The article by Wandall and colleagues in this issue of Blood shows that in humans, galactosylation does not protect cold-stored platelets from being removed from the circulation. This evidence stands in striking contrast to previously published results in mice, and is a new reminder of how exquisitely prudent we should be when extrapolating findings from animal models to humans.
In 1970, Murphy et al published a new method for preparation of platelet concentrates from whole blood donations and storage of these platelets at 22°C under continuous agitation.1 Just a year before, they had reported that transfused platelets stored at 4°C showed reduced survival. For many years, it was thought that this reduced survival was due to the shape change induced in platelets by low temperatures. However, inhibitors of the shape change induced by the cold did not preclude platelets from being cleared from the circulation, so other mechanisms had to be involved.
In 2003, Hoffmeister et al elegantly demonstrated that, in mice, reduced circulatory survival of short-term cold-stored platelets was due to clustering of the glycoprotein (GP) complex (Ibαβ-IX)2-V. The clustering of complex GP Ib-IX-V resulted in the recognition of exposed β-N-acetylglucosamine (β-GlcNAc) by the αMβ2 integrin receptor (CD11b/CD18, Mac-1) present on hepatic macrophages, which led to phagocytosis.2 The same group later showed that galactosylation of surface β-GlcNAc residues on platelet glycoproteins, mainly GPIbα, prevented the phagocytosis of chilled murine platelets; galactosylation of human platelets also prevented phagocytosis after cold storage in an in vitro phagocitic model using a human monocytic leukemia derived cell line (THP-1).3
The publication of these findings created high expectations in the transfusion medicine community, since the refrigerated storage of platelet concentrates would offer advantages compared with storage at 22°C: easier logistics and a cheap method for inhibiting bacterial growth in contaminated units. Unfortunately, the data reported by Wandall and colleagues in this issue of Blood demonstrate that, in contrast to previously published studies in mice, galactosylation of human platelets does not prolong their survival in circulation.
In vivo studies of radiolabeled platelets in healthy volunteers revealed that the mean survival of galactosylated and nongalactosylated platelets stored for 36 to 48 hours at 4°C was very similar, at 2.2 and 2.9 days, respectively; the mean survival of platelets stored at 22°C was 6.8 days. Although galactosylation was studied in platelets from only 4 individuals, the consistency of the results observed and their similarity to values obtained with standard cold-stored platelets, in this particular study as well as many others in the past, lends weight to these findings.
The authors tried to identify the causes of such a notable discrepancy between the findings of their study and previous reports on mice. Two seem likely: in the previously published murine studies, platelets were exposed to cold for only 2 hours (a very short time), and were resuspended in buffer (no plasma). It turns out that the changes induced by this short refrigerated storage, which is deleterious to platelet survival, are prevented by galactosylation. Unexpectedly, longer periods of refrigerated storage of murine and human platelets (classically for human platelets in plasma, more than 18 hours at 4°C) elicit irreversible changes that are not prevented by galactosylation.
New lines of research are being pursued in order to identify the changes in platelets caused by long-term cold storage and develop new strategies for preventing them.4,5 Meanwhile, we are left at the same point established by Murphy et al almost 40 years ago: when storing platelet concentrates for transfusion, a temperature of 22°C under continuous agitation is, still today, the only option.
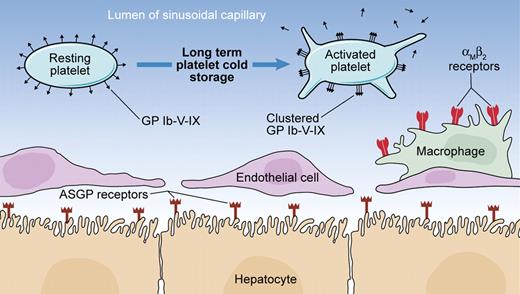
Potential mechanisms of clearance of long-term cold-stored murine platelets from the circulation. Long-term cold-stored platelets undergo clustering of the platelet GP complex (Ibαβ-IX)2-V, resulting in the recognition of exposed β-N-acetylglucosamine residues by αMβ2 receptors on hepatic macrophages and phagocytosis. It has been also reported that with long-term cold storage, galactose residues cluster sufficiently to induce recognition by hepatocyte asialoglycoprotein (ASGP) receptors and subsequent phagocytosis. The shedding of GP Ibα and GPV due to metalloproteinase ADAM17 activity observed during prolonged cold storage and rewarming is associated with a reduced survival of platelets in the circulation, although the implicated mechanisms have not been fully elucidated. Illustration by Kenneth Probst.
Potential mechanisms of clearance of long-term cold-stored murine platelets from the circulation. Long-term cold-stored platelets undergo clustering of the platelet GP complex (Ibαβ-IX)2-V, resulting in the recognition of exposed β-N-acetylglucosamine residues by αMβ2 receptors on hepatic macrophages and phagocytosis. It has been also reported that with long-term cold storage, galactose residues cluster sufficiently to induce recognition by hepatocyte asialoglycoprotein (ASGP) receptors and subsequent phagocytosis. The shedding of GP Ibα and GPV due to metalloproteinase ADAM17 activity observed during prolonged cold storage and rewarming is associated with a reduced survival of platelets in the circulation, although the implicated mechanisms have not been fully elucidated. Illustration by Kenneth Probst.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal