To the editor:
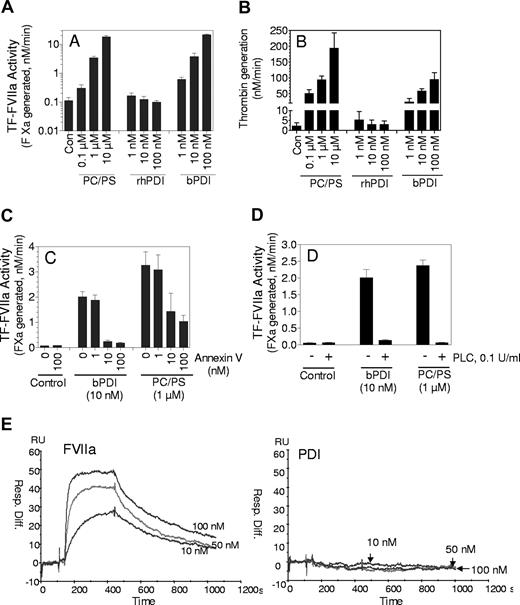
Tissue factor (TF) initiates coagulation after the binding of clotting factor VIIa (FVIIa). Only a small fraction of the total TF present on cell surfaces is coagulant-active; the majority is inactive or cryptic. At present it is not completely clear how the TF that is active in coagulation differs from the cryptic form or what mechanisms are involved in TF activation.1 It has been suggested recently that protein-disulfide isomerase (PDI), through its oxidoreductase activity, regulates TF function on cells.2,3 The validity of this hypothesis has been questioned.4 Interestingly, Versteeg and Ruf5 more recently reported that PDI, independent of its thiol-oxidoreductase activity, markedly increased TF coagulant activity. In these experiments the enhancing effect of PDI was seen only with soluble TF (sTF) or microparticle TF and used commercially obtained purified bovine PDI (Sigma-Aldrich, St Louis, MO). In contrast to the published report,5 we saw no significant effect of recombinant human PDI (rhPDI) on TF activity in our preliminary studies. In light of this discrepancy we have reexamined the effect of bovine PDI and rhPDI on sTF activity. The bovine PDI used in the present study is from the same supplier as the bovine PDI used by Versteeg and Ruf.5 Recombinant hPDI was obtained from 2 different sources, Biovision (Mountain View, CA) and RayBiotech (Norcross, GA). sTF was kindly provided by Wolfram Ruf (Scripps Research Institute, La Jolla, CA) and annexin V was provided by Jonathan Tait (University of Washington, Seattle, WA). In accordance with their report,5 bovine PDI increased the activity of sTF (Figure 1A) both markedly and in a dose-dependent manner. In contrast to the results seen with bovine PDI, rhPDI failed to increase the activity of sTF in a significant manner (Figure 1A). The dramatic difference between bovine PDI and rhPDI in their ability to enhance the activity of sTF could be a real phenomenon and reflect differences between these molecules or one of the above findings could be artifactual. It is pertinent to note here that sTF has minimal coagulant activity, which can be increased greatly in the presence of anionic phospholipids (PL).6,7 If the commercially obtained bovine PDI that was purified from liver (also a starting material for purification of PL in many cases) contained traces of PL, it would markedly increase the activity of sTF or microparticle TF without affecting the activities of relipidated or cell surface TF, exactly as observed. To test this hypothesis we have examined bovine PDI for traces of PL. The activation of prothrombin by factor Xa/Va is highly sensitive to the presence of PL. As noted with sTF, addition of bovine PDI, but not rhPDI, markedly increased prothrombin activation (Figure 1B). Addition of annexin V, a phospholipid binding protein, completely attenuated the bovine PDI-mediated increase in sTF activity (Figure 1C) and prothrombin activation (data not shown), further strengthening our hypothesis that the observed enhancing effects of bovine PDI stem from contamination of the reagent with PL. Consistent with this notion is our observation that pretreatment of bovine PDI with phospholipase C, which inhibits PL function by cleaving it at the phosphate group, abolished the enhancing effect of bovine PDI on sTF. Finally, in contrast to the data obtained with immunoprecipitation studies,5 we found no evidence for the direct interaction of PDI with TF in Biacore binding studies (Figure 1E). In summary, the enhancing effect of bovine PDI is not limited to its effects on sTF but also extends to activation of prothrombin by factors Xa/Va. Our present data suggest that the effect of bovine PDI on sTF (and prothrombin activation) is probably an artifact due to contamination of commercial bovine PDI with traces of PL.
Evidence that traces of anionic phospholipids present in commercially obtained bovine PDI is responsible for increasing sTF activity. (A) Bovine PDI but not human rPDI enhances sTF activity. The reaction mixtures were in a buffer containing 10 mM Hepes, 0.15 M NaCl, 1 mg/mL BSA, and 5 mM CaCl2 and contained sTF (10 nM), FVIIa (10 nM), and various concentrations of bovine PDI, rhPDI (0 to 100 nM) or PC/PS (80%:20% wt/wt) vesicles (0 to 10 μM). TF-FVIIa activity was measured by adding the substrate, factor X (1 μM), and measuring the amount of factor Xa generated at the end of a 10-minute reaction period in a chromogenic assay (note: y-axis is in a log scale). (B). Bovine PDI enhances prothrombin activation. Factor Xa (0.1 nM) plus FVa (10 nM) were incubated for 5 minutes with a control buffer or various concentrations of bovine PDI, rhPDI, or PC/PS vesicles, and then prothrombin (1.4 μM) was added to initiate the reaction. At the end of a 2-minute reaction period, an aliquot was removed from the reaction mixture and the amount of thrombin formed was measured in a chromogenic assay. (C) Annexin V, a phospholipid binding protein, inhibits bovine PDI-mediated increased sTF activity. Bovine PDI (10 nM) or PC/PS vesicles (1 μM) was preincubated with various concentrations of annexin V for 30 minutes and then added to sTF (10 nM). TF activity was measured as described in panel A. (D). Treatment of bovine PDI with phospholipase C abolishes the enhancing effect of bovine PDI on sTF activity. Bovine PDI (10 nM) or PC/PS vesicles (1 μM) were treated with phospholipase C (0.1 U/mL) for 15 minutes before they were added to sTF. TF activity was measured as in panel A. The data shown in panels A to D represent means plus or minus SEM (n = 3-5). (E) PDI fails to bind to sTF. A CM5 sensor chip was coated with sTF and the chip was equilibrated overnight with the buffer at a flow rate of 5 μL/min. various concentrations of FVIIa or bovine PDI (10, 50, 100 nM) were passed over the sensor chip for 5 minutes (association time), followed by 10 minutes dissociation period at a flow rate of 30 μL//min. Regeneration was performed with a 3-minute pulse of 10 mM EDTA in Hepes buffer. Similar to the data shown in Figure 1E, no binding was observed between rhPDI and sTF.
Evidence that traces of anionic phospholipids present in commercially obtained bovine PDI is responsible for increasing sTF activity. (A) Bovine PDI but not human rPDI enhances sTF activity. The reaction mixtures were in a buffer containing 10 mM Hepes, 0.15 M NaCl, 1 mg/mL BSA, and 5 mM CaCl2 and contained sTF (10 nM), FVIIa (10 nM), and various concentrations of bovine PDI, rhPDI (0 to 100 nM) or PC/PS (80%:20% wt/wt) vesicles (0 to 10 μM). TF-FVIIa activity was measured by adding the substrate, factor X (1 μM), and measuring the amount of factor Xa generated at the end of a 10-minute reaction period in a chromogenic assay (note: y-axis is in a log scale). (B). Bovine PDI enhances prothrombin activation. Factor Xa (0.1 nM) plus FVa (10 nM) were incubated for 5 minutes with a control buffer or various concentrations of bovine PDI, rhPDI, or PC/PS vesicles, and then prothrombin (1.4 μM) was added to initiate the reaction. At the end of a 2-minute reaction period, an aliquot was removed from the reaction mixture and the amount of thrombin formed was measured in a chromogenic assay. (C) Annexin V, a phospholipid binding protein, inhibits bovine PDI-mediated increased sTF activity. Bovine PDI (10 nM) or PC/PS vesicles (1 μM) was preincubated with various concentrations of annexin V for 30 minutes and then added to sTF (10 nM). TF activity was measured as described in panel A. (D). Treatment of bovine PDI with phospholipase C abolishes the enhancing effect of bovine PDI on sTF activity. Bovine PDI (10 nM) or PC/PS vesicles (1 μM) were treated with phospholipase C (0.1 U/mL) for 15 minutes before they were added to sTF. TF activity was measured as in panel A. The data shown in panels A to D represent means plus or minus SEM (n = 3-5). (E) PDI fails to bind to sTF. A CM5 sensor chip was coated with sTF and the chip was equilibrated overnight with the buffer at a flow rate of 5 μL/min. various concentrations of FVIIa or bovine PDI (10, 50, 100 nM) were passed over the sensor chip for 5 minutes (association time), followed by 10 minutes dissociation period at a flow rate of 30 μL//min. Regeneration was performed with a 3-minute pulse of 10 mM EDTA in Hepes buffer. Similar to the data shown in Figure 1E, no binding was observed between rhPDI and sTF.
Authorship
Contribution: H.K. performed the majority of the experiments described in this letter; P.S. performed the initial TF activity assays with bovine PDI and Biacore binding studies; U.R.P. and L.V.M.R. designed the research and analyzed the data; and L.V.M.R. wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao or Usha Pendurthi, Center for Biomedical Research, University of Texas Health Science Center at Tyler, 11937 US Highway 271, Tyler, TX 75708; e-mail: vijay.rao@uhtct.edu or usha.pendurthi@uthct.edu
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal