Proapoptotic Bcl-2 family member Bax is a crucial protein in the induction of apoptosis, and its activation is required for this process. Here we report that Bax is a short-lived protein in malignant B cells and Bax protein levels decreased rapidly when protein synthesis was blocked. Malignant B cells were relatively resistant to tumor necrosis factor–related apoptosis inducing ligand (TRAIL)–induced apoptosis, and this correlated with low basal Bax protein levels. Furthermore, during treatment with TRAIL, the resistant cell lines showed prominent Bax degradation activity. This degradation activity was localized to mitochondrial Bax and could be prevented by truncated Bid, a BH3-only protein; in contrast, cytosolic Bax was relatively stable. The proteasome inhibitor bortezomib is a potent drug in inducing apoptosis in vitro in malignant B-cell lines and primary chronic lymphocytic leukemic (CLL) cells. In CLL cells, bortezomib induced Bax accumulation, translocation to mitochondria, conformational change, and oligomerization. Accumulation and stabilization of Bax protein by bortezomib-sensitized malignant B cells to TRAIL-induced apoptosis. This study reveals that Bax instability confers resistance to TRAIL, which can be reversed by Bax stabilization with a proteasome inhibitor.
Introduction
Bax is a critical element in the induction of apoptosis, and having adequate levels of intracellular Bax protein is crucial for cells to die by apoptosis in response to death signals. During the apoptotic process, Bax translocates from the cytosol to the mitochondria and undergoes conformational changes. Insertion into the mitochondrial membrane is essential for the proapoptotic activity of Bax. However, Bax protein has a shortened half-life in cancer cells because of greatly increased proteasome-dependent degradation activity.1 Low levels of, or absent, Bax protein in malignant cells are associated with significant resistance to cancer therapy. Instability of Bax has been found in several types of malignant cells, including Jurkat T-cell and pre-B acute lymphoblastic leukemia 697 cell lines,2 primary chronic lymphocytic leukemic cells (CLL),3 cervical cancer Hela cell line,4 and advanced human prostate cancer.1 Decrease of Bax protein was significantly correlated with a poor prognosis in prostate cancer1 and primary superficial-spreading melanoma.5
Recently, studies have revealed that many proapoptotic molecules are substrates and targets for ubiquitin/proteasome degradation, including p53,6 truncated Bid (tBid),7 Bax,1,4 ARTS [apoptosis-related protein in the TGF-beta signaling pathway],8 NOXA,9 and Bim.10 Alteration of the stability of these proteins through the ubiquitin/proteasome-regulated pathway generally contributes to apoptosis resistance and poor prognosis in cancer cells. However, it is unclear whether the status of protein degradation by the ubiquitin/proteasome system could be altered by the treatment of cancer.
The levels of all intracellular proteins are determined by the duality of regulation:protein synthesis versus degradation. Basal Bax protein levels or the ratios of Bax/Bcl-2 have been considered to be critical factors for the sensitivity of malignant B cells to anticancer drugs.11,12 Killing of malignant cells by induction of Bax expression commonly occurs with DNA damaging agents via p53-dependent and p53-independent pathways.13,,–16 Proteasome inhibitors also have the ability to increase or maintain Bax protein levels, which are critical for Bax activation, when used alone or in combination with other agents.17,,–20 Tumor necrosis factor–related apoptosis inducing ligand (TRAIL) is a promising anticancer agent. However, malignant B cells are resistant to TRAIL-induced apoptosis.21,22 We previously found that Bax protein levels decline in leukemic cell lines during treatment with TRAIL.23 It is unclear whether this could be one of the mechanisms of the resistance of malignant B cells to TRAIL.
Bortezomib (PS-341, Velcade) is a novel, first-in-class proteasome inhibitor with antitumor activity against several hematologic and nonhematologic malignancies.24 In vitro studies showed that Bortezomib selectively inhibits proteasome-dependent degradation of p53,25 IκB,26 p21,27 Noxa,9,28 and TRAIL receptors DR4 and DR5.29 Proteasome inhibitors have been shown to be a promising approach for overcoming the resistance of tumor cells to TRAIL-induced apoptosis.29,,,,,–35
Overexpression of Bcl-2 is one of the characteristics of malignant B cells. However, the role of Bax on malignant B-cell survival and resistance to therapy has not been widely studied. This study has looked at Bax protein instability in malignant B cells and the critical role of bortezomib in the induction of Bax activation.
Methods
This study was approved by East London and The City Research Ethics Committee.
Materials
Bortezomib was provided by Millennium Pharmaceuticals (Cambridge, MA). Soluble TRAIL was from BIOMOL (Exeter, United Kingdom). Annexin V–fluorescein isothiocyanate (FITC) kit, anti-Bax clone 6A7, and clone 3 monoclonal antibodies were purchased from BD Biosciences (San Diego, CA). M-450 Rat anti–mouse immunoglobulin G1 (IgG1) Dynalbeads was from Dynal Biotech (Wirral, United Kingdom)). The anti-Bax 2D2 monoclonal antibody (clone YTH-2D2) and the recombinant caspase-8 cleaved Bid were from R&D Systems (Oxford, United Kingdom)). The anti–Bcl-2 (100), anti–Bcl-XL, and anti-Bax antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). MitoTracker red CMXRos was from Invitrogen (Carlsbad, CA). RNeasy mini kit was from QIAGEN (West Sussex, United Kingdom)). The first-strand synthesis kit was purchased from Invitrogen (Paisley, United Kingdom)). The proteasome substrate III, Suc-Leu-Leu-Val-Tyr-7-Amino-4-Methylcoumarin (Suc-LLVY-AMC), was from Alexis Biochemicals (Nottingham, United Kingdom). Cycloheximide (CHX), propidium iodide (PI), AMC, the monoclonal anti–β-actin antibody, ubiquitin, anti-ubiquitin serum, protease inhibitor cocktail, and other chemicals were from Sigma Chemical (Poole, United Kingdom).
Cell culture and clinical samples
Epstein-Barr virus (EBV)–transformed human B cell, the HRC57 cell line (indicated as HRC in this article, provided by CRUK [Cancer Research UK] cancer cell services), and the human diffuse large B-cell lymphoma cell lines, CRL,36 DoHH2,37 and DHL-438 and human leukemic cell line K562, were used in this study. Cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 25 mM HEPES, and 2.0 mM l-glutamine at 37°C in a 5% CO2 humidified incubator. Peripheral blood was collected after written informed consent was obtained from patients with CLL after clinical approval. The mononuclear cells were isolated by density centrifugation over Ficoll.
Apoptosis assays by flow cytometry
Apoptosis was determined by both annexin V and DNA content assays. The annexin V assay was performed according to the protocol of the annexin V-FITC kit. Whole cells in the binding buffer suspension were stained with 5 μL annexin V-FITC and 10 μL PI for 15 minutes at room temperature in the dark. Annexin V-FITC and PI fluorescence were measured on the FL1-H and FL3-H channels, respectively, by flow cytometry (BD Biosciences [San Jose, CA] FACScan). Annexin V–positive cells (both PI negative and positive) were defined as apoptotic. DNA content was measured by flow cytometry, as described previously.15
Preparation of cellular fractions
Cells (5 × 107) were washed in Ca2+/Mg2+-free phosphate-buffered saline (PBS) and suspended in 1 mL buffer A (250 mM sucrose, 10 mM HEPES-KOH, pH 7.4, 10 mM KCl, 1 mM ethylenediaminetetraacetic acid [EDTA], 1 mM EGTA, 1 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF), protease inhibitor cocktails, and 20 μM cytochalasin B) and incubated for 20 minutes on ice. Cells were then broken with a glass Dounce homogenizer. Nuclei were separated by spinning at 790g for 10 minutes at 4°C. The postnuclear supernatant was further spun at 10 000g for 10 minutes at 4°C to separate cytosol and mitochondria. The crude mitochondrial pellet was purified by passing through a sucrose gradient (100-300 mM) cushion at 9000g for 8 minutes.39
Proteasome activity assay
Cytosolic or mitochondrial proteins (50 μg) were diluted to 90 μL with fluorogenic assay buffer (20 mM PIPE-KOH, pH 7.4, 10 mM DTT, 10% sucrose, 1.0 mM EDTA, and 0.1% CHAPS). The reaction was initiated by the addition of 10 μL of 400 μM (final concentration was 40 μM) fluorescent substrate, Suc-LLVY-AMC for the chymotrypsin-like peptidase activity of the 20S proteasome. After incubation at 37°C for 15 minutes, the reaction was stopped by the addition of 50 μL of 1% sodium acetate trihydrate in 175 mM acetic acid and cooling on ice. The fluorescence at 380/460 nm for AMC release by the proteasome cleavage was measured using a Bio-Tek Instruments Synergy HT Multi-Detection Microplate Reader (Winooski, Vermont). Measurements were calibrated against a standard linear regression curve of AMC. Proteasome activity was defined as μM AMC release per milligram protein per minute (μM/min/mg protein).
In vitro Bax degradation assay
Mitochondria or cytosol in 5 mg/mL protein concentration were incubated in the Buffer B (250 mM sucrose, 10 mM HEPES-KOH, pH 7.4, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, protease inhibitor cocktail 1:100, 50 μg/mL creatine phosphokinase, 10 mM phosphocreatine, and 2 mM adenosine triphosphate). After the addition of 2 μg/mL ubiquitin, the reaction mixture was incubated at 37°C for up to 3 hours in the buffer B (without any detergent). Equal amounts of reaction mixtures were taken out at each indicated time point to determine the Bax protein level by Western blotting.
Detection of Bax conformational change and ubiquitination by immunoprecipitation
After treatment with bortezomib, cells were washed with PBS and lysed with the Chaps buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 1% CHAPS, 1 mM DTT, 0.1 mM PMSF, 3 μg/mL aprotinin, 25 μg/mL leupeptin, and 25 μg/mL pepstain). M-450 rat anti–mouse IgG1 Dynalbeads (20 μL) were preincubated with 1 μg anti-Bax (6A7) antibody at 4°C on the rotor for 3 hours. The cell lysates were normalized for protein content. Then 500 μg of total protein in 300 μL Chaps lysis buffer was mixed with Bax (6A7) antibody-loaded Dynalbeads. Immunoprecipitation was performed at 4°C on a rotator overnight. After rinsing 4 times with lysis buffer, beads were collected with a Dynal Magnetic Particle Concentrator. Conformationally changed Bax protein was eluted with 25 μL loading buffer for Western blotting. The anti-Bax 2D2 antibody was used to detect the conformational changed Bax,16 and the polyclonal antiubiquitin serum was used to determine Bax–ubiquitin conjugation.
Immunofluorescence analysis of Bax translocation
To detect Bax translocation to mitochondria, intact cells were first labeled with the mitochondrion-specific dye, MitoTracker red CMXRos. Cells in culture medium were incubated with 100 nM MitoTracker at 37°C for 15 minutes. After washing, cells were fixed/permeabilized on slides. Cells were incubated with the anti-Bax clone 3 antibody, which only detects active Bax, at a 1:50 dilution for 1 hour and then incubated with FITC-conjugated anti–mouse secondary antibody (Sigma) at a 1:20 dilution. Slides were washed with PBS, air-dried at 4°C in the dark, and stained with 4,6-diamidino-phenylindole (DAPI) before being viewed under a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Jena, Germany).15,17,39
Reverse transcription–polymerase chain reaction
Total RNA was extracted with the RNeasy mini kit. One to 5 μg RNA was used for reverse transcription. cDNA was synthesized with the first-strand synthesis kit and amplified with Bax primers (5′-CCCTTTTGCTTC-AGGGTTTC, and 3′ primer TGTTACTGTCCAGTTCGTCC size 151 bp) or actin primers (5′-GGAACGGTGAAGGTGACAG and 3′ primer GGGACAA AAAGGGGGAAG size 338 bp). Polymerase chain reaction (PCR) program was set up as follows: cDNA templates denatured at 94°C for 2 minutes, followed by 30 cycles of denaturation at 94°C for 15 seconds, annealing at 56°C for 20 seconds, and an extension at 72°C for 20 seconds. Additional extension was at 72°C for 10 minutes. The PCR product was detected in 2% agarose gel.
Results
Bax is a short-lived protein in malignant B-cell lines
The expression of the Bcl-2 family of proteins was determined in the malignant B-cell lines, CRL, DoHH2, and DHL-4 and compared with the EBV-transformed B-cell line HRC. Malignant B cells overexpressed Bcl-2 protein and some of them also had higher levels of Bcl-XL. Bax protein expression was significantly lower in the malignant B-cell lines, CRL, and DoHH2 compared with HRC cells. In agreement with a previous study,38 neither Bax nor Bak protein was detected in the DHL-4 cell line. Expression of Bak was not significantly altered in the CRL and DoHH2 cell lines compared with HRC (Figure 1A). Importantly, Bax mRNA levels were similar in these cell lines and were not consistent with their protein levels (Figure 1B). The DHL-4 cell line, which lacked Bax protein expression, had plentiful Bax mRNA. These results suggested that the regulation of Bax expression in these cell lines may occur at the posttranslational level.
Bax protein instability. (A) Expression of Bcl-2, Bcl-XL, Bak, and Bax proteins. A total of 25 μg proteins/each lane was loaded to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The anti–Bcl-2 (100) and anti–Bcl-XL antibodies were used at 1:200 dilution; anti-Bax (2D2) antibody was used at 1:1000 dilution, anti-Bak antibody was used at 1:200 dilution, and anti–β-actin antibody was used at 1:10 000 dilution. (B) RT-PCR detection of Bax mRNA levels. (C) The lifespan of Bax protein. HRC, CRL, or DoHH2 cell lines were preincubated with or without 50 nM bortezomib (Borte) for 2 hours and then treated with 50 μg/mL CHX for 3 hours. Proteins were extracted hourly. Bax levels were evaluated by Western blotting using Bax 2D2 antibody at 1:1000 dilution. β-Actin antibody was used at 1:10 000 dilution. Numbers under each pair of blots are the ratio of Bax/β-actin.
Bax protein instability. (A) Expression of Bcl-2, Bcl-XL, Bak, and Bax proteins. A total of 25 μg proteins/each lane was loaded to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The anti–Bcl-2 (100) and anti–Bcl-XL antibodies were used at 1:200 dilution; anti-Bax (2D2) antibody was used at 1:1000 dilution, anti-Bak antibody was used at 1:200 dilution, and anti–β-actin antibody was used at 1:10 000 dilution. (B) RT-PCR detection of Bax mRNA levels. (C) The lifespan of Bax protein. HRC, CRL, or DoHH2 cell lines were preincubated with or without 50 nM bortezomib (Borte) for 2 hours and then treated with 50 μg/mL CHX for 3 hours. Proteins were extracted hourly. Bax levels were evaluated by Western blotting using Bax 2D2 antibody at 1:1000 dilution. β-Actin antibody was used at 1:10 000 dilution. Numbers under each pair of blots are the ratio of Bax/β-actin.
The stability of Bax protein was tested in HRC, CRL, and DoHH2 cell lines after they were treated with CHX, a protein synthesis inhibitor, and the lifespan of Bax protein was examined by Western blotting. Bax protein was found to be stable in the transformed HRC cell line but not in the malignant B-cell lines (Figure 1C). However, CHX-mediated Bax degradation in both CRL and DoHH2 cell lines was completely inhibited by pretreatment with bortezomib (Figure 1D). The expression of Bak remained unchanged in all cell lines tested, suggesting that Bak protein is relatively stable (data not shown). These results indicate that Bax, but not Bak, is a short-lived protein in malignant B cells, and its instability varies among these cells. Importantly, Bax degradation can be inhibited by bortezomib.
TRAIL induces Bax degradation in malignant B-cell lines
The malignant B cells with low Bax protein expression, CRL and DoHH2, were found to be more resistant to TRAIL-induced apoptosis compared with the HRC cell line. DHL-4 cells, which had no detectable Bax protein, were highly resistant to TRAIL (Figure 2A). The inverse correlation of Bax protein levels and sensitivity to TRAIL led us to examine the stability of Bax during treatment with TRAIL. A reduction in Bax protein was detected in the TRAIL-resistant CRL and DoHH2 cell lines, but not in the sensitive HRC cell line (Figure 2B), indicating that Bax protein degradation occurs in malignant B cells during TRAIL treatment in resistant cell lines. The expression of Bcl-2 and Bak was not significantly altered (data not shown). These results demonstrate that treatment with TRAIL is associated with enhanced Bax instability in resistant B-cell lines.
Effect of TRAIL-induced Bax regulation. (A) TRAIL-induced apoptosis was assessed with the annexin V assay using flow cytometry. Data shown are means (± SD) from 3 independent experiments. Error bars indicate SD. (B) Proteins were extracted from HRC, CRL, or DoHH2 cells, which were treated with TRAIL for up to 24 hours.
Effect of TRAIL-induced Bax regulation. (A) TRAIL-induced apoptosis was assessed with the annexin V assay using flow cytometry. Data shown are means (± SD) from 3 independent experiments. Error bars indicate SD. (B) Proteins were extracted from HRC, CRL, or DoHH2 cells, which were treated with TRAIL for up to 24 hours.
Proteasome inhibition promotes apoptosis in the TRAIL-resistant DHL-4 cell line
As shown above, the DHL-4 cell line lacks Bax protein expression but not Bax mRNA (Figure 1). Bortezomib at 20 nM induced neither Bax accumulation nor apoptosis in DHL-4 cells. We were interested in whether proteasome inhibition could lead to an accumulation of Bax protein and TRAIL sensitization in the DHL-4 cell line. After DHL-4 cells were incubated with a high dose of bortezomib (100 nM) for 24 hours, Bax protein accumulation was observed by Western blotting, whereas the Bax mRNA levels remained unchanged (Figure 3A,B). DHL-4 cells were highly resistant to TRAIL-induced apoptosis, showing no apoptosis when cells were treated with TRAIL alone. High-dose bortezomib caused an accumulation of Bax protein in DHL-4 cells and sensitized these cells to TRAIL-induced apoptosis (Figure 3C). These results further confirmed that the down-regulation of Bax in malignant B cells is regulated at the posttranslational level. Bax degradation contributes to resistance of malignant B cells to TRAIL-induced apoptosis, which can be reversed by proteasome inhibition, leading to stabilization of Bax protein levels.
Bortezomib-induced Bax protein accumulation in DHL-4 cells. DHL-4 cells were treated with 100 nM/L bortezomib for 24 hours. Cells were collected for protein and mRNA extraction. (A) Bax protein expression was determined with Western blotting. Anti-Bax antibody 2D2 was used at 1:1000 dilution. Anti–β-actin antibody was used at 1:10 000 dilution. (B) Bax or actin mRNA was determined by RT-PCR. (C) TRAIL and bortezomib-induced apoptosis in the DHL-4 cell line. After DHL-4 cells were incubated with or without 100 nM/L bortezomib for 24 hours, cells were incubated with (B + T) or without 500 ng/mL TRAIL (Borte) for another 24 hours. Significantly increased sensitivity (P < .0001; t test) of cells treated with bortezomib and TRAIL was seen compared with cells treated with bortezomib or TRAIL alone. B + T indicates bortezomib plus TRAIL; and error bars, SD.
Bortezomib-induced Bax protein accumulation in DHL-4 cells. DHL-4 cells were treated with 100 nM/L bortezomib for 24 hours. Cells were collected for protein and mRNA extraction. (A) Bax protein expression was determined with Western blotting. Anti-Bax antibody 2D2 was used at 1:1000 dilution. Anti–β-actin antibody was used at 1:10 000 dilution. (B) Bax or actin mRNA was determined by RT-PCR. (C) TRAIL and bortezomib-induced apoptosis in the DHL-4 cell line. After DHL-4 cells were incubated with or without 100 nM/L bortezomib for 24 hours, cells were incubated with (B + T) or without 500 ng/mL TRAIL (Borte) for another 24 hours. Significantly increased sensitivity (P < .0001; t test) of cells treated with bortezomib and TRAIL was seen compared with cells treated with bortezomib or TRAIL alone. B + T indicates bortezomib plus TRAIL; and error bars, SD.
Bax degradation occurs in the mitochondrion and can be prevented by tBid or bortezomib
To understand why TRAIL triggers Bax degradation and whether it is associated with Bax translocation to mitochondria, the location of Bax degradation was tested. Mitochondria isolated from the human leukemic K562 cell line were used as a model because they possess an inactive form of the Bax protein.16,40 Cytosol was extracted from the DHL-4 cell line, which does not contain detectable Bax protein (Figure 1A). The resting proteasome activity was tested in both cytosolic and mitochondrial fractions. Proteasome activity was found to be mainly located in the cytosol. Interestingly, the DHL-4 cytosol had greater proteasome activity than K562 cytosol (Figure 4Ai). Bortezomib significantly inhibited proteasome activity in both DHL-4 and K562 cytosol (Figure 4Aii). Mitochondrial Bax degradation occurred when K562 mitochondria were incubated with K562 cytosol in the presence of ubiquitin, and it was prevented by tBid. Interestingly, Bax protein degradation was not observed in the K562 cytosol (Figure 4B). The interaction of tBid with Bax at the mitochondrial level is a critical intermediate step in TRAIL-induced apoptosis in type II cells.41 The effect of tBid in Bax degradation was tested at the mitochondrial level. Mitochondrial Bax protein was stable when the K562 mitochondria were incubated in the degradation buffer containing ubiquitin for 3 hours, indicating that cytosol is required for the degradation. Similarly, Bax was also relatively stable when the K562 mitochondria were incubated with K562 cytosol without ubiquitin (Figure 4C). When the K562 mitochondria were incubated in the DHL-4 cytosol in the absence of ubiquitin, the mitochondrial Bax protein disappeared within 30 minutes, indicating that the mitochondrial Bax is susceptible to the degradation system present in the DHL-4 cytosol, probably because of the higher proteasome activity in the DHL-4 cytosol. Bax degradation was prevented in the presence of tBid (Figure 4D). To test whether the mitochondrial Bax degradation is proteasome dependent, the DHL-4 cells were treated with 100 nM bortezomib for 24 hours. Cytosol was then extracted from these cells and incubated with K562 mitochondria for 5 hours. Bax degradation was diminished by bortezomib (Figure 4E). These results indicate that Bax degradation occurs at the mitochondrial level, and tBid prevents Bax degradation, probably by promoting stable Bax membrane insertion. TRAIL is known to induce Bax translocation to the mitochondria,42 and this was thought to be an essential event in the induction of apoptosis by Bax. However, our data imply that Bax degradation may be active even after translocation after TRAIL stimulation.
Prevention of Bax degradation by tBid at the mitochondrial level. (A) Determination of proteasome activity. (Ai) Proteasome activity was measured in both cytosol (Cyto) and mitochondrial (Mito) fractions in the absence of ubiquitin. *Significantly different proteasome activities (P < .0001) between DHL-4 and K562. (Aii) Inhibition of proteasome activity by bortezomib. Bortezomib (70 nM) was added to the Cyto (5 μg/μL protein) 1 hour before the reaction. Data shown were from 3 separate experiments. *Significant inhibition (P < .0001; t test). (B) Mito Bax degradation in K562 Cyto. K562 Mito were incubated with K562 Cyto in the presence of ubiquitin. Mito were mixed with Cyto according to a protein ratio of Mito/Cyto of 1:2 and incubated at 30°C for 3 hours. Bax degradation was monitored in the presence or absence of 10 nM tBid. Bax protein levels were detected by Western blotting for both Mito and Cyto. (C) K562 Mito were either incubated in the degradation buffer containing ubiquitin or in the K562 Cyto without ubiquitin for 3 hours. Bax protein levels were examined in the Mito fraction. (D) Mito Bax degradation in the DHL-4 Cyto. Mito were isolated from the K562 cells. Cyto was extracted from DHL-4 cells. In this assay, ubiquitin were omitted to test whether the DHL-4 Cyto has a higher Bax degradation activity. Mito were mixed with Cyto according to a protein ratio of Mito/Cyto of 1:2 and incubated at 30°C for up to 60 minutes in the presence or absence of 10 nM tBid. Bax protein levels were detected by Western blotting for both Mito and Cyto. (E) Bortezomib prevents Bax degradation. DHL-4 cells were treated with 100 nM bortezomib for 24 hours, and its Cyto was mixed with K562 Mito and incubated at 30°C for 5 hours. Mito Bax levels were then determined by Western blotting after separation from Cyto. Bax antibody (clone 2D2) was used for the Western blotting.
Prevention of Bax degradation by tBid at the mitochondrial level. (A) Determination of proteasome activity. (Ai) Proteasome activity was measured in both cytosol (Cyto) and mitochondrial (Mito) fractions in the absence of ubiquitin. *Significantly different proteasome activities (P < .0001) between DHL-4 and K562. (Aii) Inhibition of proteasome activity by bortezomib. Bortezomib (70 nM) was added to the Cyto (5 μg/μL protein) 1 hour before the reaction. Data shown were from 3 separate experiments. *Significant inhibition (P < .0001; t test). (B) Mito Bax degradation in K562 Cyto. K562 Mito were incubated with K562 Cyto in the presence of ubiquitin. Mito were mixed with Cyto according to a protein ratio of Mito/Cyto of 1:2 and incubated at 30°C for 3 hours. Bax degradation was monitored in the presence or absence of 10 nM tBid. Bax protein levels were detected by Western blotting for both Mito and Cyto. (C) K562 Mito were either incubated in the degradation buffer containing ubiquitin or in the K562 Cyto without ubiquitin for 3 hours. Bax protein levels were examined in the Mito fraction. (D) Mito Bax degradation in the DHL-4 Cyto. Mito were isolated from the K562 cells. Cyto was extracted from DHL-4 cells. In this assay, ubiquitin were omitted to test whether the DHL-4 Cyto has a higher Bax degradation activity. Mito were mixed with Cyto according to a protein ratio of Mito/Cyto of 1:2 and incubated at 30°C for up to 60 minutes in the presence or absence of 10 nM tBid. Bax protein levels were detected by Western blotting for both Mito and Cyto. (E) Bortezomib prevents Bax degradation. DHL-4 cells were treated with 100 nM bortezomib for 24 hours, and its Cyto was mixed with K562 Mito and incubated at 30°C for 5 hours. Mito Bax levels were then determined by Western blotting after separation from Cyto. Bax antibody (clone 2D2) was used for the Western blotting.
Stabilizing Bax by proteasome inhibition contributes to bortezomib-induced apoptosis in primary malignant B cells
It was determined whether the proteasome inhibitor bortezomib could induce apoptosis and Bax accumulation in primary malignant B cells. The sensitivity of CLL cells to bortezomib-induced apoptosis and Bax accumulation was determined in fresh cells from 10 patients. Susceptibility to bortezomib-induced apoptosis was universal, with no significant differences between cases (Figure 5A), although Bax protein levels varied, and different Bax degradation activities were shown among these samples: 5 cases showed Bax degradation, 3 were negative, and 2 were not tested (Agrawal et al, accompanying paper). This suggests that the differential Bax protein levels and Bax degradation activity were not sufficient to affect the sensitivity of CLL cells to bortezomib-induced apoptosis. Bortezomib-induced Bax accumulation was observed in most of CLL cases tested, although the degrees of accumulation varied among these samples (Figure 5B).
Bortezomib (Borte)-induced apoptosis and Bax activation in CLL cells. (A) Borte-induced apoptosis in 10 CLL samples. Fresh CLL cells were treated with different concentrations of Borte for 16 hours. Apoptotic cells were determined by the annexin V assay. (B) Borte-induced Bax protein accumulation in 9 cases of CLL patients. (C) Borte-induced Bax protein accumulation, oligomerization, and activation in 1 case of CLL patient. CLL cells were treated with Borte for 16 hours. Accumulation of Bax protein was determined by Western blotting using the anti-Bax antibody clone 2D2 at 1:1000 dilution. A total of 30 μg protein was loaded into each lane. Bax dimers and trimers are shown in the top panel blot indicated by 2× and 3×. For detection of conformationally changed Bax, the active form of Bax was immunoprecipitated (IP) by Bax 6A7 antibody and probed by Bax 2D2 antibody. (D) Bax translocation to mitochondria. CLL cells were treated with or without 20 nM Borte for 16 hours. Cells were stained with 100 nM MitoTracker for 15 minutes, washed 3 times, then fixed and permeabilized. Slides were stained with anti-Bax clone 3 antibody (1:20 dilution) for 1 hour and then stained with FITC-conjugated anti–mouse IgG (1:50 dilution) for 1 hour. Finally, slides were stained with 50 ng/mL DAPI. The red color indicates the mitochondrial location, and the yellow color represents the active Bax in the mitochondria.
Bortezomib (Borte)-induced apoptosis and Bax activation in CLL cells. (A) Borte-induced apoptosis in 10 CLL samples. Fresh CLL cells were treated with different concentrations of Borte for 16 hours. Apoptotic cells were determined by the annexin V assay. (B) Borte-induced Bax protein accumulation in 9 cases of CLL patients. (C) Borte-induced Bax protein accumulation, oligomerization, and activation in 1 case of CLL patient. CLL cells were treated with Borte for 16 hours. Accumulation of Bax protein was determined by Western blotting using the anti-Bax antibody clone 2D2 at 1:1000 dilution. A total of 30 μg protein was loaded into each lane. Bax dimers and trimers are shown in the top panel blot indicated by 2× and 3×. For detection of conformationally changed Bax, the active form of Bax was immunoprecipitated (IP) by Bax 6A7 antibody and probed by Bax 2D2 antibody. (D) Bax translocation to mitochondria. CLL cells were treated with or without 20 nM Borte for 16 hours. Cells were stained with 100 nM MitoTracker for 15 minutes, washed 3 times, then fixed and permeabilized. Slides were stained with anti-Bax clone 3 antibody (1:20 dilution) for 1 hour and then stained with FITC-conjugated anti–mouse IgG (1:50 dilution) for 1 hour. Finally, slides were stained with 50 ng/mL DAPI. The red color indicates the mitochondrial location, and the yellow color represents the active Bax in the mitochondria.
A dose-dependent Bax protein accumulation and oligomerization (dimer and trimer) were observed in bortezomib-treated CLL cells (Figure 5C top panel). The accumulation of both p18 and p21 Bax was seen after the treatment with bortezomib (Figure 5C second panel). Bax activation was initially determined by a conformational change in Bax. It was shown that bortezomib-induced Bax conformational change was also dose dependent (Figure 5C third panel). Bortezomib-induced Bax activation was also confirmed by Bax translocation to mitochondria. After treatment with bortezomib, the active Bax protein (green color) merged with the red mitochondria, becoming yellow (Figure 5D). These results show that bortezomib induces Bax protein accumulation, a change in its conformation, translocation to mitochondria, and an oligomerization. We have previously shown that Bax accumulation is sufficient to trigger its activation.16,40 It was also observed that bortezomib caused NOXA accumulation in CLL cells (data not shown).
Next, the effect of bortezomib on accumulation of ubiquitinated proteins and Bax–ubiquitin conjugation was investigated. An accumulation of polyubiquitinated proteins was detected when CLL cells were treated with bortezomib (Figure 6A). Immunoprecipitation experiments showed that active Bax can be recognized by, and conjugated with, ubiquitin when proteasome-dependent degradation was blocked by bortezomib. However, Bax–ubiquitin conjugation was also detected in the control CLL cells, but to a lesser extent compared with treated CLL cells (Figure 6B). We therefore propose that Bax degradation in CLL cells is ubiquitin/proteasome dependent.
Bortezomib-induced polyubiquitination and Bax–ubiquitin conjugation. Fresh CLL cells were treated with bortezomib for 16 hours. (A) Polyubiquitination. CLL cells from 3 different patients were lysed with the lysis buffer. A total of 50 μg proteins was loaded into each lane. The antiubiquitin antibody was used at 1:500 dilution. Multiple ladders in the blot show an accumulation of polyubiquitinated proteins. (B) CLL cells were lysed with Chaps containing lysis buffer. A total of 500 μg protein was used for immunoprecipitation (IP) with Bax 6A7 antibody overnight at 4°C, and Bax–ubiquitin conjugation was probed with the antiubiquitin antibody at 1:500 dilution. The numbers on the left side of the blot are molecular weights of standard proteins. WB indicates Western blotting.
Bortezomib-induced polyubiquitination and Bax–ubiquitin conjugation. Fresh CLL cells were treated with bortezomib for 16 hours. (A) Polyubiquitination. CLL cells from 3 different patients were lysed with the lysis buffer. A total of 50 μg proteins was loaded into each lane. The antiubiquitin antibody was used at 1:500 dilution. Multiple ladders in the blot show an accumulation of polyubiquitinated proteins. (B) CLL cells were lysed with Chaps containing lysis buffer. A total of 500 μg protein was used for immunoprecipitation (IP) with Bax 6A7 antibody overnight at 4°C, and Bax–ubiquitin conjugation was probed with the antiubiquitin antibody at 1:500 dilution. The numbers on the left side of the blot are molecular weights of standard proteins. WB indicates Western blotting.
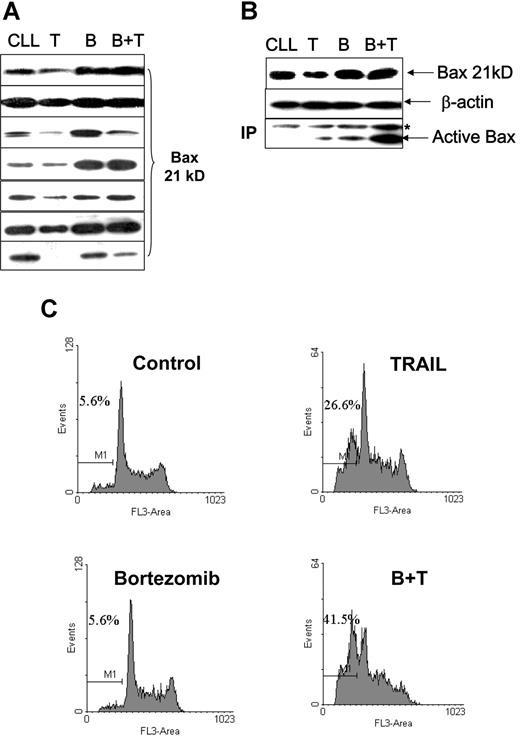
We also investigated whether the Bax degradation seen with TRAIL treatment in cell lines, and the reversal of this with bortezomib, occurred in primary malignant B cells. Fresh CLL cells treated with 500 ng/mL soluble TRAIL were also found to have reduced Bax protein levels (Figure 7A). TRAIL-induced Bax conformational change and apoptosis were low in the CLL cells (Figure 7B,C). Ten-nanomolar bortezomib alone did not show significant effects on cell viability in CLL cells. However, bortezomib and TRAIL in combination had a synergistic effect on apoptosis induction and Bax activation, with prevention of TRAIL-induced Bax degradation. As with the cell lines, Bax mRNA expression in primary CLL cells was not affected during TRAIL-mediated reduction in Bax protein expression or its reversal with bortezomib (data not shown).
Proteasome inhibitor facilitates TRAIL-induced Bax activation and apoptosis. Fresh CLL cells were pretreated with 10 nM/L bortezomib for 1 hour and then incubated with or without 500 ng/mL TRAIL for 16 hours. (A) TRAIL ± bortezomib induced changes of Bax protein levels in 7 cases of CLL patients. (B) Association between Bax protein levels and Bax conformational change. CLL indicates the control; T, TRAIL-treated; B, bortezomib-treated; B + T, treated with bortezomib and TRAIL. The anti-Bax antibody 2D2 was used for Western blotting. The active Bax proteins were immunoprecipitated (IP) with 1 μg anti-Bax 6A7 antibody and detected by Western blotting using anti-Bax 2D2 antibody at 1:1000 dilution. (C) Apoptotic cell death was measured by DNA content assay. This is 1 of 3 separate experiments. Numbers shown in the flow cytometry profiles are percentages of apoptotic cells (sub-G0/G1 population).
Proteasome inhibitor facilitates TRAIL-induced Bax activation and apoptosis. Fresh CLL cells were pretreated with 10 nM/L bortezomib for 1 hour and then incubated with or without 500 ng/mL TRAIL for 16 hours. (A) TRAIL ± bortezomib induced changes of Bax protein levels in 7 cases of CLL patients. (B) Association between Bax protein levels and Bax conformational change. CLL indicates the control; T, TRAIL-treated; B, bortezomib-treated; B + T, treated with bortezomib and TRAIL. The anti-Bax antibody 2D2 was used for Western blotting. The active Bax proteins were immunoprecipitated (IP) with 1 μg anti-Bax 6A7 antibody and detected by Western blotting using anti-Bax 2D2 antibody at 1:1000 dilution. (C) Apoptotic cell death was measured by DNA content assay. This is 1 of 3 separate experiments. Numbers shown in the flow cytometry profiles are percentages of apoptotic cells (sub-G0/G1 population).
Discussion
In this study, we demonstrate that Bax is a short-lived protein in malignant B cells. Bax degradation occurs at the mitochondrial level, probably before its stable mitochondrial membrane insertion. This affects the sensitivity of malignant B cells to TRAIL-induced apoptosis. The proteasome inhibitor, bortezomib, stabilizes Bax and enhances the proapoptotic activity of the Bax protein. Bortezomib alone, in appropriate concentrations, induces apoptosis in primary malignant B cells by stabilizing Bax protein.
The ubiquitin/proteasome-dependent degradation pathway is a major system for short-lived protein degradation in eukaryotic cells.42 Many proteins must undergo subunit separation, local unfolding, or post-translational modification before recognition by the appropriate E3 ubiquitin ligase.42,43 It was reported that Bax is a short-lived protein in tumor cells, and its degradation is ubiquitin/proteasome dependent because of an increased proteasome activity in tumor cells.1,4 The vexing problems are what is the trigger for Bax degradation, and where is Bax degraded? The native Bax is a globular protein with a hydrophobic core center. In the resting state, it localizes in the cytosol or is loosely attached to the mitochondria in some models. Bax translocates to the mitochondrial outer membrane in response to apoptotic signaling. The conformational changes in Bax enable its stable membrane insertion via the α5-α6 helices and oligomerization with Bax and/or Bcl-2. However, Bax translocation to mitochondria does not commit cells to apoptosis because Bax translocation and membrane insertion are separate events.15,44 In the experiments looking at Bax degradation in the mitochondria or cytosol, a detergent such as Triton X-100 was omitted because it can trigger Bax conformational change in a cell-free system.45 Bax protein is relatively stable in the cytosol in the absence of detergent. In the K562 mitochondria, Bax is in an inactive form, and it requires the BH3-only protein for triggering cytochrome c release.16,44 The mitochondrial Bax is vulnerable to degradation in the absence of the BH3-only protein tBid. The role of the BH3-only proteins on mitochondria is to promote Bax membrane insertion by engaging the multiple survival relatives guarding Bax and Bak.46 K562 cells have lower proteasome activity, and this may be one of the reasons that K562 mitochondria maintain an abundant Bax protein. Having a higher proteasome activity, DHL-4 cytosol degrades K562 mitochondrial Bax rapidly in the absence of artificial ubiquitin. In the presence of ubiquitin, the K562 cytosol degrades mitochondrial Bax gradually, and this is also inhibited by tBid. However, the sensitivity of Bax protein to degradation may depend on many factors, such as the specific conformation of Bax protein, fewer BH-3–only proteins, the cytosolic proteasome activity, and the coordination of ubiquitin recognizing and conjugating with Bax. The general proteasome activity is not specific for Bax degradation.
