Germinal centers (GCs) are lymphoid tissue structures central to the generation of long-lived, high-affinity, antibody-forming B cells. However, induction, maintenance, and regulation of GCs are not sufficiently understood. The F-actin–binding, Rac-interacting protein SWAP-70 is strongly expressed in activated B cells like those in B follicles. Recent work suggests that SWAP-70 is involved in B-cell activation, migration, and homing. Therefore, we investigated the role of SWAP-70 in the T-dependent immune response, in GC formation, and in differentiation into plasma and memory B cells. Compared with wt, sheep red blood cell (SRBC)–, or NP-KLH–immunized SWAP-70−/− mice have strongly reduced numbers of GCs and GC-specific B cells. However, SWAP-70−/− NP-specific B cells accumulate outside of the B follicles, and SWAP-70−/− mice show more plasma cells in the red pulp and in the bone marrow, and increased NP-specific Ig and antibody-forming B cells. Yet the memory response is impaired. Thus, SWAP-70 deficiency uncouples GC formation from T-dependent antibody and long-lived plasma cell production and causes extrafollicular generation of high-affinity plasma cells, but does not adequately support the memory response.
Introduction
B lymphocytes are central to efficient innate and adaptive immune responses. In innate immunity, B cells such as those forming the marginal zone surrounding the follicles in the spleen respond rapidly to T-independent compounds such as bacterial lipopolysaccharides.1,–3 In adaptive immunity, B cells in the spleen or the lymph node (LN) follicles are stimulated through direct contact with T cells, perform Ig class switching and somatic hypermutation, and then as plasma cells produce high-affinity antibodies.4 Memory cells develop for later revitalization of a specific immune response.5,–7 After immunization with a T-dependent antigen, an oligoclonal cohort of B cells is activated along the border of the T-cell areas of secondary lymphoid organs.8,9 Following interaction with T cells, activated B cells migrate either to extrafollicular foci or to B follicles.10,11 B cells that emigrate to extrafollicular foci within the red pulp of the spleen differentiate into short-lived Ab-secreting cells producing low-affinity Ig.12 Approximately 1 week after initial immunization, some antigen-primed B cells migrate back to the follicles and together with follicular B cells form germinal centers (GCs).13,,–16 GCs are inducible lymphoid microenvironments composed primarily of antigen-specific B cells, antigen-specific CD4+ follicular T cells,17 and follicular dendritic cells (FDCs).18,19 GCs are sites of rapid antigen-specific B-cell selection and expansion, affinity maturation by somatic hypermutation, isotype switching, and receptor editing, and are sites of apoptosis of B cells, which fail in selection.15,16,20,–22 The GC reaction generates long-lived plasma cells and memory B cells.7,13,23 GCs can be detected in situ and by fluorescence-activated cell sorting (FACS) by staining for peanut agglutinin (PNA) or with anti-GL7.24 The GC can be subdivided into the light zone enriched in noncycling B cells (centrocytes) and the dark zone containing more proliferating B cells (centroblasts). The zones can be further distinguished by staining for FDCs and stroma expressing CXCL13 besides CXCR5high B cells in the light zone, and CXCR4high centroblasts and CXCL12+ stroma in the dark zone.25 This separation into light and dark zones and their functions may not be as strict as hitherto assumed, since recent reports have shed light on GC B-cell dynamics and showed that GC B cells exhibit polarized shape, are very motile, and transit between dark and light zones.26,,,–30 Migration is therefore an important parameter for GC functions and much remains to be elucidated about GC induction and the mechanisms that control the commitment to either extrafollicular reaction or GC formation
A number of molecules involved in the transduction of signals from cell-surface receptors to adhesion molecules and to the F-actin cytoskeleton regulate migration, cell adhesion, and transmigration into the tissues. Notably, small G-proteins of the Rho family (eg, Rac-1, Rac-2), together with their regulators, are central to hematopoietic cell migration.31,32 Since B-cell migration is uniquely regulated, it is important to identify the signaling molecules involved and to characterize their functions.
SWAP-70 is a Rac-interacting protein, which carries an unusual arrangement of protein domains and motifs.33,34 The protein contains a coiled-coil region, a pleckstrin homology (PH) domain, 3 nuclear localization signals (NLSs), a nuclear exit signal (NES), a domain weakly homologous to Dbl (DH) domains, and a putative EF-hand.33,35,36 The presence of NLS and NES suggests that SWAP-70 may shuttle between the cytoplasm and the nucleus, an assumption that we showed to be correct.34 The PH domain of SWAP-70 specifically binds phosphatidylinositol 3,4,5-triphosphate (PIP3). In addition, SWAP-70 binds nonmuscle F-actin.37 DH domain–containing proteins are involved in activation of small GTPases of the Rho family. Accordingly, SWAP-70 specifically interacts with Rac, regulating levels of activated Rac and its intracellular localization.36,38,39 SWAP-70 is required for specific processes during remodeling of the F-actin cytoskeleton such as membrane ruffling and lamellipodia formation.36,38,39 In GCs of mouse spleen, lymph nodes, or human tonsils, immunofluorescence (IF) revealed strong staining for SWAP-70 in B cells and dendritic cells (DCs).33,35,36 Recently, we reported effects of SWAP-70 deficiency on B-cell adhesion and migration into secondary lymphoid organs such as reduced migration of SWAP-70−/− B cells into LN. SWAP-70−/− B cells rolled and adhered, yet accumulated in LN high endothelial venules.38 This defect is not due to impaired integrin expression or chemotaxis, which are like wt, but rather to aberrant regulation of integrin-mediated adhesion. Attached SWAP-70−/− B cells show defective polarization, and do not form uropods or stabilize lamellipodia at a defined region. Thus, SWAP-70 selectively regulates processes essential for B-cell entry into LN, such as rearrangement of the actin cytoskeleton, integrin-mediated attachment, and polarization.
In the present study, we asked for the consequences of SWAP-70 deficiency for the T-dependent immune response, and in particular for the formation and function of GCs by analyzing the natural response to sheep red blood cells (SRBCs) or NP-KLH in nontransgenic wt or SWAP-70−/− mouse strains.
Methods
Mice
Mice were bred and maintained in the animal facility at the Mount Sinai School of Medicine under specific pathogen-free conditions. All studies were approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine. SWAP-70−/− mice backcrossed onto a 129Sv background have been described previously. Some experiments were done with SWAP-70−/− mice backcrossed at least 10 times onto a C57BL/6 background. C57BL/6 mice deficient for B lymphocytes (μMT) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were used at 4 to 8 weeks of age.
Immunizations
For GC induction, mice were immunized once intraperitoneally with 1 to 5 × 108 SRBCs (Colorado Serum, Denver, CO). Some mice were immunized subcutaneously with an emulsion containing equal volumes of CFA (Pierce, Rockford, IL) and KLH (2.5 mg/mL) (CFA/KLH) in the footpads draining the popliteal LN as described previously.40 T-dependent antigen response was induced by intraperitoneal injection of 100 μg NP20-KLH (Biosearch Technologies, Novato, CA) in CFA. Secondary response was elicited by intravenous injection of 50 μg NP20-KLH in PBS.
