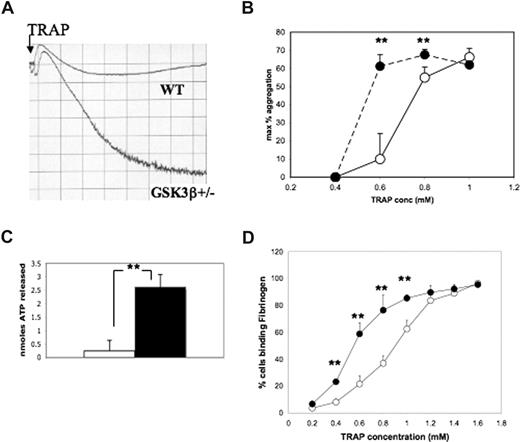
To begin to understand whether GSK3β may play a role in platelet function, we compared platelet aggregation in PRP derived from mice lacking one allele of GSK3β (GSK3β−/− mice die in utero) with that of PRP from WT mice. Platelets from GSK3β mice are hypersensitive to the PAR4 agonist AYPGKF (representative tracing of platelets stimulated with 0.6 mM TRAP is shown in Figure 3A) and thromboxane A2 analog U46619 (additional data not shown) relative to WT control mice when aggregation is directly compared in a 2-channel aggregometer. The maximal percentage aggregation was measured in response to varying concentrations of the mouse thrombin receptor (PAR4) agonist peptide in 3 independent experiments. A dose-response curve for aggregation to AYPGKF is shown in Figure 3B and demonstrates a left-shifted dose response in the GSK3β+/− platelets relative to WT controls. The difference in secretion is statistically significant at 0.6 and 0.8 mM agonist (P < .01, n = 4, unpaired Student t test). Secretion of dense granule contents is also enhanced in GSK3β+/− mice relative to WT control mice (P < .01, unpaired Student t test, n = 6). Secretion was measured in a lumi-aggregometer concurrent with aggregation. Figure 3C shows the average amount of ATP released from GSK3β+/− and WT platelets treated with 0.6 mM AYPGKF: 0.24 (± 0.39) nmol ATP was released from WT platelets compared with 2.6 (± 0.49) nmol ATP released from the same number of GSK3β+/− platelets.
Platelets from GSK3β+/− mice are hypersensitive to agonist-stimulated aggregation, secretion of dense granule contents, and fibrinogen binding. (A) PRP from WT and GSK3β+/− mice (2 × 108/mL) were stimulated with mouse TRAP AYPGKF (0.6 mM) in the presence of luciferase (100 nM). Aggregation and ATP release were recorded with an optical aggregometer. A representative aggregation trace is shown. (B) The maximal percent aggregation in response to varying concentrations of AYPGKF was measured. The mean (± SD) for 4 experiments is shown for WT platelets (○) and GSK3β+/− platelets (●). ** indicates the difference between WT and GSK3β+/− is statistically significant (P < .01, n = 4, unpaired Student t test). (C) The amount of ATP secretion (nmol/5 × 107 platelets) in response to 0.6 mM mouse TRAP was measured in 6 experiments: the mean (± SD) is shown; ** indicates the difference between WT and GSK3β+/− is statistically significant (P < .01, unpaired Student t test; WT, □; GSK3β+/− ■). (D) The percent of cells binding fibrinogen following stimulation with varying concentrations of mouse TRAP AYPGKF is shown: ○ for WT cells, ● for GSK3β+/− platelets (mean ± SD; ** indicates statistical significance, P < .01, unpaired Student t test for 3 experiments).
Platelets from GSK3β+/− mice are hypersensitive to agonist-stimulated aggregation, secretion of dense granule contents, and fibrinogen binding. (A) PRP from WT and GSK3β+/− mice (2 × 108/mL) were stimulated with mouse TRAP AYPGKF (0.6 mM) in the presence of luciferase (100 nM). Aggregation and ATP release were recorded with an optical aggregometer. A representative aggregation trace is shown. (B) The maximal percent aggregation in response to varying concentrations of AYPGKF was measured. The mean (± SD) for 4 experiments is shown for WT platelets (○) and GSK3β+/− platelets (●). ** indicates the difference between WT and GSK3β+/− is statistically significant (P < .01, n = 4, unpaired Student t test). (C) The amount of ATP secretion (nmol/5 × 107 platelets) in response to 0.6 mM mouse TRAP was measured in 6 experiments: the mean (± SD) is shown; ** indicates the difference between WT and GSK3β+/− is statistically significant (P < .01, unpaired Student t test; WT, □; GSK3β+/− ■). (D) The percent of cells binding fibrinogen following stimulation with varying concentrations of mouse TRAP AYPGKF is shown: ○ for WT cells, ● for GSK3β+/− platelets (mean ± SD; ** indicates statistical significance, P < .01, unpaired Student t test for 3 experiments).
The central event in platelet aggregation is widely regarded to be the triggering of a conformational change in the platelet-specific integrin αIIbβ3: this change is termed inside-out signaling and permits binding of circulating fibrinogen.39 To determine whether enhanced aggregation responses in GSK3β+/− platelets were attributable to GSK3β-mediated regulation of inside-out signaling resulting in fibrinogen binding to integrin αIIbβ3, AYPGKF-stimulated fibrinogen binding was compared in GSK3β+/− versus WT platelets. Gel-filtered platelets from GSK3β+/− mice and WT mice were incubated with varying concentrations of mouse (PAR4) TRAP, and Alexafluor 488–conjugated fibrinogen and surface-bound fluorescence was measured in a BD flow cytometer. The percent of fibrinogen-bound platelets was plotted relative to agonist concentration. The dose-response curve is shown in Figure 3D and demonstrates that GSK3β+/− platelets are more sensitive to agonist-induced fibrinogen binding than WT platelets.
Human platelets treated with GSK3β inhibitors display enhanced sensitivity to agonist-induced aggregation
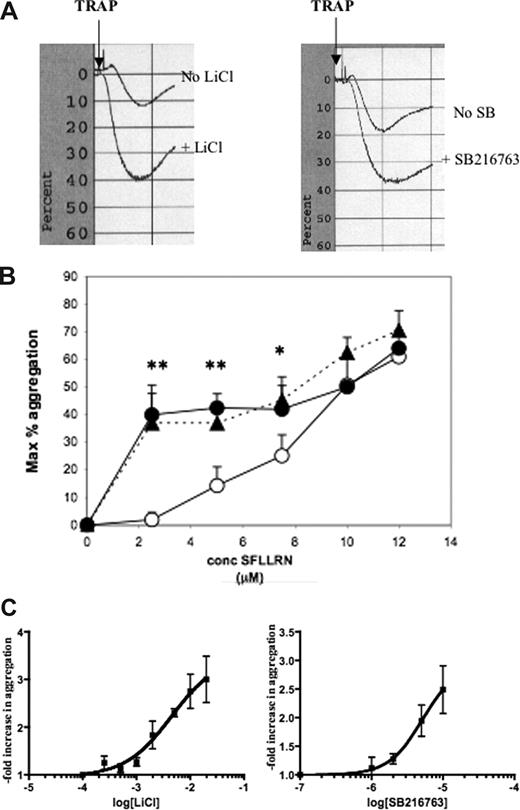
To determine if GSK3β also plays a role in human platelets, washed human platelets were treated for 2 hours with either of 2 GSK3β antagonists, LiCl or SB216763. Platelet aggregation was then tested in response to different concentrations of the PAR1 agonist SFLLRN (human TRAP). As with reduction of GSK3β expression in mouse platelets, inhibition of GSK3β in human platelets increases aggregation responses to PAR1 agonist. Representative tracings of responses of untreated human platelets compared with LiCl-treated platelets or SB216763-treated platelets compared with control are shown in Figure 4A. Responses of untreated, LiCl-, and SB216763-treated platelets to varying concentrations of SFLLRN, shown in Figure 4B, demonstrate that treatment of human platelets with either GSK3 inhibitor increases the sensitivity for agonist-induced aggregation. Dose-response curves to LiCl and SB216763 are shown in Figure 4C. The EC50 calculated using GraphPad Prizm software (San Diego, CA) for enhancement of aggregation by LiCl is 4.5 mM and 5.2 μM for SB216763.
GSK3β antagonists enhance aggregation to PAR1 agonists. (A) Washed human platelets (2.5 × 108/mL) were incubated for 2 hours with LiCl (20 mM), SB216763 (10 μM), or Tyrode buffer, then stimulated with human TRAP (PAR1 agonist) SFLLRN (5 μM), and aggregation was measured in an optical aggregometer. (B) Aggregation in response to varying concentrations of SFLLRN (1-10 μM) was measured as described in panel A. (WT, ○; LiCl (20 mM), ●; SB216763 (10 μM), ▲.) The results shown are the means (± SD) of 3 to 8 data points. The difference between LiCl and WT, or SB216763 and WT is statistically significant with **P < .01, *P < .05, unpaired Student t test. (C) Aggregation to 7 μM SFLLRN in the presence of the indicated concentrations (M) of LiCl or SB216763 was measured 3 to 16 times for each data point. Results were plotted and analyzed to fit a Sigmoidal dose-response in GraphPad Prizm to obtain the EC50s for each compound.
GSK3β antagonists enhance aggregation to PAR1 agonists. (A) Washed human platelets (2.5 × 108/mL) were incubated for 2 hours with LiCl (20 mM), SB216763 (10 μM), or Tyrode buffer, then stimulated with human TRAP (PAR1 agonist) SFLLRN (5 μM), and aggregation was measured in an optical aggregometer. (B) Aggregation in response to varying concentrations of SFLLRN (1-10 μM) was measured as described in panel A. (WT, ○; LiCl (20 mM), ●; SB216763 (10 μM), ▲.) The results shown are the means (± SD) of 3 to 8 data points. The difference between LiCl and WT, or SB216763 and WT is statistically significant with **P < .01, *P < .05, unpaired Student t test. (C) Aggregation to 7 μM SFLLRN in the presence of the indicated concentrations (M) of LiCl or SB216763 was measured 3 to 16 times for each data point. Results were plotted and analyzed to fit a Sigmoidal dose-response in GraphPad Prizm to obtain the EC50s for each compound.
Haploinsufficiency or inhibition of GSK3β in platelets reduces phosphorylation of the GSK3β substrate microtubule-associated protein-tau
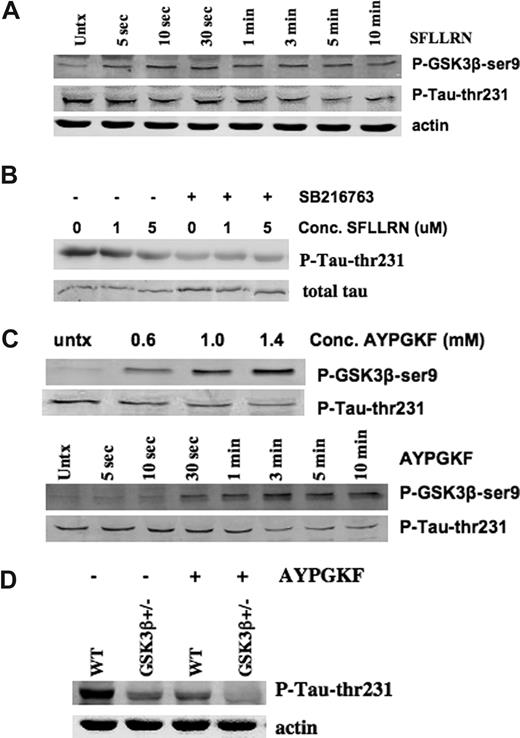
To determine the effect of agonist on GSK3β enzymatic activity, phosphorylation of the putative GSK3 substrate MAP-tau was examined. MAPtau contains many sites for phosphorylation on ser or thr residues, but phosphorylation of thr-231 has been reported to be GSK3 dependent.40-42 Therefore, we evaluated phosphorylation of this residue in mouse and human platelets with and without agonist stimulation. Since phosphorylation of GSK3β-ser9 is expected to reduce its kinase activity, phosphorylation of GSK3β substrates should decrease as GSKβ-ser9 phosphorylation increases. Figure 5A demonstrates that GSK3β becomes phosphorylated within 5 seconds of agonist (PAR1) stimulation, and its phosphorylation reaches a maximum at about 3 minutes, a time frame consistent with a role in regulating platelet aggregate formation or stability. Tau-thr231 phosphorylation decreases over this same time frame. To confirm that phosphorylation of this residue is agonist- and GSK3-dependent in human platelets, platelets were exposed to SFLLRN (or no agonist) for 3 minutes, and MAP-tau-thr231 phosphorylation was evaluated in the presence and absence of SB216763. Treatment of human platelets with (PAR1) TRAP reduced phosphorylation of this residue (Figure 5B), consistent with the hypothesis that agonist-mediated Akt activation would reduce kinase activity of GSK3β. Treatment of human platelets for 2 hours with the GSK3-selective inhibitor SB216763 also resulted in reduced phosphorylation of tau thr231, confirming that phosphorylation of this residue is GSK3 dependent (Figure 5B).
MAP-tau thr212 is dephosphorylated after agonist treatment in GSK3β+/− platelets and in human platelets treated with GSK3 inhibitors. (A) Washed human platelets (4 × 107) were incubated for the indicated times with no agonist (Untx) or SFLLRN (5 μM), lysed, and immunoblotted with antibody to phospho-GSK3β ser9, phospho-tau-thr231, or actin (the actin immunoblot shown is a reprobe of the immunoblot of phospho-GKS3β). Similar results have been obtained in 3 separate experiments. (B) Washed human platelets (4 × 107) were incubated for 2 hours in Tyrode buffer (−) or 10 μM SB216763 (+), then treated with the indicated concentration of human TRAP SFLLRN (0, 1, or 5 μM) for 3 minutes. Platelets were lysed and immunoblotted with antibody to phospho-MAPtau-thr 231. The blot was stripped and reprobed with an antibody to total MAP-tau. Similar results have been obtained in 4 experiments. (C) Washed platelets (4 × 107) from WT mice were incubated with varying concentrations of TRAP AYPGKF, lysed and immunoblottted with a phosphospecific antibody to MAP-tau thr 231 or GSK3β-ser9 (top blots). WT mouse platelets were incubated for the indicated times with 1 mM AYPFGF, lysed, and immunoblotted as above. The results shown are representative of 2 independent experiments each. (D) Washed platelets (4 × 107) from WT or GSK3β+/− mice were incubated with or without 1 mM AYPGKF, lysed, and immunoblottted with a phosphospecific antibody to MAP-tau thr 231. Blots were reprobed with an antibody to actin. A representative blot of 3 experiments is shown.
MAP-tau thr212 is dephosphorylated after agonist treatment in GSK3β+/− platelets and in human platelets treated with GSK3 inhibitors. (A) Washed human platelets (4 × 107) were incubated for the indicated times with no agonist (Untx) or SFLLRN (5 μM), lysed, and immunoblotted with antibody to phospho-GSK3β ser9, phospho-tau-thr231, or actin (the actin immunoblot shown is a reprobe of the immunoblot of phospho-GKS3β). Similar results have been obtained in 3 separate experiments. (B) Washed human platelets (4 × 107) were incubated for 2 hours in Tyrode buffer (−) or 10 μM SB216763 (+), then treated with the indicated concentration of human TRAP SFLLRN (0, 1, or 5 μM) for 3 minutes. Platelets were lysed and immunoblotted with antibody to phospho-MAPtau-thr 231. The blot was stripped and reprobed with an antibody to total MAP-tau. Similar results have been obtained in 4 experiments. (C) Washed platelets (4 × 107) from WT mice were incubated with varying concentrations of TRAP AYPGKF, lysed and immunoblottted with a phosphospecific antibody to MAP-tau thr 231 or GSK3β-ser9 (top blots). WT mouse platelets were incubated for the indicated times with 1 mM AYPFGF, lysed, and immunoblotted as above. The results shown are representative of 2 independent experiments each. (D) Washed platelets (4 × 107) from WT or GSK3β+/− mice were incubated with or without 1 mM AYPGKF, lysed, and immunoblottted with a phosphospecific antibody to MAP-tau thr 231. Blots were reprobed with an antibody to actin. A representative blot of 3 experiments is shown.
We next evaluated phosphorylation of GSK3β and MAP-tau in mouse platelets stimulated with PAR4 agonist. Figure 5C demonstrates that, as platelets are treated with increasing concentrations of (PAR4) agonist, phosphorylation of tau-thr231 decreases, while phosphorylation of GSK3β-ser9 increases. A time course for phosphorylation of GSK3β-ser9 and tau-thr231 following stimulation of mouse platelets with PAR4 agonist is also shown (Figure 5C), and again an inverse relationship between phosphorylation of GSK3β and that of tau is seen. Both events are evident within 30 seconds to 1 minute of PAR4 stimulation. To confirm that phosphorylation of MAPtau-thr231 is GSK3β dependent in mouse platelets, phosphorylation of this site was compared in WT versus GSK3β+/− platelets under both agonist-stimulated and unstimulated conditions. In unstimulated WT platelets, tau-231 is constitutively phosphorylated. Phosphorylation of this residue is reduced in GSK3β+/− platelets compared with WT platelets, consistent with the notion that phosphorylation of this site is GSK3β dependent. Phosphorylation of tau thr231 is also reduced after platelets of either genotype are stimulated with PAR4 agonist (Figure 5D), suggesting that agonist treatment induces inhibition of GSK3β kinase in platelets.
Haploinsufficiency of GSK3β increases sensitivity to thrombotic insult in mice
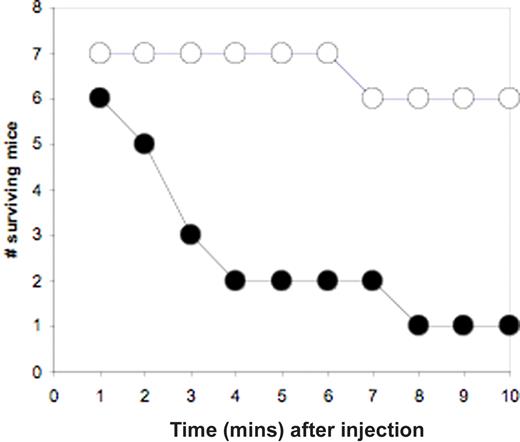
To determine whether the function of GSK3β in platelets may play a role in regulating thrombosis in vivo, responses of GSK3β+/− mice and WT age-matched controls were compared in 2 thrombosis models: a model of disseminated thrombosis (as described in Renne et al43 ) and a ferric chloride arterial injury model (as in Woulfe et al33 ). To determine the susceptibility of mice to disseminated thrombosis, mice were injected via tail vein under anesthesia (100 μg/kg pentobarbital) with a combination of collagen (170 μg/kg) and epinephrine (350 μM/kg) (methods essentially as in reference 44). The time to breathing stasis was measured, up to a total of 10 minutes, at which time the mice were killed. Figure 6 shows the number of surviving mice remaining as a function of time (in minutes). At 10 minutes, 6 of 7 WT mice survived, whereas only 1 of 6 GSK3β+/− mice survived. The number of mice surviving at 10 minutes differ significantly with P equal to .03 according to Fisher exact probability test. Hematoxylin and eosin–stained lung sections of GSK3β+/− mice compared with WT controls showed thrombi formed in the lungs of GSK3β+/− mice, but not in the lungs of surviving WT controls, indicating that all mice that died likely did so due to pulmonary embolism (data not shown), consistent with previous reports using this assay.
Disseminated thrombosis in WT versus GSK3β+/− mice. A combination of collagen (170 μg/kg) and epinephrine (350 μM/kg) was injected into the tail vein of sedated mice (7 WT, 6 GSK3β+/−), and the time until breathing stasis was recorded for 10 minutes. The number of surviving mice is graphed as a function time in minutes (WT, ○, GSK3β+/−, ●).
Disseminated thrombosis in WT versus GSK3β+/− mice. A combination of collagen (170 μg/kg) and epinephrine (350 μM/kg) was injected into the tail vein of sedated mice (7 WT, 6 GSK3β+/−), and the time until breathing stasis was recorded for 10 minutes. The number of surviving mice is graphed as a function time in minutes (WT, ○, GSK3β+/−, ●).
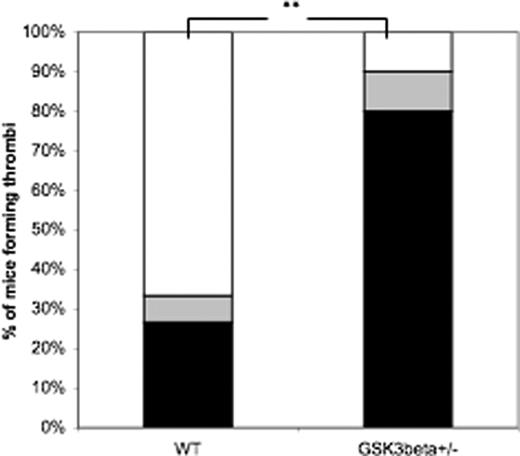
In the ferric chloride injury model, the carotid arteries of mice were exposed to a ferric chloride–soaked filter paper for 2 minutes, then arterial flow rate was measured for 30 minutes.17 Mice in which arterial flow rate decreased to no more than 10% of the original flow rate were scored as having occlusive thrombi; mice in which the flow rate returned to normal prior to the end of the assay were scored as having unstable thrombi; those in which the flow rate remained near 0 were scored as having stable thrombi. Mice whose flow rate remained in the normal range were scored as having no occlusive thrombi. As shown in Figure 7, only 33.3% of WT mice formed occlusive thrombi following 2 minutes of exposure to ferric chloride, whereas 90% of GSK3β mice formed thrombi under these conditions. The number of mice forming occlusive thrombi differed significantly between the 2 genotypes (P = .01) according to Fisher exact probability test. The flow rates in the individual mice tested are provided in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). These results suggest that GSK3β+/− mice display a hypersensitivity to thrombotic insult in models of arterial injury and agonist-induced thrombosis. These data taken together suggest that GSK3β in platelets negatively regulates platelet activation and thrombosis.
Occlusive thrombus formation following ferric chloride arterial injury. Ferric chloride was applied to the surface of the carotid artery of pentobarbital-sedated mice. Arterial flow rates were measured for 30 minutes with a Doppler flow probe (flow rates for each mouse are shown in Figure S1). Mice were scored as having stable occlusive thrombi (■), unstable occlusive thrombi (▒), or no thrombi (□). The number of mice forming occlusive thrombi differs between WT and GSK3β+/− mice (**P = .01, Fisher exact probability test). The results of 15 WT mice and 10 GSK3β mice are shown.
Occlusive thrombus formation following ferric chloride arterial injury. Ferric chloride was applied to the surface of the carotid artery of pentobarbital-sedated mice. Arterial flow rates were measured for 30 minutes with a Doppler flow probe (flow rates for each mouse are shown in Figure S1). Mice were scored as having stable occlusive thrombi (■), unstable occlusive thrombi (▒), or no thrombi (□). The number of mice forming occlusive thrombi differs between WT and GSK3β+/− mice (**P = .01, Fisher exact probability test). The results of 15 WT mice and 10 GSK3β mice are shown.
Discussion
GSK3β (glycogen synthase kinase 3β) is involved in a variety of signaling pathways and may play a contributing role in pathologies as varied as heart failure,45 Alzheimer disease,46 and sepsis.27 GSK3 was originally named for its ability to phosphorylate glycogen synthase.47 By doing so, GSK3β activity suppresses glycogen synthesis in skeletal muscle. In this context, the phosphorylation of GSK3β by Akt inhibits GSK3β activity, removing this suppressive function.24 In some respects, the roles of Akt and GSK3β in regulating platelet function would seem to parallel the roles of Akt and GSK3β in the stimulation of glycogen synthesis by skeletal muscle. That is, our results suggest that GSK3β functions as a tonic suppressor of platelet activation: its inhibition or reduced expression increases platelet aggregation and fibrinogen binding in vitro (Figures 3,Figure 4–5). Stimulation of platelets with agonists that phosphorylate and activate Akt cause phosphorylation of GSK3β on ser9 (Figure 1), and reductions in Akt expression inhibit agonist-dependent GSK3β phosphorylation (Figure 2). As in other cells, GSK3β ser9 phosphorylation is associated with inhibition of its enzyme activity, since agonist activation of platelets results in reduced phosphorylation of a known GSK3β substrate, MAP-tau (Figure 5). Inhibition of GSK3β or the absence of an allele also results in reduced phosphorylation of this putative substrate for GSK3β in platelets (Figure 5).
Although it is clear that GSK3β plays a role in phosphorylating MAP-tau in platelets, the role of MAP-tau in platelets is not defined. More than 50 substrates of GSK3 have been identified to date48 : it is not clear which of these candidate effectors may be important for regulating platelet function. We have observed that haploinsufficiency of GSK3β or treatment of platelets with LiCl or SB216763 enhances agonist-induced dense granule secretion (Figure 3C; additional data not shown) and phosphorylation of MAP-tau (Figure 5). MAP-tau is a microtubule binding protein and has been shown to regulate microtubule polymerization in neuronal cell lines.49 Thus, we are investigating a possible role for GSK3β in regulating microtubule dynamics that may contribute to platelet secretion. Microtubules have been proposed to regulate platelet secretion in the past, since inhibitors of microtubule polymerization or tubulin-blocking antibodies inhibit platelet secretion and aggregation.50,51 However, although our laboratory has also observed inhibition of platelet secretion with these agents (data not shown), the role of microtubules in platelet secretion remains controversial.52 Thus, roles for GSK3β in regulating the β3 integrin and formation of the mitochondrial permeability transition pore in platelets are also being explored. Of note, the observed enhancements in platelet function of the GSK3β+/− mice are not due to thrombocytosis: although recent reports identify a role for GSK3β in hematopoiesis,53 platelet counts in GSK3β+/− mice are normal (mean = 1.09 × 109/mL, n = 3) compared with WT control mice (mean = 1.09 × 109, n = 2).
Because our initial studies suggested a role for GSK3β in platelet activation in vitro, we next investigated a role for GSK3β in thrombosis. Haploinsufficiency of GSK3β enhanced sensitivity to thrombotic insult in 2 mouse models of thrombosis: a disseminated thrombosis model in which injection of platelet agonist results in death due to formation of pulmonary emboli, and a ferric chloride arterial injury model in which the arterial flow rate is measured following arterial injury. Reduced expression of GSK3β thus enhances thrombosis in both of these models. In contrast, the deletion or inhibition of several signaling proteins predicted to be upstream of GSK3 phosphorylation result in defects in thrombus formation: these include PI3Kg,16,17 PI3Kβ,18 Akt1,19 and Akt2.33 The suppressive effect of GSK3β on platelet function is not unexpected, given that its negative regulators Akt and PI3K each play positive roles in platelet function in vitro and in vivo. Specifically, mice lacking PI3Kγ have reduced susceptibility to thrombosis,16 and inhibitors of PI3Kβ reduce thrombus formation on collagen under flow.18 The loss of either Akt1 or Akt2 in mice (in different strain backgrounds) results in reduced sensitivity to arterial thrombosis.19,33 Building upon the results of these studies, our results suggest that platelet agonists stimulate the PI3K-dependent phosphorylation of Akt, which results in phosphorylation and inhibition of GSK3β, thereby removing its suppression of platelet activation.
Although we describe here an important role for GSK3β in the tonic inhibition of platelet activation, it is likely not the only effector of Akt to play a role in platelet signaling and function. Akt is known to phosphorylate NOS3, which has recently been demonstrated to have important effects on platelet signaling and function.20 The Akt substrate PDE3A has been shown to regulate platelet cAMP levels.22 A previous study has also suggested that GSK3β plays a role in platelets, although this study suggested a positive role for GSK3β in platelet aggregation, based upon short-term incubation of human platelets with putative GSK3β inhibitors, including LiCl.23 These results may have been due to non–GSK-selective effects, since it has previously been demonstrated that platelets incubated with LiCl require more than an hour to equilibrate to the point that intracellular concentrations of LiCl reach those in the extracellular medium.54,55
As previously mentioned, GSK3β plays myriad roles in cell signaling in diverse tissues. Accordingly, GSK3β is being studied as a therapeutic target for stroke,56 heart failure,57 Alzheimer disease,56 and sepsis.48,58 Interestingly, in each of these cases, it is the inhibition of GSK3β that is predicted to be of therapeutic benefit. Our data suggest that reducing the activity or amount of functional GSK3β may have prothrombotic consequences. Lithium has been used for many years to treat patients with bipolar disorder, and there are only rare reports of myocardial infarction (MI) following overdose.59,60 However, in a prospective study of patients treated for depressive episodes, lithium treatment was found to be associated with a higher risk of MI, although therapeutic concentrations used are well below the concentrations used to study acute platelet responses in this study.61 Our data may suggest a potential deleterious side-effect of GSK3β inhibitors in development for therapeutic use. We propose that GSK3β is a negative regulator of platelet function in vivo and in vitro. Thus, the regulation of GSK3β in platelets is an important subject for future study, as its dysregulation may predispose some individuals to thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are deeply grateful to Dr Morris Birnbaum (University of Pennsylvania, Philadelphia, PA) for providing Akt2−/− and Akt1−/− mice and Dr James Woodgett (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON) for providing GSK3β+/− mice. Thanks also to Dr Tom Force (Thomas Jefferson University, Philadelphia, PA) for critically reading the manuscript.
This work was supported by National Institutes of Health grants K01 DK066218 and R01 HL081241-01A2 (D.S.W.).
National Institutes of Health
Authorship
Contribution: D.S.W. designed, conducted, and interpreted experiments, and wrote the manuscript; D.L. conducted experiments and analyzed data; S.A. conducted experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donna S. Woulfe, Rm 408 College Bldg, 1025 Walnut St, Philadelphia, PA 19107; e-mail: donna.woulfe@jefferson.edu.