Using an antibody (6A7) to the active form of Bax, we detected Bax–ubiquitin conjugation after treatment with bortezomib, suggesting that ubiquitin recognizes the active form of Bax. However, we could not rule out that ubiquitin may conjugate with the inactive Bax because none of the available antibodies that detect inactive Bax could be used for immunoprecipitation. We have recently observed (in another study) that the exposure of Bax hydrophobic domains during its conformational changes triggers ubiquitin recognition and the degradation process. The ubiquitin-sensitive domain of Bax is within its hydrophobic core. This also suggests that Bax translocation to mitochondria and subsequent conformational changes may be physical processes. This study shows that tBid or bortezomib stabilize Bax and prevented mitochondrial Bax from degradation, suggesting Bax degradation may occur before irreversible commitment to apoptosis.
The protein levels of Bax are determined by 2 opposing forces: synthesis and degradation. Cancer cells lacking Bax protein are resistant to apoptosis. Therefore, blocking Bax degradation to increase Bax protein levels is an attractive target for anti-cancer therapy. Many anticancer reagents, such as DNA damaging agents, induce apoptosis by an increase in Bax expression and translocation,15 indicating that rapid synthesis can overwhelm Bax degradation activity. The effect of DNA damaging agents on Bax degradation activity is unknown. TRAIL induced Bax translocation and conformational change. However, rather than increased Bax expression, TRAIL treatment was associated with enhanced Bax degradation in resistant malignant B cells. It is known that tBid is required for TRAIL-induced Bax activation.47 Poor coordination between Bax mitochondrial translocation and the BH3-only proteins, such as tBid, or overexpression of Bcl-2 (a common feature of malignant B cells), would leave mitochondrial Bax vulnerable to degradation. Primary CLL cells were found to be inherently resistant to TRAIL,22 and inhibition of Bcl-2 sensitized TRAIL-induced apoptosis in human leukemia and tumor cells.47,48 Although Bax is essential for TRAIL-induced apoptosis in certain cancer cells, the regulation of Bax in response to TRAIL is still elusive. We suggest that TRAIL-induced Bax degradation may be a consequence of insufficient levels of tBid or Bcl-2 overexpression, which cause a failure in mitochondrial membrane insertion. The proteasome inhibitor Bortezomib sensitized resistant cells to TRAIL-induced apoptosis by preventing Bax degradation and therefore maintaining levels of active Bax. These results suggest that proteasome-dependent Bax degradation is one of the mechanisms underlying the resistance of malignant B cells to TRAIL-induced apoptosis and that it can be prevented by cotreatment with a proteasome inhibitor.
Increased degradation activity of proapoptotic proteins may contribute to the development of cancer, as well as resistance of cancer cells to treatment. The human leukemia CLL is currently incurable, with frequent overexpression of Bcl-2 being one of the major causes of resistance of CLL cells to therapy. Furthermore, insufficient levels of Bax protein have also been reported to be a resistance factor in CLL in response to a variety of therapies.3,49,50 Reduced Bax protein expression was observed in several malignant B-cell lines, but this may not necessarily represent increased Bax degradation activity. Low Bax protein levels could also be attributable to a change in the balance between synthesis and degradation, namely decreased synthesis combined with increased degradation activity.
Bortezomib induces apoptosis in CLL cells in vitro.51,52 We found there was no correlation between Bax degradation activity and the sensitivity of CLL cells to bortezomib-induced apoptosis. Bortezomib caused Bax accumulation, a conformational change, translocation to mitochondria, and oligomerization in CLL cells. Increased levels of NOXA protein were also observed when CLL cells were treated with bortezomib. Bax up-regulation was also seen when CLL cells were treated with both bortezomib and anti-CD20 antibody,53 whereas cotreatment of CLL cells with bortezomib plus cladribine and fludarabine induced an up-regulation of tBid, another short-lived proapoptotic protein.54 It was reported that bortezomib-induced apoptosis was significantly decreased in Bax–knockout colon cancer cells.55 We propose that Bax accumulation and activation play an important role in bortezomib-induced apoptosis.
Our results may have clinical application because inducible resistance to apoptosis occurs during TRAIL treatment, and the combination of a proteasome inhibitor with TRAIL overcomes resistance to TRAIL by blocking Bax degradation. Further studies will focus on whether Bax degradation is associated with clinical prognosis and outcome of patients with lymphoma and leukemia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are thankful to Millennium Pharmaceuticals for the generous supply of bortezomib. We are grateful for helpful advice from Professor Gerald Cohen and Dr Stephen M. Kelsey during this study. We thank Drs J. Fitzgibbon and S.J. Strauss for kindly providing the HRC, DoHH2, CRL, and DHL-4 cell lines.
This work was mainly supported by the Barts and the London Charitable Foundation to L.J. and A.C.N. and in part by Leukemia Research Fund to M.G.D.
Authorship
Contribution: F.T.L. designed experiments, performed research, and analyzed data; S.G.A. performed research on clinical studies and edited the paper; Y.H. and M.Q.D. performed research; J.G.G. and A.C.N. performed research, provided advice, and edited the paper; and L.J. designed and performed research, analyzed data, created the figures, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Li Jia, Centre for Haematology, Institute of Cell and Molecular Sciences, Queen Mary University of London, 4 Newark St, London E1 2AT, UK; e-mail: L.jia@qmul.ac.uk.