Flow cytometry
Reagents used were biotinylated PNA (Vector Laboratories, Burlingame, CA); NP-PE (Biosearch Technologies); and mAbs B220-PE-Cy5.5, CD19-PE-Cy5.5, CD38-FITC, GL7-FITC, IgG1-APC, biotinylated CD138, and streptavidin-APC (BD-Pharmingen, San Diego, CA). For many experiments, we used either CD19 or B220 staining for B cells with the same results.
Immunohistochemistry
Cryostat sections (8-10 μm) were fixed in acetone. The following reagents were used: biotinylated PNA, purified rat anti-B220, purified rat anti-IgG1, purified Armenian hamster anti-CD3, purified rat anti-CD35, biotinylated anti-CD138, and streptavidin-Cy2, F(ab′)2 anti–rat IgG-Cy3, Cy5-conjugated F(ab′)2 anti–Armenian hamster IgG (Jackson Immunoresearch Laboratories). For NP staining, rabbit IgG-NP was kindly provided by A. Achtman from A. G. Lipp laboratory (Germany), and AlexaFluor 488 anti–rabbit IgG (Molecular Probes, Eugene, OR) was used as secondary Ab. Slides were mounted with Vectashield HardSet mounting medium (Vector Laboratories). Images were acquired with a DMRA2 fluorescence microscope (Leica Microsystems, Bannockburn, IL) equiped with 5×/0.15, 10×/0.40, and 20×/0.70 dry objectives and a digital CCD camera (model ORCA-ER; Hamamatsu, Bridgewater, NJ). Images were then analyzed with OpenLab software (Improvision, Walthman, MA). The percentage of GC was determined by counting the number of B-cell follicles containing GC among the total B-cell follicles.
BrdU labeling and analysis
For BrdU in vivo labeling experiments, immunized mice receive 1 mg BrdU in PBS as a single intraperitoneal injection 5 hours before the indicated time. Spleen cell suspensions were prepared and analyzed for BrdU incorporation with the BrdU Flow Kit (BD-Pharmingen) according to the manufacturer's instructions. For tissue analysis, sections were first stained for PNA then denatured in 0.1 N HCL, followed by anti-BrdU staining.
B-cell isolation and adoptive transfer
B cells were isolated from spleen of SWAP-70+/+ or SWAP-70−/− mice (backcrossed onto a C57BL/6 background) by negative selection with DynalBeads (Invitrogen, Frederick, MD). Purity was checked by flow cytometry by staining with anti-B220. Only isolates of more than 95% purity were used. Isolated B cells (5 × 107) or total splenocytes (1.5 × 108) were adoptively transferred by intravenous injection into μMT B cell–deficient mice (C57BL/6 background).
Enzyme-linked immunosorbent assay analysis
MaxiSorp plates (Nunc, Rochester, NY) were coated with 0.5 μg/mL NP3-BSA or NP12-BSA (Biosearch Technologies). Serial dilutions of serum samples were analyzed in triplicate. The following alkaline phosphatase–conjugated Abs were used: anti–mouse IgM, IgG2b (Southern Biotech, Birmingham, AL), IgG1, or IgG2a (BD-Pharmingen). PNPP substrate (Sigma-Aldrich, St Louis, MO) was used for detection. Plates were read at A405 using Softmax software (Molecular Devices, Sunnyvale, CA). The relative affinity of anti-NP Abs was estimated by calculating the ratio of anti-NP3 (high affinity)/anti-NP12 (low and high affinity) Abs.
Enzyme-linked immunosorbent spot
Immobilon-P membrane plates (Millipore, Billerica, MA) were coated with 10 μg/mL NP3-BSA or NP12-BSA. Serial concentration of splenocytes or bone marrow (BM) cells from NP-KLH–immunized mice were cultured for 4 hours. Alkaline phosphatase–conjugated anti–mouse IgG1 was used. Spots were developed with BCIP-NBT substrate (Sigma-Aldrich) and counted with an Automated AID ELISPOT reader (Cell Technology, Columbia, MD) using once-optimized settings (from M. Pope, Population Council, NY).
BLIMP1 immunoblot
Immunoblot analysis was performed on whole-cell lysates of splenocytes from NP-KLH–immunized mice using rat anti-BLIMP1 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-Smc1α as loading control, peroxidase-conjugated goat anti–rat IgG, and peroxidase-conjugated goat antirabbit. Super signal reagents (Pierce) were used for detection.
Statistics
Statistical comparisons were made using the Student 2-tailed t test. Results are expressed as means plus or minus SD. All experiments contained 3 to 4 replicates per experimental parameter. P values less than .1 (*) are considered significant,P values less than .01 (**) are considered very significant, and P values less than .001 (***) are considered highly significant.
Results
Reduced GC formation in SWAP-70−/− mice after SRBC immunization
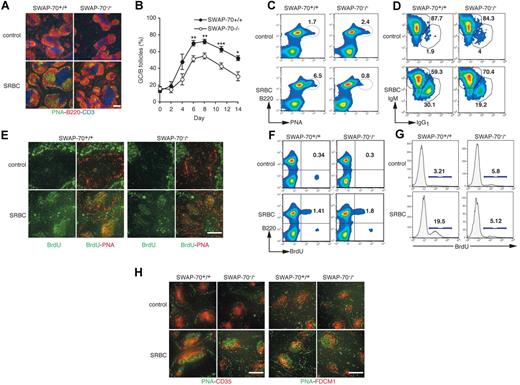
To investigate whether SWAP-70 expression affects GC formation, we immunized mice with SRBCs, a potent GC-inducing antigen. Six days after SRBC immunization, a strong GC reaction occurs in wt spleens with large clusters of PNA+ B follicles (Figure 1A). In contrast, SWAP-70−/− spleens contained fewer PNA+ clusters and the size of each cluster was reduced. Kinetic analysis (Figure 1B) shows that the number of microscopically visible GCs in SWAP-70−/− spleens never reaches wt levels, and differences are seen as early as at day 4. The overall kinetics of induction and recession of GCs is the same. This suggests that GC formation is not delayed in SWAP-70−/− mice, but less efficiently induced. This was confirmed using SWAP-70−/− mice, which were more than 10 × backcrossed onto the C57BL/6 background (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Quantification of GC B cells showed strong reduction in SWAP-70−/− mice: while in the wt, PNA+ cells increased 14 days after immunization from 1.7% to 6.5%, in immunized SWAP-70−/− mice the starting population of 2.4% did not increase but was reduced to 0.8%, possibly indicating a failure to maintain this population, which may undergo apoptosis or develop otherwise (Figure 1C). Since the GC is the site of Ig isotype class switching, we analyzed by FACS the expression of IgM and IgG1 on GC B cells (Figure 1D). SWAP-70−/− and wt PNA+ B cells both switched isotypes, but the ratio of nonswitched (IgM+) to switched IgG1+ B cells was lower in wt mice (2-fold in wt vs 3.6-fold in SWAP-70−/−), that is, more cells have switched in the wt.
Germinal center formation reduction in SWAP-70−/− mice. Wt and SWAP-70−/− mice were immunized with SRBCs, or injected with PBS as control. (A) Spleen sections were stained for GCs using PNA (green), B220 (red), and CD3 (blue) 6 days after immunization. (B) Percentage of B follicles containing GCs in spleen sections of immunized animals at the indicated time. (C) Flow cytometry of GC B cells, defined as B220+ B cells that are positive for PNA. Numbers indicate percentage of cells. Data are representative of 5 independent experiments. (D) Flow cytometry of nonswitched (IgM) and switched (IgG1) isotypes among the GC B cells gated as in panel C. Numbers in outlined areas indicate percentage of cells in each. Data are representative of 2 independent experiments. (E) Spleens stained for BrdU (green) and PNA (red) after a 5-hour pulse of BrdU 6 days after SRBC immunization. (F) B220 expression and BrdU incorporation 6 days after immunization. (G) BrdU staining in B220+PNA+ GC B cells. Numbers indicate percentage of cells. Data are representative of 2 independent experiments. (H) Spleen sections stained for PNA (green) and CD35 (red, left) or FDCM-1 (red, right) at day 6 after immunization. Data are representative of 3 independent experiments. Scale bars represent 200 μm.
Germinal center formation reduction in SWAP-70−/− mice. Wt and SWAP-70−/− mice were immunized with SRBCs, or injected with PBS as control. (A) Spleen sections were stained for GCs using PNA (green), B220 (red), and CD3 (blue) 6 days after immunization. (B) Percentage of B follicles containing GCs in spleen sections of immunized animals at the indicated time. (C) Flow cytometry of GC B cells, defined as B220+ B cells that are positive for PNA. Numbers indicate percentage of cells. Data are representative of 5 independent experiments. (D) Flow cytometry of nonswitched (IgM) and switched (IgG1) isotypes among the GC B cells gated as in panel C. Numbers in outlined areas indicate percentage of cells in each. Data are representative of 2 independent experiments. (E) Spleens stained for BrdU (green) and PNA (red) after a 5-hour pulse of BrdU 6 days after SRBC immunization. (F) B220 expression and BrdU incorporation 6 days after immunization. (G) BrdU staining in B220+PNA+ GC B cells. Numbers indicate percentage of cells. Data are representative of 2 independent experiments. (H) Spleen sections stained for PNA (green) and CD35 (red, left) or FDCM-1 (red, right) at day 6 after immunization. Data are representative of 3 independent experiments. Scale bars represent 200 μm.
As the GC is a site of strong T cell–induced B-cell proliferation, we assessed whether the impairment of GC formation is associated with a deficiency in proliferation. We treated mice with a pulse of BrdU at the peak of GC formation, and analyzed BrdU incorporation (Figure 1E,F). In wt or SWAP-70−/− control animals, no obvious BrdU incorporation was observed in primary B follicles. In contrast, SRBC immunization induced proliferation of GC B cells, and BrdU+ cells were seen within all wt GCs. In SWAP-70−/− spleen, BrdU+ cells were also present, but more dispersed as there are fewer GCs. By FACS analysis, there was no difference between wt and SWAP-70−/− animals in BrdU incorporation of total splenic B cells (Figure 1F). However, quantification of BrdU+ PNA+ GC B cells shows that the wt efficiently induces GC B-cell proliferation, while the SWAP-70−/− mice fail to do so (Figure 1G). Apoptosis of SWAP-70−/− GC B cells is not altered compared with wt (data not shown), suggesting that unlike reduced proliferative capacity, cell death does not explain GC reduction.
The GC light zone contains a network of FDCs that express CD35 and FDC-M1.15,25 Under nonimmunized conditions, FDC networks in primary follicles are normal both in wt and SWAP-70−/− animals, suggesting the GC impairment seen in SWAP-70−/− mice is not due to an a priori defect in B follicle structure (Figure 1H). After SRBC immunization, SWAP-70−/− GCs showed enriched staining for CD35+ and FDC-M1+ FDCs in the light zone with the expected zonal distinction. Thus, the few SWAP-70−/− GCs that do exist exhibit normal FDC polarity, suggesting the GC reduction is not a consequence of gross GC disorganization.
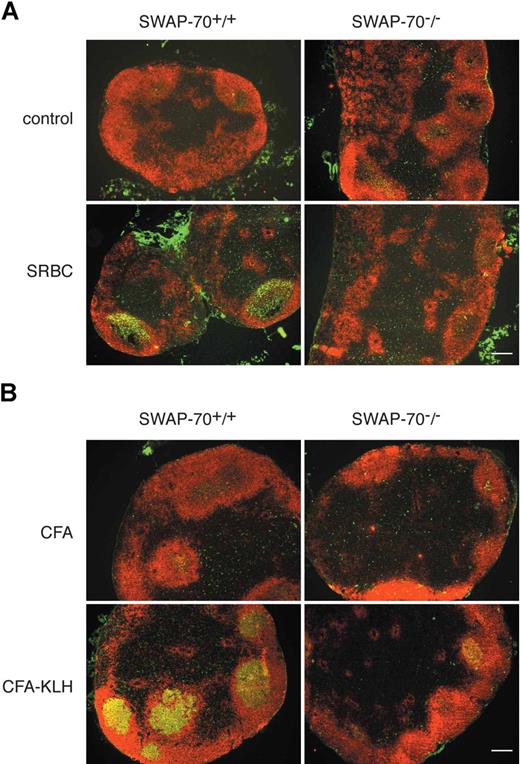
To assess whether SWAP-70 is required for GC formation at other anatomic locations, we analyzed the presence of GCs in LN after intraperitoneal SRBC immunization (Figure 2A), or after immunization of mice subcutaneously with an emulsion of CFA/KLH (Figure 2B) known to induce a robust inflammatory reaction, which results in the accumulation of B cells in the draining LN.40 GC induction in the LN of SWAP-70−/− mice was strongly reduced in both models. This shows that SWAP-70 plays a generally important role in GC formation.
GC formation in LN. (A) SWAP-70+/+ and SWAP-70−/− mice were immunized with SRBCs or PBS as control. Six days after immunization, mesenteric LN were stained for PNA (green) and B220 (red). (B) SWAP-70+/+ and SWAP-70−/− mice were immunized with CFA or CFA-KLH in the footpad. Six days after immunization, draining popliteal LN were stained for PNA (green) and B220 (red). Scale bars represent 200 μm.
GC formation in LN. (A) SWAP-70+/+ and SWAP-70−/− mice were immunized with SRBCs or PBS as control. Six days after immunization, mesenteric LN were stained for PNA (green) and B220 (red). (B) SWAP-70+/+ and SWAP-70−/− mice were immunized with CFA or CFA-KLH in the footpad. Six days after immunization, draining popliteal LN were stained for PNA (green) and B220 (red). Scale bars represent 200 μm.
The defect in GC formation in SWAP-70−/− is intrinsic to B cells
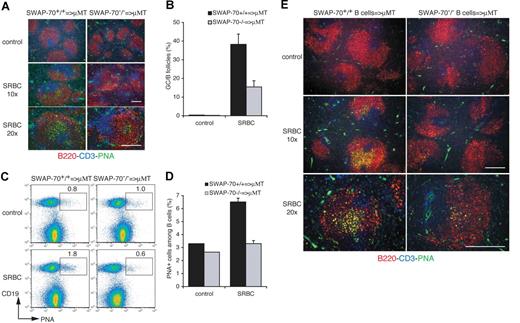
SWAP-70 is expressed not only by B cells but also by mast cells and DCs, although not by T cells.33,35 Thus, the splenic environment may be altered in SWAP-70−/− mice and therefore may contribute to the failure in these mice to efficiently develop GCs. Thus, we performed adoptive transfer experiments to test whether the observed deficiency is B-cell intrinsic. We injected 15 × 107 splenocytes from wt or SWAP-70−/− mice into B-cell deficient μMT mice.41 The recipient mice were then immunized with SRBCs, and the presence of B cells monitored at day 6. There were no GCs after SRBC immunization of μMT mice without adoptive transfer (data not shown). GCs containing B220+PNA+ cells are formed within follicles of the recipient spleen (Figure 3A,B), but adoptively transferred SWAP-70−/− splenocytes form GCs with much lower efficiency. FACS analysis of B cells in the μMT spleen showed an increase in wt PNA+ B cells after immunization, but no increase in PNA+ SWAP-70−/− B cells (Figure 3C,D). To exclude bystander effects of other cell subsets present among the transferred splenocytes, we adoptively transferred 5 × 107 purified splenic B cells from naive wt or SWAP-70−/− mice into μMT mice (Figure 3E). Both wt and SWAP-70−/− B cells reconstituted B follicles in spleen of μMT mice with the same efficiency, that is, by 24 hours after transfer (data not shown). However, wt B cells form GCs in the host spleen, whereas GCs formed by the transferred SWAP-70−/− B cells are almost undetectable. Analysis at higher magnification revealed that the size of SWAP-70−/− B cell–derived remnant GCs is strongly reduced, confirming the observations in immunized SWAP-70−/− mice (Figure 1A; Figure S1). This demonstrates that SWAP-70−/− B cells feature an intrinsic deficiency to form GCs.
GC formation defect is intrinsic to SWAP-70−/− B cells. SWAP-70+/+ or SWAP-70−/− total splenocytes (A-D) or purified B cells (E) were adoptively transferred into μMT B cell–deficient mice, and 1 day after, mice were immunized with SRBCs or injected with PBS as control. (A) Spleen sections of SRBC-immunized μMT transferred with total splenocytes were stained for GC using PNA (green), B220 (red), and CD3 (blue) 6 days after immunization. (B) Percentage of B follicles containing GC in spleen sections of immunized animals at the indicated time. (C) Flow cytometry of GC B cells defining as CD19+ B cells that are positive for PNA. Numbers indicate percentage of cells. (D) Percentage of PNA+ cells among B cells. (E) Spleen sections of control or SRBC-immunized μMT transferred with purified B cells were stained for GC. Scale bars represent 200 μm.
GC formation defect is intrinsic to SWAP-70−/− B cells. SWAP-70+/+ or SWAP-70−/− total splenocytes (A-D) or purified B cells (E) were adoptively transferred into μMT B cell–deficient mice, and 1 day after, mice were immunized with SRBCs or injected with PBS as control. (A) Spleen sections of SRBC-immunized μMT transferred with total splenocytes were stained for GC using PNA (green), B220 (red), and CD3 (blue) 6 days after immunization. (B) Percentage of B follicles containing GC in spleen sections of immunized animals at the indicated time. (C) Flow cytometry of GC B cells defining as CD19+ B cells that are positive for PNA. Numbers indicate percentage of cells. (D) Percentage of PNA+ cells among B cells. (E) Spleen sections of control or SRBC-immunized μMT transferred with purified B cells were stained for GC. Scale bars represent 200 μm.
Reduction of GC formation in SWAP-70−/− mice after NP-KLH immunization
SRBCs elicit a strong immune response with components of T-independent and T-dependent elements. We therefore decided to analyze the effect of SWAP-70 on GCs in a defined T-dependent model using the hapten NP-KLH emulsified in CFA. Histologic analysis of spleen from immunized mice at day 14 shows that GC formation is impaired in SWAP-70−/− mice (Figure 4A), with a strong decrease in the percentage of PNA+ B follicles (Figure 4B). There were also less than one third of GL7+ NP-specific GC cells among SWAP-70−/− splenocytes as seen by FACS (not shown). The NP response can be tracked by staining NP-specific cells using NP conjugated to a fluorochrome such as PE (Figure 4C). In CFA-injected wt and SWAP-70−/− mice, NP-specific B cells are almost undetectable, whereas 14 days after NP-KLH immunization NP+B220+ B cells can clearly be visualized. A detailed kinetic analysis of the appearance of NP+ B220+ cells revealed that the initial increase in NP+B220+ cells until day 7 is similar in wt and SWAP-70−/− mice (Figure 4D) in terms of time point of onset, although the percentage of NP+B220+ cells tends to be lower in SWAP-70−/− mice from early on (day 6). Beyond day 7, SWAP-70−/− mice failed to further increase NP+B220+ cells at any subsequent time point, but the percentage rather slightly decreased. In contrast, in wt mice the number increased to almost 1% of total lymphocytes before declining at day 21 (Figure 4C,D). A B220−NP+ population was observed and is discussed in “Discussion.” Analysis of GL7 expression in NP-specific B cells, an activation marker usually expressed by GC B cells, shows that in KO mice the percentage of GL7+ cells never reached the wt level, where differences again start to be seen as early as day 6 (Figure 4E). Quantification of BrdU+ cells among NP+B220+ B cells shows that the wt up-regulates B-cell proliferation approximately 10-fold after NP-KLH immunization, whereas proliferation is only 2.5-fold increased in SWAP-70−/− mice, which, although showing higher initial proliferation, do not reach wt levels (Figure 4F). In the spleen of CFA-injected mice, NP-positive cells were undetectable both in wt and SWAP-70−/− mice (Figure 4G). In immunized wt mice, NP-specific B cells are localized within GCs, whereas in immunized SWAP-70−/− mice few NP-specific B cells are found in GCs but many accumulate in the extrafollicular region.
GC formation following T-dependent immunization. SWAP-70+/+ and SWAP-70−/− mice were immunized with CFA or NP-KLH emulsified in CFA. (A) Fourteen days after NP-KLH immunization or CFA injection, spleen sections stained for PNA (green) and B220 (red). Data are representative of 3 independent experiments. (B) Percentage of B follicles containing GC in spleen sections of immunized animals. (C) Flow cytometry of NP-specific B cells in the spleen of SWAP-70+/+ or SWAP-70−/− mice 14 days after immunization. (D) Mean percentage of NP+B220+ B cells in spleen of immunized mice at the indicated time. Experiments were done 5 times. (E) Flow cytometry of GL7 expression in NP+B220+ in spleen at the indicated time. (F) Flow cytometry of BrdU in NP+B220+ cells in spleen 14 days after immunization. Numbers indicate percentage of cells. Data are representative of 2 independent experiments. (G) Spleen stained for B220 (blue) PNA (red) and NP (green) 14 days after immunization. All scale bars represent 200 μm.
GC formation following T-dependent immunization. SWAP-70+/+ and SWAP-70−/− mice were immunized with CFA or NP-KLH emulsified in CFA. (A) Fourteen days after NP-KLH immunization or CFA injection, spleen sections stained for PNA (green) and B220 (red). Data are representative of 3 independent experiments. (B) Percentage of B follicles containing GC in spleen sections of immunized animals. (C) Flow cytometry of NP-specific B cells in the spleen of SWAP-70+/+ or SWAP-70−/− mice 14 days after immunization. (D) Mean percentage of NP+B220+ B cells in spleen of immunized mice at the indicated time. Experiments were done 5 times. (E) Flow cytometry of GL7 expression in NP+B220+ in spleen at the indicated time. (F) Flow cytometry of BrdU in NP+B220+ cells in spleen 14 days after immunization. Numbers indicate percentage of cells. Data are representative of 2 independent experiments. (G) Spleen stained for B220 (blue) PNA (red) and NP (green) 14 days after immunization. All scale bars represent 200 μm.
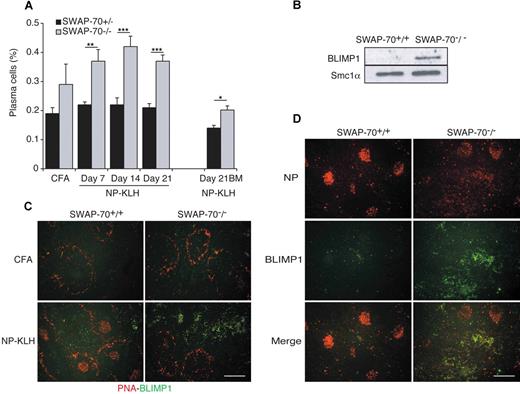
Increased Ig secretion and plasma cell production in SWAP-70−/− mice
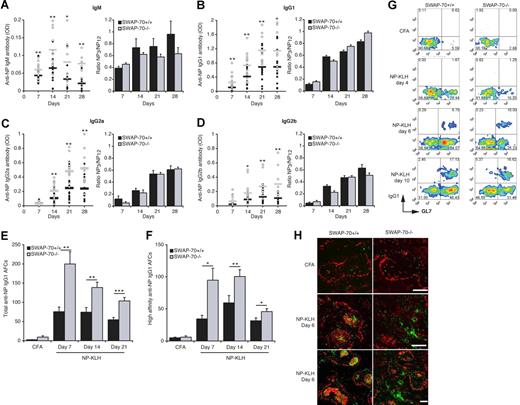
We monitored the serologic response to NP-KLH by measuring NP-specific Ig in the serum. Throughout, we observed about 2-fold higher levels of anti-NP antibody of all 4 isotypes in SWAP-70−/− mice (Figure 5A-D). However, there was no difference in the ratio of high-affinity to low-affinity antibody, indicating that all switched isotypes underwent affinity maturation as in wt mice. The slightly lower presence of high-affinity anti-NP IgM antibody is not significant.
Increased NP-specific Ig secretion in SWAP-70−/− mice. SWAP-70+/+ and SWAP-70−/− mice were immunized with NP-KLH emulsified in CFA. (A-D) Serum response to NP-KLH immunization of wt and SWAP-70−/− mice. At the indicated times, samples were collected and total NP-specific anti-IgM (A), IgG1 (B), IgG2a (C), and IgG2b (D) Ab titers were determined by ELISA with NP12-BSA as capture antigen. Black and white circles represent individual mouse for wt and SWAP-70−/−, respectively. Black and gray bars represent the mean average for wt and SWAP-70−/−, respectively. Values are expressed as arbitrary optical-density units at 405 nm. The optical-density values for CFA-injected mice are less than 0.001. Right panels represent the mean ratios of high-affinity anti-NP3 versus total anti-NP12 serum Abs. Data are from 5 independent experiments. ELISPOT analysis of total (E) and high-affinity (F) IgG1 secretion by splenocytes from wt and SWAP-70−/− mice at the indicated times after NP-KLH immunization. Data represent the mean number of spots. (G) Flow cytometry of GL7 and IgG1 expression in NP+B220+ cells in spleen at the indicated times. (H) Spleen stained for PNA (red) and IgG1 (green) 6 days after immunization. All scale bars represent 200 μm.
Increased NP-specific Ig secretion in SWAP-70−/− mice. SWAP-70+/+ and SWAP-70−/− mice were immunized with NP-KLH emulsified in CFA. (A-D) Serum response to NP-KLH immunization of wt and SWAP-70−/− mice. At the indicated times, samples were collected and total NP-specific anti-IgM (A), IgG1 (B), IgG2a (C), and IgG2b (D) Ab titers were determined by ELISA with NP12-BSA as capture antigen. Black and white circles represent individual mouse for wt and SWAP-70−/−, respectively. Black and gray bars represent the mean average for wt and SWAP-70−/−, respectively. Values are expressed as arbitrary optical-density units at 405 nm. The optical-density values for CFA-injected mice are less than 0.001. Right panels represent the mean ratios of high-affinity anti-NP3 versus total anti-NP12 serum Abs. Data are from 5 independent experiments. ELISPOT analysis of total (E) and high-affinity (F) IgG1 secretion by splenocytes from wt and SWAP-70−/− mice at the indicated times after NP-KLH immunization. Data represent the mean number of spots. (G) Flow cytometry of GL7 and IgG1 expression in NP+B220+ cells in spleen at the indicated times. (H) Spleen stained for PNA (red) and IgG1 (green) 6 days after immunization. All scale bars represent 200 μm.
ELISPOT assays confirmed the up to 3-fold increased presence of NP-specific antibody-forming B cells (AFCs) in SWAP-70−/− mice after immunization (Figure 5E). The highest number of IgG1+ AFCs was seen at day 7, after which numbers for wt and SWAP-70−/− AFCs decreased, although the decrease was more pronounced in SWAP-70−/− animals. This may reflect apoptosis of short-lived plasma cells. AFCs producing high-affinity anti-NP IgG1 were also increased in SWAP-70−/− mice (Figure 5F) and showed delayed kinetics compared with total NP-specific AFCs, consistent with their extensive maturation. IgG1+NP+B220+ cells are not seen at day 4, but appear as early as day 6 after immunization in both wt and SWAP-70−/− mice, which show more IgG1+ cells than wt, and they all express GL7 (Figure 5G). By day 10, some IgG1+GL7− cells are detectable and the percentage is doubled in ko compared with wt mice. When they start appearing at day 6, IgG1+ cells localize mainly within the GC in immunized wt mice, whereas in SWAP-70−/− mice IgG1+ cells are almost all found outside of the follicles (Figure 5H).
These results suggested that in immunized SWAP-70−/− mice more plasma cells are generated, and this was confirmed by FACS (Figure 6A). As some AFCs may be CD138−,42,43 we also used BLIMP1, a key transcriptional factor driving plasma cell differentiation,44,45 to diagnose plasmablasts and plasma cells. BLIMP1 is strongly expressed in total spleen from immunized SWAP-70−/− mice, whereas in immunized wt mice its expression is almost undetectable (Figure 6B). IF staining of spleen sections (Figure 6C) for BLIMP1 confirmed the presence of large deposits of plasma cells in the red pulp of SWAP-70−/− mice. Costaining of spleen with BLIMP1 and NP (Figure 6D) shows that most NP+ cells that accumulated in the red pulp of the spleen (Figure 4F) are BLIMP1+, suggesting that they are plasma cells.
Plasma cell differentiation. (A) Flow cytometry of CD138+B220low plasma cells in the spleen and BM of SWAP-70+/+ or SWAP-70−/− mice at the indicated times after immunization with NP-KLH. Data represent the mean percentage of 4 independent experiments. (B) Immunoblot of lysates from splenocytes of wt and SWAP-70−/− mice 14 days after NP-KLH immunization. Blots were probed with anti-BLIMP1. SMC1α is used as loading control. (C) Spleens stained for BLIMP1 (green) and B220 (red) at 14 days after immunization. (D) Spleens stained for BLIMP1 (green) and NP (red). All scale bars represent 200 μm. Data are representative of 3 independent experiments.
Plasma cell differentiation. (A) Flow cytometry of CD138+B220low plasma cells in the spleen and BM of SWAP-70+/+ or SWAP-70−/− mice at the indicated times after immunization with NP-KLH. Data represent the mean percentage of 4 independent experiments. (B) Immunoblot of lysates from splenocytes of wt and SWAP-70−/− mice 14 days after NP-KLH immunization. Blots were probed with anti-BLIMP1. SMC1α is used as loading control. (C) Spleens stained for BLIMP1 (green) and B220 (red) at 14 days after immunization. (D) Spleens stained for BLIMP1 (green) and NP (red). All scale bars represent 200 μm. Data are representative of 3 independent experiments.
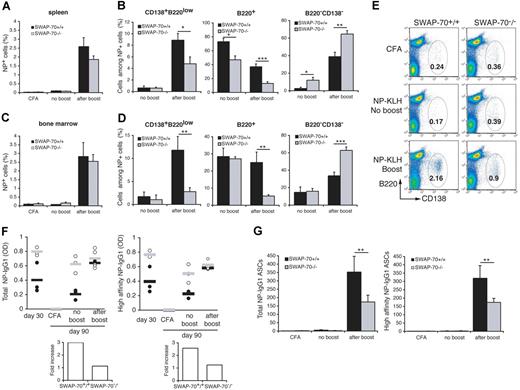
We also observed 1.4-fold increased numbers of CD138+B220low cells in the bone marrow at day 21 after immunization (Figure 6A), indicating that more of the plasma cells home into the bone marrow in SWAP-70−/− mice to possibly become long-lived.
Efficient memory B-cell development requires SWAP-70
The above-described impairment in the GC response suggests that development of memory B cells may be reduced. We measured the frequency of NP-specific B cells after boosting NP-KLH–immunized wt or SWAP-70−/− mice with the soluble antigen. NP-specific cells were almost undetectable after 90 days without boost in the spleen (Figure 7A) or BM (Figure 7C). Analysis of the phenotype of the NP+ cells in the spleen without boost (Figure 7B) shows that in wt approximately 70% of cells are presumably memory cells, since they express high levels of B220 (B220+), whereas in SWAP-70−/− mice these cells represent less than 50%. A controversial B220− cell population (“Discussion”) is increased in the spleen of SWAP-70−/− mice as seen in Figure 4. In the BM, frequencies of NP-specific cell populations before the boost are similar between both genotypes (Figure 7D). After the boost, the NP+ cells rapidly expand to almost 3% in both spleen and BM of wt or SWAP-70−/− mice. However, analysis of NP-specific cell phenotypes show drastic difference between wt and SWAP-70−/− mice (Figure 7B-D). SWAP-70+/+ mice developed a distinct population of CD138+B220low NP-specific plasma cells constituting around 10% of the NP+ cells in spleen or BM, but this population was 2-fold or 4-fold reduced in SWAP-70−/− spleen or BM, respectively. B220+NP-specific cells were clearly reduced in SWAP-70−/− mice. In contrast, the B220− cell population is enhanced in SWAP-70−/− spleen and BM after the boost. FACS analysis following day 85 + 5 with or without boost detected at least 2-fold fewer total CD138+B220low plasma cells in spleens of SWAP-70−/− mice and thus confirmed an impaired memory response in these animals (Figure 7E). ELISA measurements of total and high-affinity NP-specific IgG1 in the serum of wt or SWAP-70−/− mice at day 30 and at day 85 + 5 with or without boost revealed that the initially observed higher levels of NP-specific antibody in SWAP-70−/− mice (Figure 5) are maintained, but after boosting do not further increase in these mice, while they clearly increase in wt mice (Figure 7F). In addition, numbers of NP-specific IgG1+ AFCs increased in SWAP-70−/− mice after boosting but were surpassed by those in wt mice (Figure 7G). Altogether these results suggest that consistent with the impaired GC formation and despite the presence of initially more plasma cells and more specific Ab, the memory response (ie, the differentiation of memory B cells into expanding plasma cells) is impaired in SWAP-70−/− mice.
Memory B-cell response is reduced in SWAP-70−/− mice. Mice were immunized with NP-KLH emulsified in CFA, rested for 85 days, and reimmunized with NP-KLH for 5 days. (A) Flow cytometry of NP+ B cells in spleen. (B) Percentage of CD138+B220low, B220high, and B220−CD138+ cells among the NP+ cells in the spleen. (C) Flow cytometry of NP+ B cells in BM. (D) Percentage of CD138+B220low, B220high, and B220− cells among the NP+ cells in the BM. Data represent the mean percentage of cells in 3 independent experiments. (E) Flow cytometry of total plasma cells CD138+B220low in the spleen. Numbers indicate the percentage among total lymphocytes. (F) NP-specific total (left panel) and high-affinity (right panel) anti-IgG1 titers were determined by ELISA with NP12-BSA and NP3-BSA as capture antigen. Black and white circles represent individual mouse for wt and SWAP-70−/− respectively. Black and gray bars represent the mean average for wt and SWAP-70−/−, respectively. Values are expressed as arbitrary optical-density units at 405 nm. Fold increase in NP-IgG1 titers between no boost and after boost (low panels). (G) ELISPOT analysis of total (left panel) and high-affinity (right panel) IgG1 secretion by splenocytes from wt and SWAP-70−/− mice. Data represent the mean number of spots.
Memory B-cell response is reduced in SWAP-70−/− mice. Mice were immunized with NP-KLH emulsified in CFA, rested for 85 days, and reimmunized with NP-KLH for 5 days. (A) Flow cytometry of NP+ B cells in spleen. (B) Percentage of CD138+B220low, B220high, and B220−CD138+ cells among the NP+ cells in the spleen. (C) Flow cytometry of NP+ B cells in BM. (D) Percentage of CD138+B220low, B220high, and B220− cells among the NP+ cells in the BM. Data represent the mean percentage of cells in 3 independent experiments. (E) Flow cytometry of total plasma cells CD138+B220low in the spleen. Numbers indicate the percentage among total lymphocytes. (F) NP-specific total (left panel) and high-affinity (right panel) anti-IgG1 titers were determined by ELISA with NP12-BSA and NP3-BSA as capture antigen. Black and white circles represent individual mouse for wt and SWAP-70−/− respectively. Black and gray bars represent the mean average for wt and SWAP-70−/−, respectively. Values are expressed as arbitrary optical-density units at 405 nm. Fold increase in NP-IgG1 titers between no boost and after boost (low panels). (G) ELISPOT analysis of total (left panel) and high-affinity (right panel) IgG1 secretion by splenocytes from wt and SWAP-70−/− mice. Data represent the mean number of spots.
Discussion
This communication provides evidence for a key role of SWAP-70 in formation and function of GCs, in plasma cell development, and in the B-cell memory response. In the absence of SWAP-70, GCs do not form efficiently, and generation of GC B cells is strongly impaired. Despite the large reduction in GC formation, titers of specific Abs including high-affinity Abs and plasma cell numbers were elevated in immunized SWAP-70−/− mice. No significant numbers of NP-specific B cells were detected in GCs of SWAP-70−/− mice, but rather NP-specific B cells accumulated in the red pulp and expressed BLIMP1, diagnostic for plasma cells. A model summarizing these results and hypothesizing about the function of SWAP-70 is shown in Figure S2.
The underlying molecular cause for the GC deficiency is unknown, but the properties of SWAP-70 provide some indications. How may a protein, which binds to PIP3, Rac, and F-actin, cause such phenotype? To our knowledge this is the first report implicating a protein involved in cytoskeletal rearrangements in GC formation and function without grossly altering spleen architecture as seen in WASP-deficient mice.46 SWAP-70 controls certain rearrangements of F-actin, particularly at the cytoplasmic membrane, such as membrane ruffling and lamellipodia formation, as well as polarization of B cells into a head/uropod shape.36,38,39 Formation of GCs depends initially on the presence of naive B cells in primary follicles, which are similar in naive wt and SWAP-70−/− mice as well as after adoptive transfer of purified naive B cells into B cell–deficient mice. T cell–to–B cell interaction is also critical for GC formation. This contact requires formation of immunologic synapses, which involve integrin clustering, CD40/CD40L clustering, and B-cell MHCII-peptide/TCR interactions.14,47 Thus, receptor/ligand dynamics,48,–50 which depend on rearrangements of the underlying F-actin network, need to be properly supported. Formation of GCs further depends on migration of follicular B cells toward the T-cell zone and back to form the polarized GC. A level of mobility and flexibility in shape higher than previously appreciated was recently shown by 3 groups for LN GC B cells.26,,,–30 These papers propose that dark and light zone cells are morphologically similar, proliferation occurs in both zones with exchange between and/or within these compartments, and GC B cells compete for T-cell help as well as Ag. Allen et al26 showed that GC B cells have highly dynamic shapes, extending dendritic processes as they move, whereas naive follicular B cells and plasma cells exhibit a more round phenotype. Cell polarization to acquire proper shape and cell migration are thus likely essential for proper GC formation and function. Our group previously showed that SWAP-70−/− B cells aberrantly regulate integrin-mediated adhesion, show defective polarization, and do not form uropods and stabilized lamellipodia. LFA-1 integrin function, F-actin rearrangements, and adhesion are impaired in the absence of SWAP-70 leading to a defect in migration and homing of B cells.38 It is therefore likely, that these deficiencies constitute at least one major reason for the GC phenotype seen in SWAP-70−/− mice. Our hypothesis, which shall now be addressed molecularly, is that SWAP-70 regulates specific F-actin rearrangements and thus receptor/ligand clustering and thereby activity and avidity of these systems. Without SWAP-70, these systems do not function properly and cannot support GC formation and function.
In addition to the GC deficiency, we observed preferential localization of NP-specific cells in the red pulp of SWAP-70−/− spleen, which fits to the above hypothesis of altered adhesion and tissue homing of SWAP-70–deficient B cells. About 2-fold more high-affinity long-lived plasma cells reside in the spleen of NP-KLH–immunized SWAP-70−/− mice, where BLIMP1 expression is strongly up-regulated. The known extrafollicular reactions, which produce mostly low-affinity, short-lived plasma cells from marginal zone B cells, from some follicular B cells or from plasmablasts seem to occur with high efficiency in the GC-impaired environment of the SWAP-70−/− mouse. Plasmablast differentiation into long-lived plasma cells producing high-affinity antibody has been described as a minor reaction, and those cells rarely give rise to long-lived plasma cells in the bone marrow. The majority of extrafollicular splenic plasmablasts die within 3 days, and extrafollicular growth is finished 4 days after initiation of immunization.12,51 Only under extreme conditions (eg, in transgenic mice producing extraordinarily high numbers of antigen-specific B cells) are plasmablast seen in the red pulp.52 Under less artificial circumstances, the extrafollicular B cells are located at the T-cell zones on the border of the white pulp, and locate only very transiently to the red pulp. This contrasts with the large number of antigen-specific plasma cells in the red pulp seen in SWAP-70−/− mice over the course of several weeks, where at the same time very few antigen-specific cells are seen in the follicles. How does SWAP-70 interfere with the decision between extrafollicular reaction and GC formation? The mechanisms determining this choice in general remain still poorly understood. Gene expression programs of antigen-activated GC B cells are considerably different from those of plasma cells, and may be mutually exclusive. For example, BCL-6 and PAX-5 are required for GC formation, whereas BLIMP1 drives differentiation into plasma cells.23 We observed increased BLIMP1 expression in SWAP-70−/− NP-specific B cells (Figure 6), but it is unclear whether this is cause or consequence of increased red pulp plasma cell development. Future work may assess molecularly if SWAP-70 interferes with signaling involved in expression of these transcription factors.
Recent studies using BCR transgenic mice with varying affinity for antigen53,54 have shown that high-affinity specificities are more prevalent among the extrafollicular plasma cells versus GC B cells. Conversely, B cells with weaker antigen reactivity are primarily directed to GC, producing memory B cells and affinity-matured long-lived plasma cells. In contrast to these studies, SWAP-70−/− mice generate long-lived high-affinity plasma cells. Moreover the percentage of cells with low or high affinity for NP, measured by ELISPOT (Figure 5E,F), is comparable between wt and SWAP-70−/− naive B cells. Experiments to analyze total tyrosine phosphorylation upon BCR stimulation also revealed no significant difference between wt and SWAP-70−/− cells (R.J. et al, unpublished observation, 2006). It is thus unlikely that antigen recognition strength is the major factor regulating preferential differentiation toward plasma cells versus GC B-cell differentiation in SWAP-70−/− mice but rather, as we discussed above, altered migration and adhesion of SWAP-70−/− B cells that cause their accumulation in the red pulp.
It is generally thought that high-affinity Abs and the respective plasma cells are mostly generated in a GC-dependent follicular reaction.13,,–16,23 However, GCs do not appear to be absolutely required for affinity maturation because mice deficient in GCs, such as LT-α–deficient,55 Lyn-deficient,56 and Cr2-deficient mice,57 exhibit measurable affinity maturation with certain types of immunization. Consistent with that, SWAP-70 deficiency reveals that plasma cell and high-affinity Ab formation can occur—at even higher levels—when GC formation is strongly impaired. Although IgG1+NP+ cells express the activation marker GL7, which is associated with a GC phenotype, they localize outside of the GC in SWAP-70−/−, whereas in SWAP-70+/+ mice IgG1+ cells are found within the GC at the time of their initial appearance. This indicates that the SWAP-70−/− B cells in the red pulp at least maintain initially a GC B cell–like phenotype. It is difficult to exclude that these cells have very rapidly moved through a remnant GC-like structure before accumulating in the red pulp. At a time point as early as day 6, however, there is not yet a clearly visible GC in wt animals. Thus, if the SWAP-70−/− cells experienced a GC-like reaction in the follicles, it must have been in an emerging “pre-GC” structure. Schwickert et al29 report that follicular B cells are able to transit through and scan any given GC. Dynamic scanning would increase competition for Ag and stringency of affinity maturation. Scanning ensures that a large fraction of the B-cell repertoire is exposed to Ag trapped in GC and that rare high-affinity B cells not initially recruited into the GC reaction might have the opportunity to participate in the Ab response. These data would suggest that even in a setting were GC formation is reduced, as in SWAP-70–deficient mice, dynamic B cells would still be able to achieve GC-triggered affinity maturation. Based on the kinetics and the expression of GL7 and IgG1, however, it seems as likely that some of the maturation occurred extrafollicularly.
SWAP-70 is also involved in Ig class switching, for SWAP-70−/− mice are specifically impaired in the switch to the IgE isotype in vitro upon anti CD40/IL-4 stimulation of splenocytes and in vivo upon nematode infection.58 Since switching to other Ig isotypes was not much affected in either the former experiments or in the experiments reported here, the nuclear role of SWAP-70 in class switching is probably separate from its role in GC formation and function.
The generation of memory B cells and long-lived plasma cells is a major function of the GC reaction. Long-lived plasma cells enter the BM, where they reside and produce antibody for long periods, while memory B cells exit the GC and are then found in the circulation, where they may encounter antigen and be reactivated. The impaired memory response seen in SWAP-70−/− mice suggests that the high numbers of extrafollicularly generated plasma cells present in the BM, although long-lived and producing high-affinity Abs, cannot provide proper memory function. Thus, GC-driven plasma cell development may be necessary for a normal memory response. In an initial experiment58 we used NIP-OVA immunization and analyzed the memory response at day 118 without going into further detail. In agreement with the current report, the primary response was mildly enhanced in SWAP-70−/− mice, and the increase upon boost was lower than in wt. Classical memory B cells are CD19+B220+, and a CD19−B220− population, which has been described,6,59 appears to consist of CD138− antigen-capturing non-B cells, probably of myeloid phenotype.60,61 In our analysis, we also observed a B220−NP+CD138− population, which was enhanced in SWAP-70−/− mice. The B220−CD138−NP-specific cell population was characterized as CD4−CD8−F4/80−CD11clowCD11blow and thus may be assigned to the myeloid phenotype. The increase in this population may be explained by the 2-fold increased production of NP-specific non-IgM Ab in these mice, and consequently more binding of the Ig/antigen complexes by capturing cells, which use the FcγRI or FcϵRI to bind such complexes, assuming that the available Ab/Ag complex is a limiting factor.
SWAP-70−/− mice show a mild autoimmune phenotype, since antinuclear autoantibodies are detected at higher frequency in 22-week-old mice.58 We speculate that selection is less efficient in the red pulp and thus more autoreactive B-cell clones may arise. This is somewhat reminiscent of data from Shlomchik and coworkers (William et al62,63 ) who reported high-affinity autoantibody generation outside the GC in the autoimmune MRL.Faslpr model, into which a rheumatoid factor IgH chain encoding transgene has been introduced, causing an increased frequency of rheumatoid factor autoantibody producing B cells. Most of these transgene-specific B cells located to the T-cell zone/red pulp border instead of to GCs. As these B cells are resistant to Fas-mediated apoptosis, and are artificially enhanced in numbers, this system may reflect natural responses to a limited extent only. We intentionally analyzed immune responses in mice whose antigen receptor loci have not been altered, for the ensuing massive T-cell and B-cell activation processes occurring in such transgenic mouse models—as useful as they certainly are—constitute an artificial situation, which, for example, may greatly alter the cytokine milieu and cellular environment.
Altogether, we show that SWAP-70 is required for proper GC formation and function and memory response, and we hypothesize that the regulatory role played by SWAP-70 through Rho GTPases on F-actin cytoskeletal rearrangements and integrin activity determines this requirement. Aberrant migration and adhesion may cause B cells to accumulate in the red pulp, to receive improper signals, and to develop into high-affinity Ab-producing plasma cells but not into memory cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Annette Garbe, Karsten Kretschmer, and Glen Pearce for reading and commenting on the paper.
This work was supported by a grant from the National Institutes of Health (NIAID R01 49407), and from the DFG (SFB 655).
National Institutes of Health
Authorship
Contribution: L.Q., V.A., and M.C. performed experiments; L.Q. analyzed results and made the figures; and L.Q. and R.J. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rolf Jessberger, Institute of Physiological Chemistry, Dresden University of Technology, Fiedlerstr 42, D-01307 Dresden, Germany; e-mail: rolf.jessberger@tu-dresden.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal