Abstract
After a decade of the “modern era” of zebrafish hematology research, what have been their major contributions to hematology and what challenges does the model face? This review argues that, in hematology, zebrafish have demonstrated their suitability, are proving their utility, have supplied timely and novel discoveries, and are poised for further significant contributions. It presents an overview of the anatomy, physiology, and genetics of zebrafish hematopoiesis underpinning their use in hematology research. Whereas reverse genetic techniques enable functional studies of particular genes of interest, forward genetics remains zebrafish's particular strength. Mutants with diverse and interesting hematopoietic defects are emerging from multiple genetic screens. Some mutants model hereditary blood diseases, occasionally leading to disease genes first; others provide insights into developmental hematology. Models of malignant hematologic disorders provide tools for drug-target and pharmaceutics discovery. Numerous transgenic zebrafish with fluorescently marked blood cells enable live-cell imaging of inflammatory responses and host-pathogen interactions previously inaccessible to direct observation in vivo, revealing unexpected aspects of leukocyte behavior. Zebrafish disease models almost uniquely provide a basis for efficient whole animal chemical library screens for new therapeutics. Despite some limitations and challenges, their successes and discovery potential mean that zebrafish are here to stay in hematology research.
Introduction
Zebrafish (Danio rerio) have contributed to hematologic research for more than 50 years. Interest in zebrafish embryology dates to the 1930s,1 and they have long been used for zoology and toxicology research.2 Their developmental hematology entered the literature in 1963 with the second appearance of zebrafish in the journal Nature, as a representative teleost fish with an intra-embryonic origin for blood.3 The first descriptions of zebrafish blood cell morphology appeared in the 1970s.4-6 The “modern” phase of zebrafish hematology research, driven by genetic experimental approaches, started just over 10 years ago with the collection of zebrafish mutants with hematopoietic defects, mostly recognized for their anemia.7-9
Genetic approaches to studying hematopoiesis were pioneered in the mouse. Originally, spontaneous mutants provided the majority of models,10 but functional studies of specific genes, using transgenic and gene targeting methods in vivo, have dominated murine research for the last 25 years. However, the genes selected for such reverse genetic functional analyses are by definition biased by prior knowledge, and a reverse genetic gene-by-gene approach is unlikely to reveal the full set of genes contributing to hematopoiesis. Indeed, the genetic basis of many congenital human blood diseases remains unknown.
Unbiased forward genetic approaches, particularly saturation mutagenesis and phenotype-based screening for mutants, sample the genome for all genetically interruptible, functionally mandatory steps in complex biologic pathways and have been of proven value in nonmammalian models (eg, Caenorhabditis elegans, Drosophila).11 Unfortunately, the hematologic system of Drosophila is different from that of vertebrates such as mammals.12 At a genetic level, hematologically important transcription factor families and signaling pathways are represented,12 but at a cellular level, Drosophila hematology is primitive compared with mammals and adaptive immunity is entirely lacking.
Outcomes from forward genetic screens in mice looking for hematopoietic mutants are now being reported, with mutants in genes both expected (Ikaros,13 c-Myb,14 Gata-115 ) and unexpected (C1galt1,16 Bcl-x(L)17 ). Although these examples show that murine genetic screens are feasible, they are logistically difficult because of their high cost and resource requirements and hence are typically scaled well below saturation screening and are currently limited to a few large projects.
Zebrafish combine an affordable, genetically tractable vertebrate model with biology particularly suited to studying early development (eg, rapid ex vivo development, high fecundity, and optical transparency). Key reverse genetic techniques elsewhere reviewed18,19 for functionally studying genes of interest in zebrafish include transient gene overexpression and knockdown, stable transgenesis,20 and recovering stable mutated alleles by TILLING (Targeting Induced Local Lesions In Genomes),21 or from catalogued libraries of insertional mutants.22 However, generally it has been zebrafish mutants from forward genetic screens that have provided the most novel hematology discoveries. Since the first reports of the large-scale forward genetic screens in zebrafish,23,24 which included collections of hematopoietic mutants,8,9 at least 8 other groups have reported undertaking forward genetic screens of diverse designs looking primarily for zebrafish hematopoietic mutants,25-32 indicating the practicality of forward genetic approaches in zebrafish. These genetic techniques are complemented by an increasing repertoire of cell biology techniques (eg, flow cytometry and transplantation33 ) and methods for in vivo functional studies of specific cell types.
This review focuses on zebrafish hematopoiesis (not lymphopoiesis or immunology). It presents an integrated picture of the anatomic, physiologic, and genetic basis for using zebrafish in hematologic research. It discusses the strengths and weaknesses of zebrafish for hematology research and disease modeling and provides examples of their most significant and novel contributions to hematology.
Normal zebrafish hematology
Embryonic hematopoiesis
The anatomy of zebrafish embryonic hematopoiesis has been frequently reviewed.18,34,35 However, several recent detailed fate-mapping and ontologic studies enable assembly of a more integrated picture than was previously possible.
Primitive/embryonic hematopoiesis occurs at 2 anatomically separate mesodermal sites in the zebrafish embryo (Figure 1A-C). Collectively, these function analogously to mammalian yolk sac hematopoiesis. Erythroid-fated cells map to the most ventral pole of the pregastrulation embryo36 ; these cells are destined to form the caudal/posterior lateral plate mesoderm (LPM) from the onset of segmentation,6 sequentially initiating expression of an hierarchy of lineage-specification and differentiation genes.37 Through morphogenetic movements, the LPM forms an axial hematopoietic tissue expressing gata1, the intermediate cell mass (ICM; Figure 1B), which contributes the first erythrocytes to the circulation.
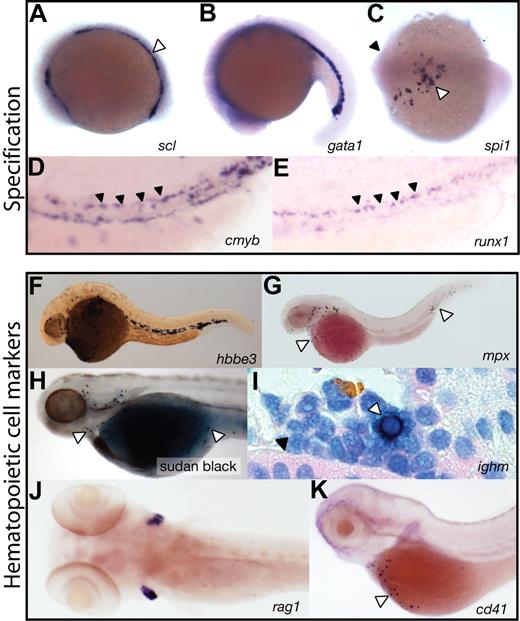
Hematopoietic specification and specific cell lineages in the zebrafish embryo. (A-E) Markers of hematopoietic specification. (A) The earliest site of primitive hematopoiesis can be identified in the posterior lateral plate mesoderm using a riboprobe for tal1(scl) (blue, open arrowhead). (B) gata1 expression (blue) in the developing intermediate cell mass (ICM). (C) spi1 (pu.1) expression (blue) in the anterior lateral plate mesoderm (open arrowhead), below the head (closed arrowhead), defines the region producing the first wave of primitive macrophages. (D,E) Definitive hematopoiesis, marked by cmyb and runx1 (blue), commences in the ventral wall of the dorsal aorta (arrowheads). (F-K) Markers of specific hematopoietic cell lineages. (F) Lineage-committed erythroid cells expressing hbbe3 (globinβe3) (blue) in the ICM and circulating cells over the yolk. (G) mpx-expressing granulocytes (blue) in circulation over the yolk and in the ventral tail (open arrowheads). (H) Sudan black cytochemistry marks the same dispersed granulocyte population (open arrowheads). (I) ighm (IgM) expression in a B lymphocyte (open arrowhead) in a kidney section, adjacent to a renal tubule (closed arrowhead). (J) T lymphocytes marked by rag1 expression (blue) in the bilateral thymi. (K) CD41 (itga2b) expression (blue) in circulating thrombocytes over the yolk (open arrowhead). (A-G,I-K) Gene expression by whole mount in situ hybridization. Microscopy was performed using a Nikon SMZ-1500 microscope (Nikon, Melville, NY) equipped with a 0.75-11.25× objective (A-H,J-K) and a Nikon Optiphot-2 microscope equipped with a 100×/1.40 oil objective (I). Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss, Thornwood, NY) with Axiovision AC software (Release 4.5). Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems, San Jose, CA).
Hematopoietic specification and specific cell lineages in the zebrafish embryo. (A-E) Markers of hematopoietic specification. (A) The earliest site of primitive hematopoiesis can be identified in the posterior lateral plate mesoderm using a riboprobe for tal1(scl) (blue, open arrowhead). (B) gata1 expression (blue) in the developing intermediate cell mass (ICM). (C) spi1 (pu.1) expression (blue) in the anterior lateral plate mesoderm (open arrowhead), below the head (closed arrowhead), defines the region producing the first wave of primitive macrophages. (D,E) Definitive hematopoiesis, marked by cmyb and runx1 (blue), commences in the ventral wall of the dorsal aorta (arrowheads). (F-K) Markers of specific hematopoietic cell lineages. (F) Lineage-committed erythroid cells expressing hbbe3 (globinβe3) (blue) in the ICM and circulating cells over the yolk. (G) mpx-expressing granulocytes (blue) in circulation over the yolk and in the ventral tail (open arrowheads). (H) Sudan black cytochemistry marks the same dispersed granulocyte population (open arrowheads). (I) ighm (IgM) expression in a B lymphocyte (open arrowhead) in a kidney section, adjacent to a renal tubule (closed arrowhead). (J) T lymphocytes marked by rag1 expression (blue) in the bilateral thymi. (K) CD41 (itga2b) expression (blue) in circulating thrombocytes over the yolk (open arrowhead). (A-G,I-K) Gene expression by whole mount in situ hybridization. Microscopy was performed using a Nikon SMZ-1500 microscope (Nikon, Melville, NY) equipped with a 0.75-11.25× objective (A-H,J-K) and a Nikon Optiphot-2 microscope equipped with a 100×/1.40 oil objective (I). Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss, Thornwood, NY) with Axiovision AC software (Release 4.5). Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems, San Jose, CA).
A separate hematopoietic domain maps dorsally in the blastula, anterior to cardiac-fated cells.38,39 These cells contribute to the rostral/anterior LPM,40 where they express spi1(pu.1) but not gata1 (Figure 1C),38,41 from which the first wave of primitive myeloid cells migrate.38,40 Some leukocytes enter the circulation.38 These actively phagocytic, embryonic macrophages distribute over the yolk and throughout the embryo, assuming specialized phenotypes as resident macrophages,42 and by 48 hours postfertilization (hpf) also form primitive neutrophils.43
The mutant cloche lacks all early tal1(scl)-dependent hematopoietic cells in both domains,44 although scattered hematopoietic gene expression still initiates later in the most posterior ICM (also called the caudal hematopoietic tissue [CHT]).45 Mutants, such as spadetail and lost-a-fin/alk8, selectively retain or lose anterior/caudal myelopoiesis, indicating that the 2 domains have independent genetic regulation.25,38,43,44
Definitive hematopoiesis initiates by 48 hpf in cellular clusters closely applied to the ventral wall of the dorsal aorta in a Notch- and Hedgehog-signaling-, runx1-dependent manner,46,47 although markers of this population (cmyb and runx1, Figure 1D,E) show much earlier (11-12 hpf) posterior LPM and ICM expression.37,44,48 This phase of zebrafish hematopoiesis is analogous to the mammalian aorta-gonad-mesonephros phase. runx1 is essential for development of aortic hemogenic precursors.46-49 From the larval stage through adulthood, the kidney is the primary hematopoietic organ.6,50-52
Fate-mapping and time-lapse imaging have collectively demonstrated the ontologic relationships between these various embryonic hematopoietic sites and their derivative cells.36,38,43,45,49 Most interestingly, the hemogenic aortic cells seed the CHT, from which arises the cells that seed adult hematopoietic organs.45,53 Before its seeding by aortic definitive hematopoietic stem cells (HSCs), the CHT appears to contain an independent, short-lived population with erythroid and myeloid potential but not lymphoid, which wanes as definitive cells fill the CHT niche.54 The circulation-dependent translocation of hemogenic cells in zebrafish unequivocally addresses a long-standing quandary in hematopoietic development55 by providing in vivo evidence that serial cell seeding, and not just serial specification at different hematopoietic sites, contributes to the relocation of hematopoiesis during vertebrate development.
Morphology and physiology of zebrafish blood cells
Because of the small size of zebrafish, quantification of normal adult hematologic indices is not as straightforward as in larger species. Peripheral blood smears demonstrate circulating erythrocytes, granulocytes, monocytes, lymphocytes, and thrombocytes (Figure 2A-D).51,52,56,57 Wide discordance in the reported relative proportions of leukocyte types probably reflects technical differences in blood collection and smear preparation.
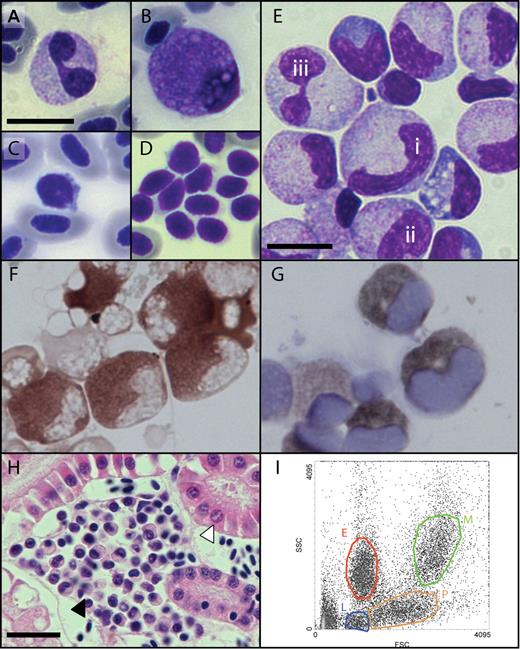
Morphology of adult zebrafish hematopoietic cells. (A-D) Peripheral blood smears showing: (A) bilobed neutrophil, (B) eosinophil, (C) lymphocyte and nucleated erythrocytes, and (D) aggregate of thrombocytes with visible cytoplasmic projections. (E-G) Kidney marrow cell cytospin showing: (E) progression of granulocyte maturation from immature (i,ii) to mature (iii) forms, (F) identification of granulocytes by myeloperoxidase cytochemistry, and (G) identification of granulocytes by Sudan black cytochemistry. (H) A cluster of hematopoietic cells (closed arrowhead) nestled between renal tubules (open arrowhead) (hematoxylin and eosin stained section). (I) FACS analysis of kidney marrow cells by forward scatter (FSC) and side scatter (SSC) separates erythroid (red), myelomonocytic (green), lymphocytic (blue), and progenitor (orange) cell populations. Bars represent 10μm (A-G) and 20μm (H). Microscopy was performed using a Nikon Optiphot-2 microscope equipped with a 40×/1.0 and 100×/1.40 oil objective. Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss) with Axiovision AC software (Release 4.5). Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems).
Morphology of adult zebrafish hematopoietic cells. (A-D) Peripheral blood smears showing: (A) bilobed neutrophil, (B) eosinophil, (C) lymphocyte and nucleated erythrocytes, and (D) aggregate of thrombocytes with visible cytoplasmic projections. (E-G) Kidney marrow cell cytospin showing: (E) progression of granulocyte maturation from immature (i,ii) to mature (iii) forms, (F) identification of granulocytes by myeloperoxidase cytochemistry, and (G) identification of granulocytes by Sudan black cytochemistry. (H) A cluster of hematopoietic cells (closed arrowhead) nestled between renal tubules (open arrowhead) (hematoxylin and eosin stained section). (I) FACS analysis of kidney marrow cells by forward scatter (FSC) and side scatter (SSC) separates erythroid (red), myelomonocytic (green), lymphocytic (blue), and progenitor (orange) cell populations. Bars represent 10μm (A-G) and 20μm (H). Microscopy was performed using a Nikon Optiphot-2 microscope equipped with a 40×/1.0 and 100×/1.40 oil objective. Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss) with Axiovision AC software (Release 4.5). Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems).
Table 1 summarizes how specific zebrafish blood cell types can be identified by marker gene expression (Figure 1F,G,I-K), cytochemistry (Figures 1H,2F,G), immunohistochemistry, or more simply using appropriate fluorescent transgenic lines to see fluorescent cells directly (Figure 3A-D).58-62 Specific hematopoietic cells can also be identified in kidney marrow cell suspensions by flow cytometry using their physical properties (Figure 2I)59 or lineage-specific fluorescence in transgenic lines,59 and in single cell suspensions prepared from whole embryos.26,49,63
Methods for identifying hematopoietic cells in zebrafish
| Cell type . | Histochemical stain(s) . | Flow cytometry59 . | Selection of riboprobe markers for whole mount in situ hybridization*† . | Antibody . | Transgenic fluorescent strain . | |
|---|---|---|---|---|---|---|
| Transcriptional marker(s) . | Lineage marker(s) . | |||||
| Hemangioblast/stem cell | — | FSCintermediate SSClow | tal1(scl)88,89 | — | — | tal1:EGFP49,156 |
| Hoechst dye efflux154 | hhex155 | |||||
| Definitive hematopoiesis onset | — | — | runx148 | — | — | cd41:EGFPlow62 |
| cmyb44 | lmo2:EGFP97 | |||||
| lmo2:dsRED97 | ||||||
| lmo244 | ||||||
| Erythrocyte | o-Dianisidine41 | FSClow SSChigh | gata141 | Globins68 | β-spectrin122 | gata1:EGFP159 |
| Diaminofluorene8 | klf485,157 | alas2125 | ||||
| znfl293 | jak2a112 | gata1:dsRED160 | ||||
| fech158 | ||||||
| Early myeloid cell | — | — | spi1(pu.1)38 | — | spi143 | spi1:EGFP58,161 |
| fli1a:GFP162 | ||||||
| Neutrophil | Myeloperoxidase51,52 | FSChigh SSChigh | cebpa163 | mpx51,52 | mpx164 | mpx:EGFP61,136 |
| Sudan black43 | mych28 | CLGY463:YFP28 | ||||
| CLGY383:YFP28 | ||||||
| Monocyte/macrophage | Neutral red42 | FSChigh SSChigh | drl40 | csf1r101 | WCL151459 | |
| Myelomonocytic‡ | — | FSChigh SSChigh | — | lcp1(l-plastin)40 | l-plastin42,72 | lyz:EGFP144 |
| lyz165 | ||||||
| mmp9166 | lyz:dsRED144 | |||||
| ncf1167 | ||||||
| Eosinophil | Periodic acid-Schiff51 | FSChigh SSChigh | — | — | gata2:EGFP59,168 | |
| T lymphocyte | — | FSClow SSClow | ikzf1(ikaros)75 | rag174 | — | lck:EGFP60 |
| rag274 | rag2:EGFP60 | |||||
| rag2:dsRED130 | ||||||
| B lymphocyte | — | FSClow SSClow | ighm76 | — | — | |
| Thrombocyte | — | FSClow SSClow | zfpm1(fog1)98 | itga2b(CD41)62 | GPIIb/IIIa80 | CD41:EGFP62 |
| mpll62 | ||||||
| Cell type . | Histochemical stain(s) . | Flow cytometry59 . | Selection of riboprobe markers for whole mount in situ hybridization*† . | Antibody . | Transgenic fluorescent strain . | |
|---|---|---|---|---|---|---|
| Transcriptional marker(s) . | Lineage marker(s) . | |||||
| Hemangioblast/stem cell | — | FSCintermediate SSClow | tal1(scl)88,89 | — | — | tal1:EGFP49,156 |
| Hoechst dye efflux154 | hhex155 | |||||
| Definitive hematopoiesis onset | — | — | runx148 | — | — | cd41:EGFPlow62 |
| cmyb44 | lmo2:EGFP97 | |||||
| lmo2:dsRED97 | ||||||
| lmo244 | ||||||
| Erythrocyte | o-Dianisidine41 | FSClow SSChigh | gata141 | Globins68 | β-spectrin122 | gata1:EGFP159 |
| Diaminofluorene8 | klf485,157 | alas2125 | ||||
| znfl293 | jak2a112 | gata1:dsRED160 | ||||
| fech158 | ||||||
| Early myeloid cell | — | — | spi1(pu.1)38 | — | spi143 | spi1:EGFP58,161 |
| fli1a:GFP162 | ||||||
| Neutrophil | Myeloperoxidase51,52 | FSChigh SSChigh | cebpa163 | mpx51,52 | mpx164 | mpx:EGFP61,136 |
| Sudan black43 | mych28 | CLGY463:YFP28 | ||||
| CLGY383:YFP28 | ||||||
| Monocyte/macrophage | Neutral red42 | FSChigh SSChigh | drl40 | csf1r101 | WCL151459 | |
| Myelomonocytic‡ | — | FSChigh SSChigh | — | lcp1(l-plastin)40 | l-plastin42,72 | lyz:EGFP144 |
| lyz165 | ||||||
| mmp9166 | lyz:dsRED144 | |||||
| ncf1167 | ||||||
| Eosinophil | Periodic acid-Schiff51 | FSChigh SSChigh | — | — | gata2:EGFP59,168 | |
| T lymphocyte | — | FSClow SSClow | ikzf1(ikaros)75 | rag174 | — | lck:EGFP60 |
| rag274 | rag2:EGFP60 | |||||
| rag2:dsRED130 | ||||||
| B lymphocyte | — | FSClow SSClow | ighm76 | — | — | |
| Thrombocyte | — | FSClow SSClow | zfpm1(fog1)98 | itga2b(CD41)62 | GPIIb/IIIa80 | CD41:EGFP62 |
| mpll62 | ||||||
FSC indicates forward scatter; SSC, side scatter; —, not applicable.
More details and a comprehensive literature listing about individual genes and strains can be found on the Zebrafish Information Network (ZFIN).169
Most specific marker in bold.
Precise leukocyte lineage specificity uncertain.
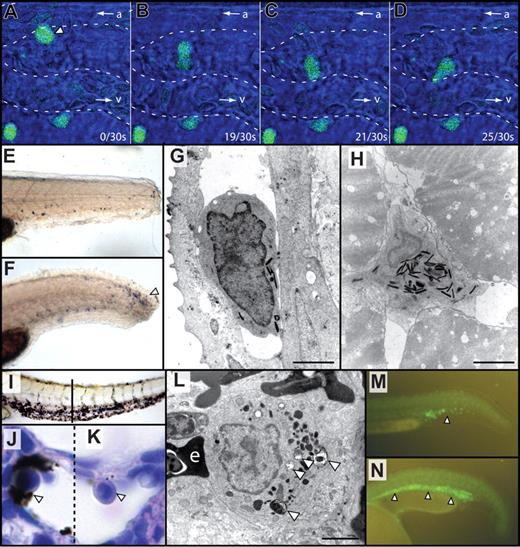
Demonstrations of hematologic cell function in zebrafish embryos. (A-D) Using spi1:EGFP transgenic animals,58 in vivo time-lapse confocal microscopy over 30 seconds shows: (A) migration of a leukocyte (arrowhead) from the arteriole (a) and its movement between cells of the extravascular compartment toward a venule (v) (B-D). Video S1 is the movie from which these 4 still images were selected; it displays more clearly the leukocyte assuming a “dumbbell” morphology as it passes between extravascular supporting cells. (E,F) Neutrophil chemotaxis mpx-expressing neutrophils (by whole mount in situ hybridization, blue) are not abundant at the site of injury 1 hour after tail transection (E) but accumulate at the injured site after 8 hours (F, arrowhead). (G,H) By electron microscopy of 7 dpf embryos, neutrophils identified by their pathognomonic electron-dense granules are demonstrated within the vasculature (G) and muscle (H) in the vicinity of a sterile wound. (I-L) Macrophage phagocytic function. After injection of India ink, there is nonembolic accumulation of carbon particles in the ventral tail (I), within a marginated phagocytic cell (open arrowhead, J), in a field including a marginated bilobed granulocyte (K) for comparison. Dashed line divides different focal planes of the same tissue section. Electron microscopy demonstrates phagosomes (open arrowheads) within an adult splenic macrophage (L). “e” indicates an adjacent erythrocyte; I is a brightfield unstained image; J and K are hematoxylin and eosin-stained sections. (M,N) Expansion of hematopoiesis by jak2a overexpression. Normal fluorescence of the ICM (arrowhead) in Tg(spi1:EGFP) embryo at 24 hpf (M). Expansion of the ICM (arrowheads) is demonstrated after injection of constitutively active zebrafish tel-jak2a (N). Bars represent 2 μm (G,H,L). Microscopy was performed using a Bio-Rad MRC1024 confocal microscope (Bio-Rad, Hercules, CA; A-D), Siemens Elmiscope 102 transmission electron microscope (Siemens, Munich, Germany; G,H,L), Nikon SMZ-1500 microscope (Nikon) equipped with a 0.75-11.25× objective (E,F,I), a Nikon Optiphot-2 microscope equipped with a 100×/1.40 oil objective (J,K) and a Leica MZFIII fluorescence microscope (Leica, Wetzlar, Germany) equipped with a 0.8-10.0× objective (M,N). Electron microscopy images were printed to photographic paper and then digitised. Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss) with Axiovision AC software (Release 4.5) or an Olympus DP70 digital camera (Olympus-Australia, Melbourne, Australia) with DP controller 1.2.1.108 software. Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems).
Demonstrations of hematologic cell function in zebrafish embryos. (A-D) Using spi1:EGFP transgenic animals,58 in vivo time-lapse confocal microscopy over 30 seconds shows: (A) migration of a leukocyte (arrowhead) from the arteriole (a) and its movement between cells of the extravascular compartment toward a venule (v) (B-D). Video S1 is the movie from which these 4 still images were selected; it displays more clearly the leukocyte assuming a “dumbbell” morphology as it passes between extravascular supporting cells. (E,F) Neutrophil chemotaxis mpx-expressing neutrophils (by whole mount in situ hybridization, blue) are not abundant at the site of injury 1 hour after tail transection (E) but accumulate at the injured site after 8 hours (F, arrowhead). (G,H) By electron microscopy of 7 dpf embryos, neutrophils identified by their pathognomonic electron-dense granules are demonstrated within the vasculature (G) and muscle (H) in the vicinity of a sterile wound. (I-L) Macrophage phagocytic function. After injection of India ink, there is nonembolic accumulation of carbon particles in the ventral tail (I), within a marginated phagocytic cell (open arrowhead, J), in a field including a marginated bilobed granulocyte (K) for comparison. Dashed line divides different focal planes of the same tissue section. Electron microscopy demonstrates phagosomes (open arrowheads) within an adult splenic macrophage (L). “e” indicates an adjacent erythrocyte; I is a brightfield unstained image; J and K are hematoxylin and eosin-stained sections. (M,N) Expansion of hematopoiesis by jak2a overexpression. Normal fluorescence of the ICM (arrowhead) in Tg(spi1:EGFP) embryo at 24 hpf (M). Expansion of the ICM (arrowheads) is demonstrated after injection of constitutively active zebrafish tel-jak2a (N). Bars represent 2 μm (G,H,L). Microscopy was performed using a Bio-Rad MRC1024 confocal microscope (Bio-Rad, Hercules, CA; A-D), Siemens Elmiscope 102 transmission electron microscope (Siemens, Munich, Germany; G,H,L), Nikon SMZ-1500 microscope (Nikon) equipped with a 0.75-11.25× objective (E,F,I), a Nikon Optiphot-2 microscope equipped with a 100×/1.40 oil objective (J,K) and a Leica MZFIII fluorescence microscope (Leica, Wetzlar, Germany) equipped with a 0.8-10.0× objective (M,N). Electron microscopy images were printed to photographic paper and then digitised. Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss) with Axiovision AC software (Release 4.5) or an Olympus DP70 digital camera (Olympus-Australia, Melbourne, Australia) with DP controller 1.2.1.108 software. Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems).
Erythrocytes and erythropoiesis.
Like avian and reptilian erythrocytes, zebrafish erythrocytes are elliptical (typically 7 ×10 μm) and nucleated9 (Figure 2A-D). As in mammals, erythroid maturation is reflected by changes in cytoplasmic shape, staining, nuclear size, and chromatin density.64 In the adult, erythropoiesis occurs primarily in the interstitium of the anterior and posterior kidneys (Figure 2H).6 Embryonic erythropoiesis is transcriptionally regulated by familiar factors, such as tal1(scl) and GATA-family transcription factors, but is also responsive to erythropoietin signaling via the erythropoietin receptor,65 JAK kinases,63 and STAT signaling molecules.66 The zebrafish globin loci are complex,67,68 encoding a range of α- and β-chains, creating considerable scope for hemoglobin switching. Histology suggests that the adult spleen functions as a reservoir of erythrocytes and a site of their destruction, rather than as a lymphoid organ. Erythrocytes serve similar functions of oxygen transport,65,69 and presumably also in metabolic regulation and blood rheology, as in mammals. Indeed, defects in enzymatic, metabolic, and cytoskeletal proteins generally result in erythrocyte abnormalities that, despite their nuclei, replicate human erythrocyte disease phenotypes (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Myeloid cells and myelopoiesis.
In the zoologic literature, the most abundant fish granulocyte has variously been called a “heterophil” or “neutrophil” granulocyte.70 The mature adult zebrafish neutrophil has a segmented nucleus with 2 or 3 lobes, expresses myeloperoxidase (mpx) abundantly, and carries cytoplasmic, multilamellated granules with a distinctive appearance electron-microscopic.51,52 Maturation from blasts to mature segmented granulocytes with accumulating granule synthesis and progressive nuclear condensation occurs in the kidney interstitium, the adult site of granulopoiesis (Figure 2E-H).50,51 Myeloperoxidase expression flags the appearance of the first recognizable granulocytes in the second day of zebrafish development.52 The number of myeloperoxidase-expressing granulocytes increases from 60 to164 per embryo over 2 to 4 days postfertilization (dpf).61 Even in early embryos, neutrophils participate in sterile acute inflammation (Figure 3E-H).52 The extent to which adult zebrafish neutrophils are phagocytic remains to be determined; in the embryo, they show only very modest phagocytic activity.43
Adult zebrafish also have eosinophilic granulocytes (Figure 2B), which carry much larger, ultrastructurally more amorphous granules.52 The function of this eosinophil granulocyte has not been demonstrated, there are no molecular markers for it, its ontogeny is not described, and one must not infer function from nomenclature. In gata2:EGFP transgenic zebrafish, eosinophils can be separated from other granulocytes in kidney hematopoietic cell suspensions on the basis of GFP expression.59
Recently, preliminary reports of a third type of granulocyte expressing carboxypeptidase have emerged,71 but a full description of this putative basophil/mast cell granulocyte has not been published.
Phagocytically active macrophages are the first leukocytes appearing in the zebrafish embryo, expressing genes such as drl, spi1(pu.1), and lcp1 (l-plastin).38,40 As other leukocyte types appear, the specificity of individual markers for specific leukocyte subtypes is uncertain, despite a range of individual cells examined for marker gene coexpression in early reports. Embryonic macrophages mark with neutral red by pinocytosis,42 exhibit avid motility, phagocytosis for cellular debris, bacteria, and foreign particles (Figure 3I-K), and participate in the inflammatory response on wounding.40,72 Adult kidney and spleen macrophages display phagosomes containing erythrocyte and other cellular debris (Figure 3L).52 Fate mapping of individual embryonic macrophages demonstrates that, as they migrate and take up residence in specific tissues, they assume specialized functions (eg, as microglia in the brain).42 The similarities and differences between embryonic and adult macrophages, and whether function specialization such as M1/M2 polarization occurs for zebrafish macrophages, remains to be determined.
Larval zebrafish lymphopoiesis initiates by day 4 in the thymus.73 Riboprobe markers have been developed for lymphocyte subtypes (eg, T lymphocytes, combining thymic location and rag1, ikaros expression,74,75 and B lymphocytes, immunoglobin gene expression76 ) (Figure 1I,J). Although some polyclonal zebrafish-specific or cross-reacting monoclonal antibody reagents have appeared en passant in the literature (Table 1), the lack of characterized monoclonal antibodies to leukocyte surface molecules presents a significant limitation to studying lymphocyte subtypes and leukocyte biology in zebrafish and precludes their use for a sophisticated cell biologic analysis of the adaptive immune response.
Thrombocytes, thrombopoiesis, and coagulation.
Nucleated zebrafish thrombocytes are present in peripheral blood and are easily distinguished by their dense nuclear chromatin, cytoplasmic projections, and aggregation in peripheral blood smears (Figure 2D). Electron microscopy reveals a canalicular structure similar to human platelets and pseudopodia-like projections in thrombocyte aggregates.57 CD41 (glycoprotein(Gp)IIb, the product of the itga2b gene) expression is first detected at 2 dpf in the CHT and by 3 dpf thrombocytes have entered circulation, where they remain in addition to clustering in the developing kidney.45,62 Fluorescent cells from cd41:EGFP transgenic zebrafish separate into 2 distinct populations of cells: CD41high cells, which are mature thrombocytes, and CD41low cells with a more undifferentiated morphology, suggesting they are earlier progenitors. It remains to be shown if CD41low cells represent thrombocyte-lineage specified cells (ie, prothrombocytes) or the pluripotent precursors seen in mammals. Zebrafish do not have megakaryocytes.
Zebrafish thrombocytes function analogously to platelets in adherence, secretion, and aggregation. Thrombocytes express surface GpIb, agglutinate without secretion in response to ristocetin, and adhere to the endothelium after injury.57 The prostaglandin pathway is functionally conserved, arachidonic acid initiates platelet aggregation, cyclooxygenases (COX)-1 and COX-2 are present, and selective COX-2 inhibition does not affect platelet aggregation. Nonspecific COX inhibition by aspirin or indomethacin inhibits thrombocyte function, indicating that zebrafish thrombocytes are dependent on functional COX-1 for aggregation/secretion.57,77 Thrombocytes aggregate in vitro in response to agonists, such as collagen, and ADP with ATP secretion and activation can be blocked by specific inhibition of the ADP receptor P2Y1.78 Although the GpIIb/IIIa complex is present on the thrombocyte surface and is probably the mediator of aggregation, this has only been directly shown in other teleost fish.79
Direct in vivo observation of arterial thrombus formation in embryos after laser-induced injury demonstrated for the first time that young thrombocytes initiate clot formation, followed by more mature thrombocytes.80 Global hemostatic function, assessed by this technique, has been used in a genetic screen for hemostatic mutants. Although not yet reported in full, one mutant mapped close to the prothrombin locus.78
Zebrafish possess all the major proteins of the coagulation system, including both vitamin K–dependent procoagulant factors, anticoagulant factors, and the fibrinolytic pathway.81 The function of the system has been measured with assays equivalent to the activated partial thromboplastin time, prothrombin time, and dilute Russell Viper Venom Time (DRVVT).81,82 Vitamin K carboxylase is present and warfarin prolongs the prothrombin time in a dose-dependent fashion when administered via the tank water.83
Regulation of zebrafish hematopoiesis
Transcriptional regulation of hematopoiesis
The transcriptional regulation of zebrafish hematopoiesis has been comprehensively reviewed.84 Overall, a high degree of conservation in the factors themselves and the sequence of their functional domains translates into a high degree of functional conservation. However, outside the DNA binding domains, there can be considerable sequence divergence (eg, the Krüppel-like factors85 ), and within some families there is gene duplication (eg, fli1a and fli1b86 ) or fish-specific members (eg, etsrp, ETS-related protein87 ).
As in mammals, the basic helix-loop-helix transcription factor tal1(scl) and its partner lmo2 sit at the apex of hematopoietic and endothelial development.88-90 gata1 and spi1(pu.1) have conserved lineage-specific erythroid and myeloid fate-specifying roles.91 runx1 and cmyb retain their connection with definitive hematopoiesis.44,48 Detailed analyses of the staggered temporal expression of hematopoietic transcription factors imply a conserved regulatory hierarchy.37,90 Probing the transcriptional regulation of hematopoiesis in zebrafish is hence probably informative for mammalian hematopoiesis, and zebrafish have proven to be a useful heterologous model for examining human disease-associated transcription factor mutations and fusion proteins involving them.48,92
Several transcription factor examples illustrate how zebrafish genetic approaches can contribute to studying hematopoietic regulatory mechanisms. New factors can be characterized functionally, and genetic interactions and epistatic relationship quickly determined in vivo (eg, znfl2a/znfl2b93,zbp-8994 ; the interplay of gata1 and spi1 for erythroid vs myeloid fate determination in vivo,91 the role of the TATA box binding protein trf3 in hematopoietic specification from mesoderm via its downstream targets mespa and cdx495 ). Regulatory elements and mechanisms have been studied (eg, separating the elements responsible for neuronal, endothelial, and hematopoietic tal1(scl) expression,64,96 identifying a functional ETS binding site in the lmo2 promoter,97 and demonstrating gata1 autoregulation by self-association98 ). Expression profiling can point to candidate gene targets (eg, klf499 ). Mutants may point to new transcriptional components critical in hematopoietic cells (eg, moonshine identified trim33 (TIF1γ), a previously poorly characterized transcriptional cofactor, as necessary for primitive and definitive erythropoiesis100 ).
Humoral regulation of hematopoiesis and cytokine signaling
In contrast to the close parallelism between mammals and zebrafish in the transcriptional regulation of hematopoiesis, there appears to be greater divergence in hematopoietic growth factor regulation.
The zebrafish type III receptor tyrosine kinase receptors csf1r and kita101,102 imply the existence of macrophage colony-stimulating factor (CSF) and stem cell factor ligands, although the ligands have not been recognized yet. The panther and sparse mutants (affecting csf1r and kita, respectively) are adult-viable, and although they both display prominent pigmentation phenotypes, they lack hematopoietic defects seen in the analogous mouse mutants.101,102
Only one csf1r homolog has been identified; it shows embryonic expression both in cells along the neural crest and in a dispersed cell population probably macrophages.101,103 The early embryonic migration of yolk-sac macrophages into tissues to become resident macrophages is defective in panther (ie, is cfms-dependent42 ), implying ligand activation of this receptor already occurs at 2 dpf.
In the case of KIT homologs, the situation is more complex, reflecting the legacy of a hypothesized whole genome duplication in fish soon after the divergence of teleost and quadruped radiations.104 kita and kitb paralogues comprise an apparently subfunctionalized gene pair, together covering the expression domains of mammalian KIT, except for primordial germ cell expression.102,105,106 Although kita shows hematopoietic expression, sparse (a kita mutant) does not have an obvious adult hematopoietic defect.102 It is proposed that “function shuffling”107 among type III receptor tyrosine kinases may have partitioned hematopoietic functions to another family member. Again, the existence of a stem cell factor homolog can be inferred, but it has not yet been characterized.
There is evidence of both substantial similarity and difference between mammalian and fish nontyrosine kinase hematopoietic growth factor receptors.108 The most comprehensive data exist for erythropoietin signaling. Zebrafish erythropoietin (epo) and its receptor (epor) have low amino acid identity compared with mammalian species and recombinant human erythropoietin is inactive in fish, but there is conservation of critical functional residues and a conserved physiologic role in the regulation of erythropoiesis.65,109
Zebrafish thrombopoietin is yet to be identified, but its role in thrombopoiesis can be inferred from the identification its receptor mpll, which shows thrombocyte-specific expression, and the similarity between the mpll zebrafish morpholino-knockdown phenotype and the mouse c-Mpl knockout phenotype.62
A granulocyte-CSF (G-CSF) receptor homolog has been recognized and confirmed as expressed by reverse-transcribed polymerase chain reaction.108 However, functional evidence for a role of this receptor in granulopoiesis is awaited. Comprehensive database mining for class I cytokine receptors suggests that multiplication of interleukin-3 receptor family members is a mammalian specialization.108 It seems probable that in zebrafish, as in chicken and other fish, myelopoiesis is regulated by a single myelomonocytic growth factor CSF3 (ie, G-CSF) ortholog.110 Hence, although a level of humoral regulation of granulopoiesis seems probable in zebrafish, they appear unlikely to be useful for dissecting nuances of interleukin-3, interleukin-5, and granulocyte-macrophage CSF signaling.
Zebrafish intracellular cytokine signaling pathways appear more conserved overall than the ligands and receptors. There are zebrafish orthologs of JAK kinases (jak1111,112,jak2a and jak2b,112 jak3113,114 ), STAT molecules (stat1 and stat3,115 stat4,116 stat5.1 and stat5.2117 ), and SOCS negative regulators (socs1,118 socs3,119 ; ZFIN lists 16 SOCS gene family members120 ). Descriptive and functional data point to their involvement in cytokine and growth factor signaling pathways mediating conserved biologic responses. Examples include hematopoietic-restricted expression of jak2a112 and the consequences of its misexpression (Figure 3M,N),63 heterologous rescue of Stat1-deficiency in vitro,115 hematopoietic expansion from stat5.1 misexpression,66 and up-regulation of socs1 and socs3 expression after lipopolysaccharide injection.118,119
Zebrafish models of hematologic disease
Zebrafish models of congenital hematologic disease
Random ENU mutagenesis has provided many zebrafish mutants with hematopoietic phenotypes. Those that are mutants of known human disease gene orthologs generally recapitulate the key human disease features, validating the use of zebrafish to model hereditary hematopoietic disorders. Novel mutant alleles of familiar genes may also provide new insights into gene function or pathogenesis. Mutants affecting novel genes point to possible new human disease loci or pathways (Table S1; Figure 4). Anemic zebrafish mutants model congenital disorders resulting from defects of structural, metabolic, and iron metabolism proteins; some representative examples follow.
Zebrafish hematopoietic mutants. (A) cloche, a mutant in the earliest acting gene in hematopoiesis, upstream of the hemangioblast marker tal1(scl), shows a lack of circulating red hemoglobinized erythrocytes circulating over the yolk compared with wild-type (arrowheads) in brightfield images (left panels), and lacks expression of gata1 (red, erythrocytes) and lcp(l-plastin) (blue, myelomonocytes) in 2-color whole mount in situ hybridization (right panels). (B) The dorsalized alk8 mutant laf gl2 lacks anterior expression of the early myeloid marker spi1(pu.1). (C) The ventralized chordin mutant dino has expansion of the intermediate cell mass with increased expression of gata1 (blue). (D) Blood smears comparing wild-type and retsina (a scl4a1 (erythrocyte band 3) mutant) erythrocytes; retsina erythrocytes have distinctive binucleate erythroblasts (closed arrowhead), reminiscent of congenital dyserythropoietic anemia type II. (A-C) Gene expression by whole mount in situ hybridization. Microscopy was performed using a Nikon SMZ-1500 microscope (Nikon) equipped with a 0.75-11.25× objective (A-C). Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss), with Axiovision AC software (Release 4.5). Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems).
Zebrafish hematopoietic mutants. (A) cloche, a mutant in the earliest acting gene in hematopoiesis, upstream of the hemangioblast marker tal1(scl), shows a lack of circulating red hemoglobinized erythrocytes circulating over the yolk compared with wild-type (arrowheads) in brightfield images (left panels), and lacks expression of gata1 (red, erythrocytes) and lcp(l-plastin) (blue, myelomonocytes) in 2-color whole mount in situ hybridization (right panels). (B) The dorsalized alk8 mutant laf gl2 lacks anterior expression of the early myeloid marker spi1(pu.1). (C) The ventralized chordin mutant dino has expansion of the intermediate cell mass with increased expression of gata1 (blue). (D) Blood smears comparing wild-type and retsina (a scl4a1 (erythrocyte band 3) mutant) erythrocytes; retsina erythrocytes have distinctive binucleate erythroblasts (closed arrowhead), reminiscent of congenital dyserythropoietic anemia type II. (A-C) Gene expression by whole mount in situ hybridization. Microscopy was performed using a Nikon SMZ-1500 microscope (Nikon) equipped with a 0.75-11.25× objective (A-C). Images were obtained using a Zeiss Axiocam MRc5 digital camera (Carl Zeiss), with Axiovision AC software (Release 4.5). Images were processed using Adobe Photoshop CS2 9.0 and Adobe Illustrator CS2 12.0.1 (Adobe Systems).
riesling (sptb, β-spectrin) and merlot/chablis (epb41, band 4.1) mutants display the typical erythrocyte abnormalities seen in human hereditary spherocytosis and elliptocytosis, respectively.122,123 Unlike the mouse β-spectrin mutant Jaundiced, riesling is homozygous adult viable, better modeling the adult viable human disease.
The retsina (slc4a1, erythrocyte band 3) mutant has binucleate erythrocytes reminiscent of congenital dyserythropoietic anemia type II (Figure 4D). Although human congenital dyserythropoietic anemia type II patients have not been found to have mutations in SCL4A1, retsina points to this pathway for further investigation as the basis for its pathogenesis.124
The heme biosynthesis mutants yquem and dracula model human porphyrias, and sauternes (an alas2 mutant) was the first animal model of congenital sideroblastic anemia.125 Although the 2 zebrafish globin loci are complex with 6 embryonic and at least 4 adult globin genes, zinfandel, which maps closely to the chromosome 3 globin locus68 without being a mutation in a globin gene per se, is potentially a model of thalassemia resulting from mutation in a cis-acting globin regulatory element.
Mutants with defects of iron metabolism have provided new physiologic insights. Although weissherbst was identified resulting from red cell hypochromia rather than for a disturbance of iron metabolism, it carries a mutation in the then novel iron transporter slc40a1 (ferroportin 1), a gene subsequently shown to be mutated in type IV autosomal dominant human hemochromatosis.126 The processes of heme biosynthesis and formation of iron-sulphur (Fe-S) clusters both require iron but had been thought to occur independently. The mutant shiraz, with a deficiency of glutaredoxin 5 (grx5, required in yeast for Fe-S cluster formation), leads to a block in heme biosynthesis at the level of alas2 leading to hypochromic microcytic anemia, linking these 2 pathways for the first time.127 Subsequently, a recessive mutation in human GRX5 has been identified in a patient with a similar phenotype.128
Several genetic screens of widely varied design have sought mutants with perturbed myeloid development, resulting in diverse outcomes, some of which have now been reported: a cohesin mutant, from an ENU screen for modifiers of runx1 expression27 ; a line with prominent YFP expression in neutrophils, resulting from an enhancer trap insertion into the promoter of the novel MYC family member mych without apparent disruption of gene function28 ; and a hai1 (hepatocyte growth factor activator inhibitor 1) mutant with a proclivity to chronic inflammation, identified in a shelf screen of insertional mutants for mpx expression.29
Several early developmental mutants without direct human disease parallels have advanced understanding of early vertebrate hematopoietic development (Figure 4). kugelig, a zebrafish mutant in the caudal-related homeobox gene cdx4, identified its pivotal role in hematopoietic specification before studies in other species.129 cloche (Figure 4A), a normally patterned mutant with both total early hematopoietic failure and an absence of anterior endothelial development, has provided genetically sound biologic evidence for the existence of the long-postulated bipotential hemangioblast.7,44 Mutants with early patterning defects, such as dino, support the notion of hematopoiesis as an early ventrally patterned fate (Figure 4C).38 Conversely, an allele of the mildy dorsalized mutant lost-a-fin, a mutant of the type I bone morphogenetic protein-receptor alk8 (Figure 4B), was surprisingly found in a screen for early myeloid failure, implicating bone morphogenetic protein signaling in early myeloid specification.25
Zebrafish models of malignant hematologic disease
Malignant hematologic diseases, including acute leukemia and myelodysplastic and myeloproliferative disorders, have been modeled in zebrafish. Many models recapitulate key pathophysiologic features of the genetically analogous human disease (Table S2).
The development of lymphoblastic leukemia models was facilitated by the early availability of promoters, providing appropriately selective spatiotemporal regulation for expressing oncogenes in lymphoid cells (eg, the rag2 promoter74 ). Early disease lethality, such as occurs with rag2:EGFP-Myc-driven acute lymphoblastic leukemia, complicated propagation of transgenic lines and fueled the need for conditional transgene expression methodologies.130 As most oncogenes act dominantly, the inclusion of fluorescent tags in oncogenic transgenes greatly facilitates both pedigree management and disease phenotype analysis. Pathways of cooperative leukemogenesis can also be assembled in zebrafish, either to test hypotheses or to build more sophisticated disease model platforms for further studies. As an example, rag2:EGFP-Myc transgenic fish develop a lethal transplantable leukemia,131 and rag2:EGFP-bcl2 fish develop an expanded lymphoid compartment without frank leukemia or lymphoma.132 Combining bcl2 overexpression with the enforced Myc expression generated a transplantable leukemia with increased resistance to γ-irradiation–induced apoptosis.132 The ETV6-RUNX1 (TEL-AML1) fusion oncogene associated with t(12;21) pediatric B-acute lymphoblastic leukemia (ALL) did not result in ALL when driven from the rag2 promoter, although when expressed ubiquitously, an ALL-like disease with the phenotypic features of B-ALL was observed at low penetrance.133
Unexpectedly, zebrafish with insertional mutations in multiple ribosomal genes showed increased tumor susceptibility, even in heterozygotes, including several occurrences of lymphoma.134 Particularly in the context of Diamond-Blackfan and Shwachman-Diamond syndromes, where disruption of ribosomal function leads to constitutional bone marrow failure and predisposition to cancer,135 this unexpected finding suggests that ribosomal gene mutation may be an important contribution to cooperative oncogenesis.
Zebrafish myeloid leukemia model development has lagged behind the lymphoid leukemia models. This is partly the result of the difficulty of finding suitable myeloid-restricted promoters; only recently has the mpx promoter been successfully used for zebrafish transgenesis.61,136 However, the experience from myeloid leukemia modeling in mice points to the importance of expressing the oncogene in the right cell at the right time, no more and no less,137 and it is probable that the same constraints apply for zebrafish myeloid leukemogenesis. Several myeloid leukemia oncogenes have been overexpressed transiently in zebrafish (eg, tel-jak2a; Figure 3M,N138 ; RUNX1-RUNX1T1(AML1-ETO)48 ). Although the observed hematopoietic perturbations indicate biologic activity of the pathways, their relevance to human disease phenotypes remains uncertain.
For any oncogenic pathway, forward genetics can be used to discover new pathway regulators; reverse genetic approaches can assess pathway cooperativity. These genetic approaches can lead to candidate therapeutic targets. However, zebrafish disease models provide a direct tool for chemical library screens,139 looking for “hit” molecules with a desired, selective in vivo pharmacologic activity.19 For example, a forward genetic screen for mutants affecting cell proliferation recovered mutants in separase and bmyb on the basis of increased phosphohistone-H3 immunostaining.140,141 Heterozygous carriers of each of these mutations have increased tumor susceptibility, indicating, albeit unsurprisingly, that tight regulation of the cell cycle is necessary for homeostasis in zebrafish also, and cell-cycle dysregulation is an important cooperative factor in oncogenesis. crash and burn (crb) was then used as the basis for a “chemical genetic” screen, in which individual whole embryos are exposed to compounds from a small molecule library looking for a salient phenotypic alteration, in this case, suppression of excessive phosphohistone-H3 immunostaining.139 This identified persynthamide, a novel compound, as a modifier of the crb phenotype.142 This approach could be successfully applied to any cell cycle or cancer pathway. The major advantage of this whole animal approach over screening for drug compounds in cell lines is that it incorporates a requirement not only for efficacy but also screens for bioavailability and toxicity. This is particularly relevant in testing potential new chemotherapeutic agents, where toxicity often limits efficacy.
Another recently reported zebrafish chemical screen, aimed at identifying new modulators of HSC, identified the prostaglandin pathway as a novel pathway critical in formation of dorsal aorta definitive HSC. Reduction of prostaglandin E2 (PGE2) decreased numbers of HSC and, conversely, PGE2 treatment increased HSC numbers. PGE2 treatment of either the whole animal (in a zebrafish model) or of bone marrow ex vivo (in a murine model) enhanced hematopoietic recovery after irradiation. The observations suggest a possible role of this pathway in leukemogenesis and suggest prostaglandin agonists as a potential new therapy for acquired bone marrow failure or after HSC transplantation to improve recovery.143
Collectively, these examples demonstrate the capability of zebrafish models for modeling malignant hematologic disease and their potential for screening for chemical and/or genetic enhancers or suppressors of malignant phenotypes, which may lead to new drugs or drug-able targets.
Zebrafish models of inflammation and infection
Inflammation and infection studies exemplify the utility of zebrafish for studying hematopoietic cell function in vivo. Leukocyte behaviors, such as migration and endothelial rolling, are readily observed by direct microscopy,40,42 but this is facilitated in zebrafish with fluorescently marked leukocytes (Figure 3A-D). At least 9 transgenic lines with fluorescent leukocytes are now available (Table 1).
Zebrafish leukocytes, even in embryos, function in host defense (Figure 3E-K). Primitive neutrophils rapidly accumulate at sterile wounds52,61 and bacterial foci43 but are less actively phagocytic than macrophages.43 Primitive macrophages avidly phagocytose particles52,144 and bacteria.40 Live cell imaging of fluorescent leukocyte trafficking permits study of the kinetics of inflammation and the cellular behaviors during it. Such studies have provided in vivo evidence that apoptosis61 and retrograde chemotaxis136,144 contribute the resolution of acute inflammation and revealed that some individual leukocytes even return to an inflammatory site after having left it.136,144 The combination of differently colored fluorescent leukocytes and pathogens promises to provide further insights into the in vivo microanatomy and cellular physiology of host-pathogen interactions.28,144 The first mutant characterized by excessive neutrophil accumulation and aberrant baseline neutrophil trafficking has recently been reported, although this functional perturbation of neutrophils does not appear to be cell autonomous.29
Infection models using bacterial and viral pathogens are being developed in zebrafish.145 The interaction of Mycobacterium marium and macrophages has been studied in particular detail, exploiting the optical transparency of zebrafish embryos and using transgenic fluorescent pathogens.146 Infection of mutants demonstrated that macrophage migration to sites of M marium infection is csfr1-independent,146 and that, in adults, adaptive immunity contributes to the host response.147
These scenarios illustrate how zebrafish enable fully integrated leukocyte function to be evaluated in vivo, and set the scene for using zebrafish genetics or chemical screening to identify new pathways and/or compounds that accelerate inflammation resolution or infection eradication.
An appraisal
Using current tools, zebrafish have the potential to offer the most when the experimental question requires a genetic approach in vivo, and when it exploits their rapid ex vivo development and/or optical transparency. Table 2 lists some current limitations to using zebrafish in hematologic research. Some of these can be overcome by systematic technical development, such as completion of the zebrafish genome project. Particularly limiting for hematologic research is the limited repertoire of antibody reagents for cell biologic and biochemical studies in zebrafish systems.
Limitations of the zebrafish model in hematology research
| Area of research . | Problem . | Possible solution(s) . |
|---|---|---|
| Anatomy | Different morphology of blood cells, eg, erythrocytes and thrombocytes are nucleated | Abstraction in experimental design, ie, seeing similarity rather than difference and learning from difference |
| Different gross anatomy, eg, what is the equivalent of the marrow stroma? | Improving depth of understanding of zebrafish physiology and anatomy will likely narrow rather than widen the apparent gaps | |
| Physiology | Lack of cell markers/antibodies | Partially addressed by rapidly expanding toolbox of fluorescently labeled cell compartments, but generation of a repertoire of zebrafish antibodies is highly desirable |
| Lack of hematopoietic cell lines | Not currently addressed | |
| Lack of biochemical reagents, eg, purified cytokines | Transient factor production based on genetic approaches | |
| Lack of in vitro differentiation system (hematopoietic cell culture assays) | Identification and purification of zebrafish hematopoietic growth factors with optimization of zebrafish cell culture techniques | |
| Lack of inbred strains, eg, for transplantation studies; to facilitate gene mapping | Limited attempts to generate inbred lines which retain hybrid vigor | |
| Genetics | Genetic divergence between fish and humans | Completion of zebrafish genome sequencing project will provide more information about missing genes, but some genetic diseases will prove difficult to model due to genome duplication and divergent evolution |
| Despite divergence in gene structure and function, zebrafish bioassays for human gene activity can still have validity | ||
| No technique for targeted gene modification by homologous recombination | TILLING (Targeting Induced Local Lesions In Genomes) allows ″off the shelf″ ordering of mutants in any gene of interest | |
| Library of characterized insertional mutants; random retroviral insertions disrupt the function of genes and ″tag″ the insertion site within the genome | ||
| Transient knockdown of any gene easily achieved using morpholino antisense oligonucleotides (stable mutant line is preferable because of the possibility of nonspecific/off target morpholino effects) | ||
| Positional cloning challenging while Genome Project still incomplete | Sanger Centre committed to complete Genome Project | |
| Limited options for conditional transgenesis or gene modification | Temperature sensitive alleles | |
| hsp70 (heat shock) promoter | ||
| Gal4-UAS transgenic approaches | ||
| Cre-recombinase or transposon-mediated recombination | ||
| Mutation of fish gene has not always recapitulated the human disease | Consider possibility of quirky phenotypes of individual alleles | |
| Despite some exceptions, fish mutants generally model the human disease persuasively, especially at the level of genetic interactions |
| Area of research . | Problem . | Possible solution(s) . |
|---|---|---|
| Anatomy | Different morphology of blood cells, eg, erythrocytes and thrombocytes are nucleated | Abstraction in experimental design, ie, seeing similarity rather than difference and learning from difference |
| Different gross anatomy, eg, what is the equivalent of the marrow stroma? | Improving depth of understanding of zebrafish physiology and anatomy will likely narrow rather than widen the apparent gaps | |
| Physiology | Lack of cell markers/antibodies | Partially addressed by rapidly expanding toolbox of fluorescently labeled cell compartments, but generation of a repertoire of zebrafish antibodies is highly desirable |
| Lack of hematopoietic cell lines | Not currently addressed | |
| Lack of biochemical reagents, eg, purified cytokines | Transient factor production based on genetic approaches | |
| Lack of in vitro differentiation system (hematopoietic cell culture assays) | Identification and purification of zebrafish hematopoietic growth factors with optimization of zebrafish cell culture techniques | |
| Lack of inbred strains, eg, for transplantation studies; to facilitate gene mapping | Limited attempts to generate inbred lines which retain hybrid vigor | |
| Genetics | Genetic divergence between fish and humans | Completion of zebrafish genome sequencing project will provide more information about missing genes, but some genetic diseases will prove difficult to model due to genome duplication and divergent evolution |
| Despite divergence in gene structure and function, zebrafish bioassays for human gene activity can still have validity | ||
| No technique for targeted gene modification by homologous recombination | TILLING (Targeting Induced Local Lesions In Genomes) allows ″off the shelf″ ordering of mutants in any gene of interest | |
| Library of characterized insertional mutants; random retroviral insertions disrupt the function of genes and ″tag″ the insertion site within the genome | ||
| Transient knockdown of any gene easily achieved using morpholino antisense oligonucleotides (stable mutant line is preferable because of the possibility of nonspecific/off target morpholino effects) | ||
| Positional cloning challenging while Genome Project still incomplete | Sanger Centre committed to complete Genome Project | |
| Limited options for conditional transgenesis or gene modification | Temperature sensitive alleles | |
| hsp70 (heat shock) promoter | ||
| Gal4-UAS transgenic approaches | ||
| Cre-recombinase or transposon-mediated recombination | ||
| Mutation of fish gene has not always recapitulated the human disease | Consider possibility of quirky phenotypes of individual alleles | |
| Despite some exceptions, fish mutants generally model the human disease persuasively, especially at the level of genetic interactions |
Reverse genetic zebrafish studies provide an efficient, relatively quick way of evaluating gene function, even for genes first attracting interest in other models.148 Morpholino oligonucleotide gene knock-down experiments are easy to perform but are vulnerable to misinterpretation and require careful attention to controls.149 However, it is their suitability for forward genetics and in vivo chemical screening that most distinguishes zebrafish from other animal models. Indeed, the most novel hematologic contributions from zebrafish result from forward genetics (eg, identification of new human disease loci firstly in fish via the mutants weissherbst126,150,151 and shiraz127,128 ; the role of cdx4 in hematopoiesis via the mutant kugelig129 ; and strengthening the case for ribosomal gene dysfunction in oncogenesis134 ) and from chemical genetics (eg, the role of prostaglandins in stem cell biology143 ).
Since the description of the anemic mutants resulting from the initial screens,8,9 at least 8 groups have reported results from forward genetic screens that collectively encompass the genetic pathways regulating development of all major hematopoietic lineages,25-32 and there are others under way. Although some forward genetic screens looking for hematologic phenotypes are being undertaken in other models, such as the mouse14,152 and medaka,153 such a decentralized, multipronged forward genetic effort at understanding the genetic regulation of hematopoiesis is difficult to envisage in any model organism other than zebrafish. Newer zebrafish screens exploit efficiencies provided by fluorescent transgenic lines and incorporate refinements, such as using mutant and transgenic strains as sensitized backgrounds. Screens that focus on hematopoietic cell function (rather than just development, proliferation, and differentiation) are also envisaged. With zebrafish, it is feasible for a small research group to contemplate a forward genetic experimental approach.
In conclusion, enough is now known about zebrafish hematology to validate using them for hematologic research and for modeling hematologic disease. Ultimately, though, it is the capacity of the model for discovery rather than for replication of what is already known by which its usefulness will be judged. This survey argues that, for hematologic research, zebrafish have demonstrated their suitability, are proving their utility, have already supplied timely and novel discoveries, and are well poised to make further significant contributions.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Barry Paw for the retsina images in Figure 4, Judy Layton and Luke Pase for images used in Figures 1, 2, and 4, Wendy Hertan for sourcing early zebrafish papers, Czesia Markiewicz for excellent photography, Judy Layton for comments on the manuscript, and Stephen Cody for help with taking the images in Figure 3A-D and Video S1. We acknowledge the cooperative spirit of the zebrafish research community and apologize to our colleagues whose work is not cited because of space constraints.
D.C. is supported by a National Health and Medical Research Council scholarship (406695), and work in the laboratory of G.J.L. is supported by the National Health and Medical Research Council 461222, 461208, and 516750 and National Institutes of Health (HL079545).
National Institutes of Health
Authorship
Contribution: D.C. and G.J.L. worked together planning, drafting, and revising the text, figures, and tables. This is a review and does not present new research. Some figure panels were generated in the course of our research. All research in the Lieschke Laboratory is conducted under appropriate approval from the Walter and Eliza Hall Institute Animal Ethics Committee.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Graham J. Lieschke, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC 3050, Australia; e-mail: lieschke@wehi.edu.au.