The Rho GTPase family member Rac1 functions in the migration of hematopoietic stem/progenitor cells in the embryo and is required for the emergence of intraembryonic hematopoiesis.
Although it is generally agreed that migrating hematopoietic stem cells (HSCs) seed the developing fetal liver, where they differentiate along most blood lineages, their origin during ontogeny has been in dispute for nearly 15 years. Moore and Metcalf proposed that both embryonic and adult hematopoiesis arise in the yolk sac of the mouse embryo, and that HSCs are carried through the bloodstream from the yolk sac to the fetal liver.1 Later work from the labs of Dzierzak, Cumano, and others pointed to an intraembryonic origin for the earliest HSCs and, because cells from yolk sac do not engraft in and reconstitute adult mice, it was argued that these cells did not play an important role in definitive hematopoiesis (reviewed in Speck et al2 ). The controversy was stirred up further by the discoveries that multipotent progenitors from the yolk sac can repopulate neonatal mice, suggesting that they need to mature outside that tissue to acquire HSC activity, and that the placenta contains HSCs (see a recent review3 and references cited therein). In this issue of Blood, Ghiaur and colleagues have used conditional genetic ablation to explore the source of the first HSCs that seed the developing fetal liver, and they provide compelling evidence in support of an extraembryonic origin for these cells.
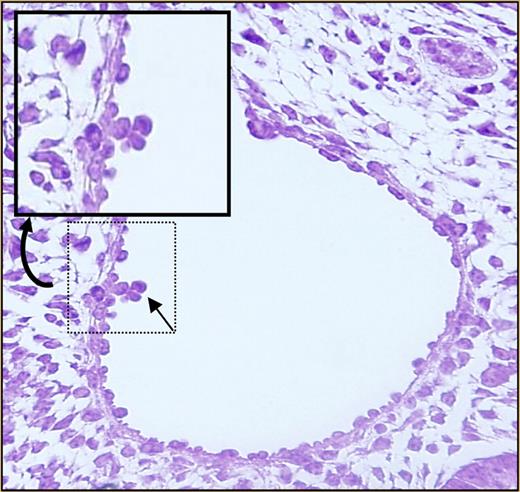
Almost nothing is known about the mechanisms underlying HSC migration to fetal tissues such as the liver, or whether this process is driven by any of the same molecules that guide homing to and engraftment within the adult bone marrow. Such molecules include stromal derived factor-1α (SDF-1α) and its receptor, CXCR4; c-kit; CD44; and β1 integrin.4 Intracellular pathways downstream from these molecules are integrated by Rho GTPases such as the Rac proteins. Previous work from Ghiaur and colleagues identified defects in engraftment of Rac1-deficient HSCs and progenitor cells (Ps) following transplantation.5 However, deletion of Rac1 after the cells had engrafted did not affect steady-state hematopoiesis.5 The authors therefore hypothesized that, in the absence of Rac1, hematopoiesis would be normal in tissues where hematopoiesis occurs de novo, but seeding of secondary sites such as the fetal liver would be reduced or absent. Using a hematopoietic tissue–specific conditional knockout approach, these investigators now show that Rac1 is not required for development of HSCs/Ps in the yolk sac, but it is required for intraembryonic hematopoiesis. Progenitor assays revealed a dramatic reduction in colony-forming units from fetal liver cells deficient for Rac1. No clusters2 were found within the aorta-gonad-mesonephros (AGM) region (see figure for a photograph of wild-type aortic clusters). The hematopoietic defect was not due to apoptosis or abnormalities in cell proliferation. Rac1-deleted HSCs/Ps in yolk sac and in the circulation showed altered migration properties in vitro. On the basis of these observations, the authors conclude that Rac1 plays a critical role in the migration of HSCs/Ps into embryonic sites, including the fetal liver.
Definitive hematopoiesis of embryos at day E10.5. See the complete figure in the article beginning on page 3313.
Definitive hematopoiesis of embryos at day E10.5. See the complete figure in the article beginning on page 3313.
The most straightforward interpretation of these results is that the yolk sac (and perhaps the placenta, another extraembryonic tissue that was not examined here) is the source of the first HSCs, and their migration into the embryo is required for normal hematopoietic development. It has not been formally excluded that HSCs do normally form de novo within the AGM region and major blood vessels,2 but not in Rac1-deficient embryos. Such a scenario would be consistent with both extraembryonic and intraembryonic locations for the emergence of HSCs. In either case, this provocative study resurrects the yolk sac as a biologically relevant source of HSCs during ontogeny.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal