Activated protein C (APC) signals in endothelial cells ex vivo through protease activated receptor-1 (PAR1). However, it is controversial whether PAR1 can mediate APC's protective effects in sepsis because the inflammatory response results in thrombin generation and thrombin proteolytically activates PAR1 much more efficiently than APC. Here we show that APC can induce powerful barrier protective responses in an endothelial cell monolayer in the presence of thrombin. Using cell surface immunoassays with conformation sensitive monoclonal anti-PAR1 antibodies we analyzed cleavage of endogenous PAR1 on the endothelial cell surface by APC in the absence and presence of thrombin. Incubation with APC caused efficient PAR1 cleavage and upon coincubation with thrombin APC supported additional PAR1 cleavage. Thrombin-cleaved PAR1 rapidly disappeared from the cell surface whereas, unexpectedly, the APC-cleaved PAR1 remained and could be detected on the cell surface, even when thrombin at concentrations of up to 1 nM was also present. Our findings demonstrate for the first time directly that APC can generate a distinct PAR1 population on endothelial cells in the presence of thrombin. The data suggest that different trafficking of activated PAR1 might explain how PAR1 signaling by APC can be relevant when thrombin is present.
Introduction
Recombinant human activated protein C (APC) has powerful protective effects in systemic inflammation that led to its approval to treat patients with severe sepsis.1 Protein C (PC) is physiologically activated on the endothelial cell surface by the key procoagulant enzyme thrombin and APC down-regulates thrombin formation in a negative feedback loop.2 However, this anticoagulant effect of APC is unlikely to explain its benefit in systemic inflammation because other anticoagulants with comparable effect do not improve survival in septic patients3,4 or in animal models.5
More recently, APC signaling through protease activated receptor-1 (PAR1) emerged as an alternative mechanism for APC's beneficial effects.6,7 In cultured endothelial cells PAR1 mediates protective effects of APC on gene expression,6,8 survival,9,10 and barrier integrity.11 PAR1 has also been implicated in mouse models analyzing neuroprotective effects of APC in vivo.9,12,13 PAR1 is a 7-transmembrane G-protein–coupled receptor that is enzymatically cleaved after Arg41 to expose a new extracellular N-terminus that acts as a tethered activating ligand.14 PAR1 is the prototypical thrombin receptor and thrombin cleaves and activates PAR1 with high efficiency because it directly binds to PAR1 in an orientation that favors cleavage.15 In contrast, APC needs to be recruited to a coreceptor, the endothelial protein C receptor (EPCR), to induce PAR1-dependent signaling6 but APC remains less efficient than thrombin.16 Systemic inflammation leads to generation of thrombin and in view of the relatively low efficiency of PAR1 activation by APC it has been argued that a role of APC-PAR1 signaling in sepsis is unlikely.16,17
Here we demonstrate that APC can mediate significant PAR1 cleavage even in the presence of thrombin. In contrast to thrombin-cleaved PAR1, the APC-cleaved PAR1 accumulates on the endothelial cell surface. The findings support the concept that PAR1 can mediate protective APC signaling in conditions where thrombin is also present.
Methods
Reagents and assays
Human thrombin was as described.6,18 Human plasma–derived APC and PC were from Haematologic Technologies (Essex Junction, VT). Recombinant human wild-type APC and mutant APC S360A were a gift from Dr John Griffin (Scripps Research Institute, La Jolla, CA).19 All experiments involving stimulation with APC included hirudin (Calbiochem, La Jolla, CA) unless indicated otherwise or if cells were coincubated with APC and thrombin. Control experiments demonstrated that hirudin alone had no effect in any of our assays. Brefeldin A was from Calbiochem. Monoclonal anti-PAR1 ATAP2, WEDE15, and SPAN11 were as described.20,21 Monoclonal rat anti-EPCR RCR-92 (nonblocking) and RCR-252 (blocking) were provided by Dr Kenji Fukudome (Saga Medical School, Saga, Japan) and were used at 25 μg/mL.22 Amidolytic assays for APC activity were as described previously.23
Cell culture, permeability assay, and surface immunoassays
EA.hy926 cells24 and primary human umbilical vein endothelial cells (HUVEC; Cascade Biologics, Portland, OR) were cultivated and macromolecular monolayer permeability was analyzed in a dual chamber system using Evans blue-labeled bovine serum albumin (BSA) as described previously.11 For cell-surface enzyme linked immunoassays (ELISA), the cells were fixed with 2% paraformaldehyde, blocked with 1% BSA and probed with anti-PAR1 antibodies at 0.5 μg/mL for 30 minutes. A horseradish peroxidase (HRP)–coupled goat anti-mouse antibody and tetrametylbenzidine were used for spectrophotometric quantification of anti-PAR1 binding. To facilitate the comparison of different experiments the quantification of antibody binding was normalized. Specific blocking peptides TFLLRNPNDK (for ATAP2), KYEPFWEDEEKNES (for WEDE15), and NATLDPRSFFLR (for SPAN11) were custom made (Invitrogen, Carlsbad, CA) and all peptides completely blocked the respective specific anti-PAR1 staining. Residual unspecific staining was found to be unaffected by agonists and was subtracted to correct for background (0% staining). In all experiments nonagonist-treated cells were included and PAR1 staining in these cells was defined as 100%. The shown data were generated using EA.hy926 cells and results were confirmed in HUVECs. For immunofluorescence microscopy EA.hy926 cells were grown, fixed, and stained on glass cover slides using identical conditions as for ELISA. Alexa Fluor 488-coupled goat antimouse (Invitrogen) was used as the secondary antibody. The cover slides were extensively washed, mounted (Gelmount; Sigma-Aldrich, St Louis, MO), and immediately analyzed using an Olympus BX60 fluorescence microscope (Olympus, San Diego, CA).
Biotinylation of cell-surface proteins and Western blotting
After agonist incubation, proteases were quenched, EA.hy926 cells were washed twice on ice and kept at 4°C for all subsequent steps. Cell-surface proteins were biotinylated (0.2 mg/mL Sulfo-NHS-SS-biotin for 30 minutes; Pierce, Rockford, IL) before extraction in RIPA buffer supplemented with Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN), 20 μM bestatin, and 0.1 mg/mL phenylmethylsulfonyl fluoride. Biotinylated proteins were collected using streptavidin agarose (Invitrogen). Proteins were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, transferred to Immobilon-P-membranes (Millipore, Bedford, MA), blocked with dry milk, and probed with WEDE15 or ATAP2 followed by HRP-coupled goat antimouse and visualization using the Femto detection system (Pierce). In blots showing non–cell-surface proteins loading was assessed using anti–β-actin (Sigma-Aldrich) followed by HRP-coupled goat antirabbit. Optical density of immunoreactive bands was assessed using Scion Image Alpha 4.0.3.2 software (Frederick, MD).
Statistical analysis
Data analysis was performed using the NCSS Statistical & Power Analysis or SigmaStat 3.5 (Systat Software, San Jose, CA) software. A 2-sample 2-tailed homoscedastic t test was used to calculate the indicated P values.
Results
APC can induce endothelial barrier protective signaling in the presence of thrombin
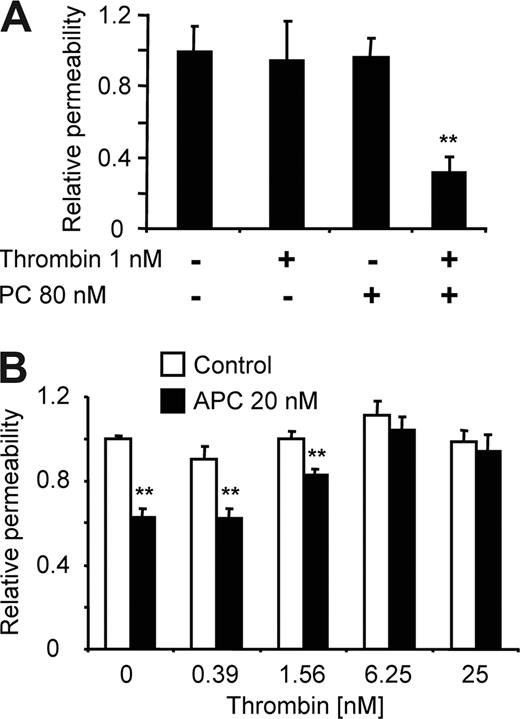
Enhancement of endothelial barrier integrity is a sensitive readout for PAR1-dependent signaling by exogenous and endogenously generated APC.11,23 To test if APC-PAR1 signaling still occurs when thrombin is present at concentrations that are expected to lead to rapid cleavage of the available PAR1, we determined whether locally generated APC can still mediate protective effects in the presence of 1 nM thrombin. Neither thrombin alone nor PC zymogen affected permeability of a monolayer of endothelial cells in a dual chamber system after 3 hours of incubation. However, when the cells were coincubated with thrombin and PC, the generated APC significantly enhanced the barrier function (Figure 1A). We next studied the effect of increasing thrombin concentrations on APC's barrier enhancing effects. To only vary the thrombin but not the APC concentration, APC was exogenously added in these experiments, although exogenous APC is less efficient than endogenously generated APC. Again APC enhanced the barrier integrity despite the presence of up to low-nanomolar thrombin concentrations (Figure 1B). These data indicate that exogenous and endogenously generated APC still mediate barrier protective effects even if thrombin is also present. The possibility that thrombin becomes inhibited or sequestered, allowing APC to induce protective effects through newly externalized PAR1,25 was ruled out in control experiments that demonstrated that thrombin's proteolytic activity in the cell medium was stable under our experimental conditions (not shown).
Protective signaling by APC in the presence of thrombin. EA.hy926 cells in a dual-chamber system were incubated for 3 hours with the indicated agonists in the top chamber followed by analysis of permeability. Means plus or minus SEM with n = 5 (A) and 10 (B). **P < .005.
Protective signaling by APC in the presence of thrombin. EA.hy926 cells in a dual-chamber system were incubated for 3 hours with the indicated agonists in the top chamber followed by analysis of permeability. Means plus or minus SEM with n = 5 (A) and 10 (B). **P < .005.
Quantification of endogenous PAR1 on the endothelial cell surface by immunoassays
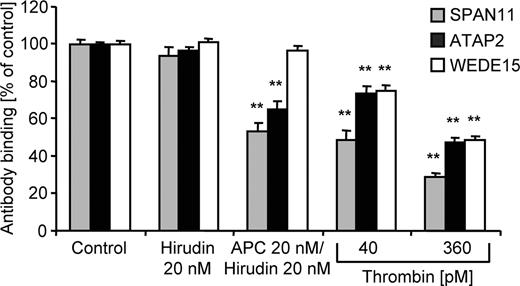
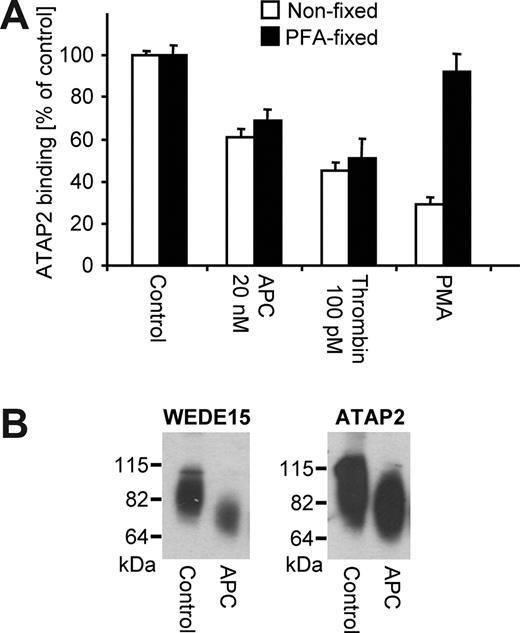
Because it is not possible to directly prove that APC's barrier enhancing effects in the presence of thrombin-PAR1 signaling still depend on PAR1 cleavage, we established assays to assess cleavage of endogenous PAR1 by APC and thrombin alone and in combination. A tagged PAR1 construct has been used to measure PAR1 cleavage in a previous report.16 However, such transfected PAR1 constructs were later shown to have decreased susceptibility toward cleavage by APC as compared with endogenously expressed PAR1, most likely because of relative unavailability of EPCR.26 We therefore established a quantitative cell-surface ELISA to analyze how cleavage and/or conformation of endogenous endothelial cell PAR1 are affected. Control experiments using immunofluorescence microscopy demonstrated that the anti-PAR1 antibodies under conditions used for the ELISA indeed only bind to cell surface PAR1 and do not detect intracellular pools (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thrombin dose–dependently down-regulated binding of 3 monoclonal antibodies that were raised against the N-terminal exodomain of human PAR1, ie, SPAN11, ATAP2, and WEDE1520,21 (Figure 2), consistent with the expected cleavage and internalization of PAR1.27 APC incubation strongly decreased binding of the cleavage-sensitive SPAN11, indicating that APC reduces surface availability of native, uncleaved PAR1. Binding of the non–cleavage-sensitive WEDE15, which binds to an epitope downstream from the Arg41 cleavage site in PAR1's N-terminal exodomain, was not affected by APC, indicating that levels of total surface PAR1 remain constant. These data demonstrate for the first time directly that incubation with APC leads to cleavage of endogenous endothelial cell PAR1. APC down-regulated ATAP2 binding although ATAP2 also binds to an epitope downstream from the Arg41 cleavage site and is regarded as a non–cleavage-sensitive anti-PAR1. This unexpected result indicates that the APC-cleaved PAR1 is detected on the cell surface by WEDE15 but not by ATAP2. ATAP2 recognizes PAR1's Leu44-Arg46 amino acid stretch while WEDE15 binds further downstream at Phe55-Glu60.20,21 Previous data indicate that APC can only directly cleave PAR1's N-terminal exodomain at Arg41.28 Incubation with APC might lead to activation of other cellular proteases that in turn might cleave PAR1 at additional sites and cause shedding of the ATAP2 but not the WEDE15 epitope. However, effects of APC on ATAP2 staining were found to be comparable in native cells and cells that have been fixed before agonist incubation (Figure 3A), suggesting that loss of ATAP2 binding is mediated by a conformational change after cleavage. Western blotting of biotinylated cell-surface proteins was used to test whether the ATAP2 epitope can be detected in denatured cleaved PAR1 after APC incubation. The surface biotinylation–based assay does not detect intracellular PAR1, as shown in control experiments (Figure S1B). PAR1 migrated as an elongated immunoreactive band as reported earlier and prolonged incubation with APC led to a cleavage product at the expected smaller size.28,29 Both WEDE15 and ATAP2 anti-PAR1 similarly detected native and cleaved PAR1 (Figure 3B). The ATAP2 epitope in APC-cleaved PAR1 is thus not shed or degraded and becomes available for ATAP2 binding in denatured PAR1. In conclusion, WEDE15 is a non–cleavage-sensitive antibody and detects total PAR1 in both native (ELISA) and denaturing (Western blotting) conditions. ATAP2 does also bind to cleaved and uncleaved denatured PAR1; however, it is conformation sensitive and does not bind to the cleaved PAR1 in the cell-surface immunoassay.
Quantification of cell surface–exposed PAR1. Confluent EA.hy926 cells were incubated for 3 hours with the indicated agonists. Apical expression of PAR1 was analyzed in a cell-surface ELISA using monoclonal anti-PAR1 antibodies SPAN11, ATAP2, and WEDE15. Results are shown relative to control (means ± SEM, n = 9, **P < .005).
Quantification of cell surface–exposed PAR1. Confluent EA.hy926 cells were incubated for 3 hours with the indicated agonists. Apical expression of PAR1 was analyzed in a cell-surface ELISA using monoclonal anti-PAR1 antibodies SPAN11, ATAP2, and WEDE15. Results are shown relative to control (means ± SEM, n = 9, **P < .005).
ATAP2 does not detect PAR1's active conformation generated after scissile bond cleavage. (A) ATAP2 binding was quantified by cell-surface ELISA after incubation for 3 hours with the indicated agonists in cells before and after fixation with paraformaldehyde (PFA). PMA indicates phorbol myristate acetate (0.1 μg/mL). Means plus or minus SEM are shown (n = 12). (B) Cells were incubated for 3 hours with control or APC (20 nM) and biotinylated surface proteins were analyzed by Western blotting with WEDE15 or ATAP2 as indicated. A representative experiment of 3 is shown.
ATAP2 does not detect PAR1's active conformation generated after scissile bond cleavage. (A) ATAP2 binding was quantified by cell-surface ELISA after incubation for 3 hours with the indicated agonists in cells before and after fixation with paraformaldehyde (PFA). PMA indicates phorbol myristate acetate (0.1 μg/mL). Means plus or minus SEM are shown (n = 12). (B) Cells were incubated for 3 hours with control or APC (20 nM) and biotinylated surface proteins were analyzed by Western blotting with WEDE15 or ATAP2 as indicated. A representative experiment of 3 is shown.
PAR1 cleavage by APC
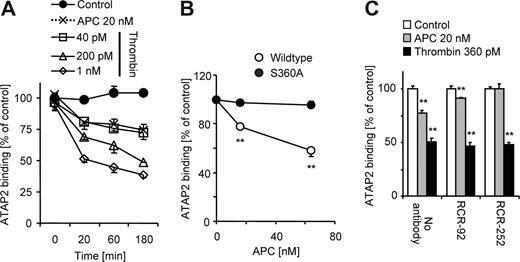
Given that ATAP2 is commercially available and sensitive to the conformational change upon cleavage of PAR1 we used this antibody to assess cleavage of endogenously expressed PAR1. Results were confirmed using SPAN11 as indicated below. APC and thrombin down-regulated the binding of ATAP2 in a time dependent manner and 20 nM of APC was similarly efficient as 40 pM thrombin (Figure 4A). A proteolytically inactive recombinant APC variant with an S→A substitution at the active center did not affect ATAP2 binding (Figure 4B). As expected, PAR1 cleavage by APC was completely inhibited in the presence of an antibody against EPCR that blocks APC binding to EPCR (Figure 4C). In contrast, APC significantly down-regulated ATAP2 binding in the presence of nonblocking anti-EPCR, albeit to a lesser extent than in the absence of antibody. A possible explanation for the effect of nonblocking anti-EPCR might be that the antibody leads to clustering and internalization of EPCR during the 3-hour experiment. Down-regulation of ATAP2 binding in response to APC thus requires both APC's proteolytic activity and APC binding to EPCR. For all subsequent experiments analyzing effects of exogenously added APC a concentration of 20 nM was used and agonist incubation was for 3 hours to obtain significant EPCR-dependent cleavage of PAR1.
APC down-regulates ATAP2 binding dependent on proteolytic activity and EPCR binding. (A) ATAP2 binding was quantified by ELISA after incubation with the indicated agonists in time course experiments. (B) Cells were incubated for 3 hours with the indicated concentrations of recombinant wild-type APC or proteolytically inactive APC S360A and ATAP2 binding was quantified. (C) Cells were preincubated (10 minutes) in the absence and presence of nonblocking (RCR-92) or blocking (RCR-252) anti-EPCR (25 μg/mL) followed by 3 hours of incubation with the indicated agonists and quantification of ATAP2 binding. Means plus or minus SEM are shown (n = 6 in panels A,C; n = 9 in panel B. **P < .005).
APC down-regulates ATAP2 binding dependent on proteolytic activity and EPCR binding. (A) ATAP2 binding was quantified by ELISA after incubation with the indicated agonists in time course experiments. (B) Cells were incubated for 3 hours with the indicated concentrations of recombinant wild-type APC or proteolytically inactive APC S360A and ATAP2 binding was quantified. (C) Cells were preincubated (10 minutes) in the absence and presence of nonblocking (RCR-92) or blocking (RCR-252) anti-EPCR (25 μg/mL) followed by 3 hours of incubation with the indicated agonists and quantification of ATAP2 binding. Means plus or minus SEM are shown (n = 6 in panels A,C; n = 9 in panel B. **P < .005).
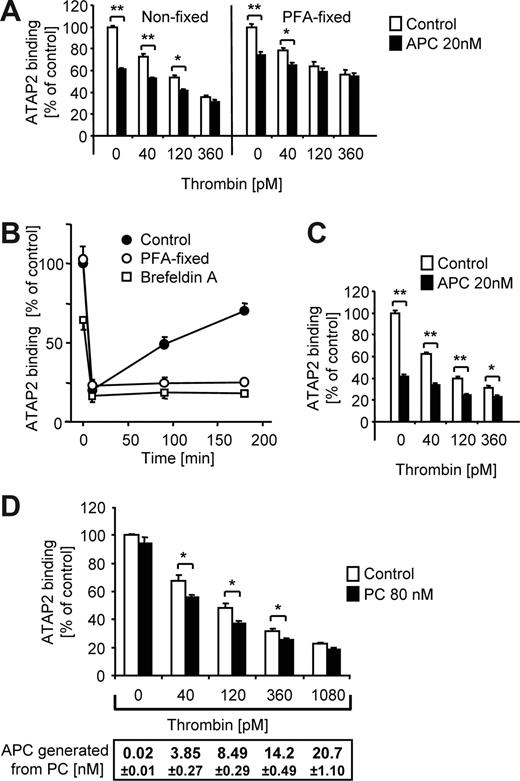
APC can mediate additional PAR1 cleavage even if thrombin is also present
When APC was coincubated with thrombin an additional loss of ATAP2 binding was detected (Figure 5A). APC also caused an additional loss of ATAP2 binding in cells that were fixed before agonist incubation consistent with direct effects of APC on PAR1. Endothelial cells have intracellular pools of PAR1 and previous studies have shown that both native and cleaved PAR1 are translocated to the cell surface upon agonist treatment.20,25 As shown in Figure 5B, the cell surface was repopulated with PAR1 after a high-dose thrombin challenge with similar kinetics in the EA.hy926 cell line as described previously for HUVECs. Brefeldin A, an inhibitor of vesicle trafficking that blocks exocytosis but not internalization of membrane proteins,30 prevented repopulation of the cell surface with ATAP2 binding sites (Figure 5B). When surface repopulation was blocked with brefeldin A, APC caused a significant additional loss of ATAP2 binding in the presence of up to 360 pM thrombin (Figure 5C). Similar results were obtained using the cleavage-sensitive antibody SPAN11 (not shown). We have previously shown that the endogenous PC activation pathway is mechanistically linked to efficient PAR1-dependent protective signaling.23 Consistent with these results, when cells were incubated with thrombin in the presence of PC zymogen, ATAP2 binding (and SPAN11 binding; not shown) was further decreased even when only low nM APC was generated (Figure 5D).
Both exogenous and endogenously generated APC support additional PAR1 cleavage in the presence of thrombin. (A) Nonfixed or PFA-fixed cells were incubated for 3 hours with different concentrations of thrombin in the absence or presence of APC as indicated, followed by analysis of ATAP2 binding. (B) Cells were incubated with 20 nM thrombin for 10 minutes followed by quenching of the protease with 50 nM hirudin. Recovery of ATAP2 binding upon incubation at 37°C over 3 hours is plotted. Where indicated, the cells were PFA-fixed or pretreated with brefeldin A (1 μM, 10 minutes) before the addition of thrombin. A representative experiment (of 3) is shown. (C) Brefeldin A–treated cells were incubated for 3 hours as indicated followed by analysis of ATAP2 binding. (D) Nonfixed cells were incubated with the indicated concentrations of thrombin in the absence or presence of 80 nM PC. After 3 hours the APC concentration in the conditioned medium was determined by chromogenic assay and ATAP2 binding was analyzed. Means plus or minus SEM are shown (n = 9 in panels A,C,D. *P < .05, **P < .005).
Both exogenous and endogenously generated APC support additional PAR1 cleavage in the presence of thrombin. (A) Nonfixed or PFA-fixed cells were incubated for 3 hours with different concentrations of thrombin in the absence or presence of APC as indicated, followed by analysis of ATAP2 binding. (B) Cells were incubated with 20 nM thrombin for 10 minutes followed by quenching of the protease with 50 nM hirudin. Recovery of ATAP2 binding upon incubation at 37°C over 3 hours is plotted. Where indicated, the cells were PFA-fixed or pretreated with brefeldin A (1 μM, 10 minutes) before the addition of thrombin. A representative experiment (of 3) is shown. (C) Brefeldin A–treated cells were incubated for 3 hours as indicated followed by analysis of ATAP2 binding. (D) Nonfixed cells were incubated with the indicated concentrations of thrombin in the absence or presence of 80 nM PC. After 3 hours the APC concentration in the conditioned medium was determined by chromogenic assay and ATAP2 binding was analyzed. Means plus or minus SEM are shown (n = 9 in panels A,C,D. *P < .05, **P < .005).
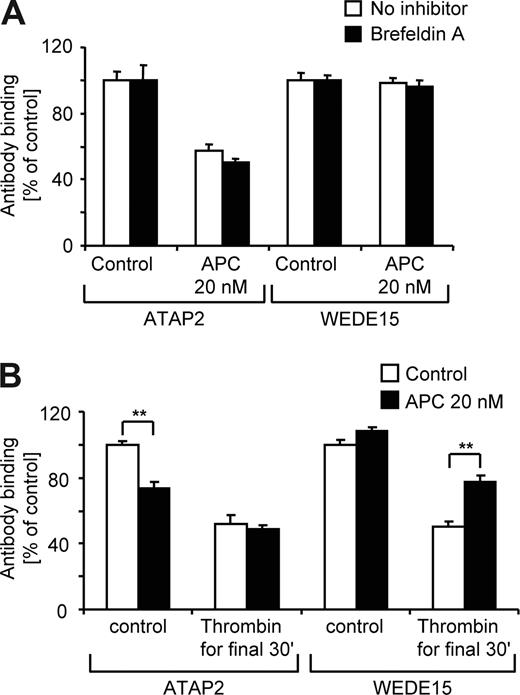
APC-cleaved PAR1 is inefficiently removed from the cell surface
Given that EA.hy926 cells can rapidly externalize PAR1 to the cell surface after agonist treatment (Figure 5B), our finding that APC does not affect WEDE15 binding (Figure 2) could be explained by efficient repopulation of the cell surface with cleaved and/or uncleaved PAR1 from intracellular pools. However, APC did not down-regulate WEDE15 staining even in the presence of brefeldin A (Figure 6A), demonstrating that, in contrast to thrombin-cleaved PAR1, APC-cleaved PAR1 is retained on the cell surface. The cleaved PAR1 present on the cell surface after APC incubation was still retained after a subsequent incubation with high-dose thrombin (Figure 6B).
APC cleaved PAR1 remains on the cell surface. (A) Cells were incubated with agonists for 3 hours in the absence or presence of brefeldin A followed by analysis of ATAP2 or WEDE15 binding by surface ELISA as indicated. Means plus or minus SEM are shown (n = 9). (B) Cells were incubated for 3.5 hours with 100 pM hirudin without or with additional 20 nM of APC. For the final 30 minutes of incubation either control or 500 pM of thrombin was added and cell surface–expressed PAR1 was quantified by analysis of ATAP2 and WEDE15 binding. Means plus or minus SEM are shown (n = 9, **P < .005 comparing results without and with thrombin for final 30 minutes).
APC cleaved PAR1 remains on the cell surface. (A) Cells were incubated with agonists for 3 hours in the absence or presence of brefeldin A followed by analysis of ATAP2 or WEDE15 binding by surface ELISA as indicated. Means plus or minus SEM are shown (n = 9). (B) Cells were incubated for 3.5 hours with 100 pM hirudin without or with additional 20 nM of APC. For the final 30 minutes of incubation either control or 500 pM of thrombin was added and cell surface–expressed PAR1 was quantified by analysis of ATAP2 and WEDE15 binding. Means plus or minus SEM are shown (n = 9, **P < .005 comparing results without and with thrombin for final 30 minutes).
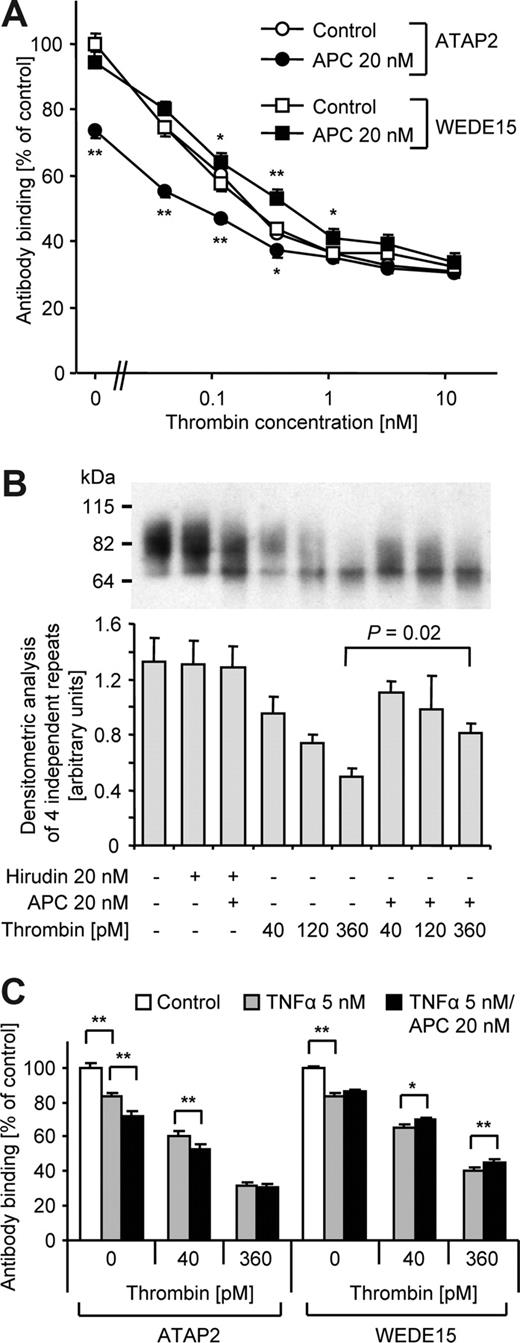
These results raise the possibility that APC-cleaved PAR1 might accumulate on the cell surface even in the presence of thrombin. When ATAP2 (detects only uncleaved PAR1) and WEDE15 (detects both cleaved and uncleaved PAR1) binding was analyzed in parallel experiments after incubation with increasing concentrations of thrombin alone, staining with both antibodies was almost identical (Figure 7A). This finding indicates that no detectable cleaved PAR1 is retained on the cell surface upon thrombin incubation. If the cells were coincubated with APC, ATAP2 binding was further decreased, consistent with additional cleavage of PAR1 by APC in the presence of thrombin. In contrast, coincubation with APC led to enhanced WEDE15 binding, demonstrating that total (cleaved and uncleaved) PAR1 is increased. Because the differential between WEDE15 and ATAP2 staining corresponds to the amount of cleaved PAR1 these findings show that treatment with APC results in the retention of cleaved PAR1 on the cell surface in the absence as well as in the presence of up to 1 nM thrombin. Analysis of biotinylated cell-surface proteins by Western blotting confirmed that coincubation with APC reproducibly led to higher levels of PAR1 on the cell surface compared with thrombin alone (Figure 7B). To test whether similar results are also obtained in inflammatory cytokine-perturbed endothelial cells, we analyzed cleavage and surface retention of PAR1 in response to incubation with APC and thrombin in cells that were pretreated with a high concentration of tumor necrosis factor-α (TNFα; Figure 7C). TNFα reduced the amount of surface-exposed native PAR1 and PAR1 cleavage by APC was found to be slightly less efficient in TNFα-induced cells most likely because of decreased EPCR expression. However, APC still supported significant PAR1 cleavage in both the absence and presence of thrombin and the APC-cleaved PAR1 was surface retained, entirely consistent with our results in quiescent cells. Although the differences were relatively small in TNFα-pretreated cells, the data show that APC leads to a unique population of cleaved PAR1 on the cell surface that might mediate APC's beneficial effects in systemic inflammation.
APC-cleaved PAR1 is retained on the cell surface even in the presence of thrombin. (A) Nonfixed cells were incubated for 3 hours with the indicated agonists and cell surface–expressed PAR1 was quantified by analysis of ATAP2 and WEDE15 binding. (B) After incubation with the indicated agonists for 3 hours, surface proteins were biotinylated and isolated with streptavidin agarose. PAR1 was detected by Western blotting using anti-PAR1 WEDE15. A representative experiment is shown in the top panel. Optical density of immunoreactive bands was measured in 3 independent experiments and means plus or minus SEM are shown in the bottom panel. Coincubation with APC led to detection of more surface PAR1 at all thrombin concentrations, a finding that was borderline significant at lower thrombin concentrations but significant at 360 pM thrombin. (C) As indicated, cells were induced with TNFα for 2 hours and thrombin and/or APC added for an additional 3 hours followed by quantification of ATAP2 and WEDE15 binding. Means plus or minus SEM are shown in panels A,C (n = 15 in panel A and 7 in panel C, *P < .05, **P < .005, comparing results without and with APC in panel A).
APC-cleaved PAR1 is retained on the cell surface even in the presence of thrombin. (A) Nonfixed cells were incubated for 3 hours with the indicated agonists and cell surface–expressed PAR1 was quantified by analysis of ATAP2 and WEDE15 binding. (B) After incubation with the indicated agonists for 3 hours, surface proteins were biotinylated and isolated with streptavidin agarose. PAR1 was detected by Western blotting using anti-PAR1 WEDE15. A representative experiment is shown in the top panel. Optical density of immunoreactive bands was measured in 3 independent experiments and means plus or minus SEM are shown in the bottom panel. Coincubation with APC led to detection of more surface PAR1 at all thrombin concentrations, a finding that was borderline significant at lower thrombin concentrations but significant at 360 pM thrombin. (C) As indicated, cells were induced with TNFα for 2 hours and thrombin and/or APC added for an additional 3 hours followed by quantification of ATAP2 and WEDE15 binding. Means plus or minus SEM are shown in panels A,C (n = 15 in panel A and 7 in panel C, *P < .05, **P < .005, comparing results without and with APC in panel A).
Discussion
In sepsis the efficient PAR1 activator thrombin is generated, and therefore the question how any beneficial effects of APC can be PAR1 mediated is at the root of the current controversy. So far it was difficult to explain how APC can mediate any effects through PAR1 because thrombin is much more efficient in cleaving this receptor. Our data directly demonstrate for the first time that APC can cleave endogenous PAR1 on the surface of human endothelial cells, corroborating conclusions from studies using overexpressed tagged PAR1 constructs16 or cleavage blocking antibodies against PAR1.6 Exogenous and endogenously generated APC can mediate both endothelial barrier protective signaling and additional cleavage of endogenous PAR1 on the endothelial cell surface even in the presence of up to 1 nM of thrombin. The absence of detectable additional cleavage of epitope tagged PAR1 by generated APC in a previous study16 could be explained by preferential cleavage of the overexpressed receptor by thrombin that binds directly to PAR1 and does not require a coreceptor.26 Our finding that APC has no detectable additional effect on PAR1 cleavage in the presence of high thrombin concentrations (> 1 nM) argues that any distinct PAR1 population that can only be cleaved by APC but not by thrombin would have to be very small. Consistent with the findings by Bae et al26 we propose that there is a PAR1 population that is colocalized with EPCR and that is cleaved by APC. Even though thrombin can also cleave this PAR1 population, cleavage by APC is efficient enough to lead to significant additional cleavage at up to high-picomolar thrombin concentrations.
Whereas thrombin-cleaved PAR1 is rapidly internalized and degraded,27 our data show that APC-activated PAR1 remains on the cell surface and accumulates upon prolonged incubation even when thrombin is present. It has been recently established that downstream G-protein coupling of PAR1 differs for specific agonists.31 Therefore, it is conceivable that even though thrombin and APC activate PAR1 by cleaving the same scissile bond28 differences in the interaction of the tethered ligand with PAR1 could lead to the activation of distinct downstream signaling pathways and to unique biologic responses, including distinct trafficking of thrombin- and APC-cleaved PAR1. Such differences might also explain the finding that thrombin and APC can have distinct effects on gene expression through PAR1 signaling in cytokine-perturbed endothelial cells.8 Our previous results demonstrate that low (∼40 pM) but not high thrombin concentrations can induce endothelial barrier protection similar to APC,11 suggesting that differences in the PAR1 cleavage rate translate into distinct downstream signaling. Our present results that APC can mediate protective effects in the presence of thrombin concentrations much higher than 40 pM indicate that differences in the cleavage rate alone cannot explain all the findings. One possibility is that APC binding to EPCR leads to distinct signaling of the colocalized PAR1.32 Although extensive research will be required to elucidate molecular details of the downstream signaling pathways and to establish that the surface-retained PAR1 indeed mediates protective APC effects in the presence of thrombin, our results provide conceptually novel insight into the paradoxical condition that the 2 key coagulation proteases thrombin and APC, linked by a negative feedback loop, can mediate opposite effects on endothelial biology through the same receptor PAR1.
Our finding that cellular trafficking of thrombin- and APC-cleaved PAR1 is distinct suggests how receptor signaling by a very inefficient protease can be relevant in the presence of the much stronger agonist thrombin. Because of the irreversibility of proteolytic activation PAR1 signaling must be regulated through mechanisms such as receptor trafficking.27 Although the rate of thrombin-PAR1 cleavage at any given point in time might be much higher than the rate of APC-PAR1 cleavage, the thrombin-cleaved receptor is rapidly internalized and degraded whereas the APC-cleaved receptor accumulates on the surface and can potentially mediate relevant signaling in the presence of thrombin. This illustrates how the efficiency of induction of a specific biologic response does not necessarily correlate with efficiency of cleavage. This concept cannot only explain our finding that APC enhances endothelial barrier integrity in the presence of thrombin, but it might help to clarify the complex roles of PAR1 in vivo. PAR1 deficiency did not affect survival in mouse models of endotoxemia,33,34 even though PAR1 has well-established proinflammatory effects in other models, such as glomerulonephritis, inflammatory bowel disease, or ischemia-reperfusion injury.35,–37 This argues that any detrimental effects of PAR1 deficiency in systemic inflammation might be offset by the absence of proinflammatory PAR1 signaling. Very recent results indeed support the conclusion that PAR1 has such dual roles during different stages of the inflammatory response in mouse models of severe sepsis.38,39 These findings suggest that PAR1 mediates protective signaling by the PC pathway in vivo under conditions where thrombin is also present. Our in vitro data suggest that relevant protective PAR1 signaling by the PC pathway might be possible in the presence of up to high-picomolar or low-nanomolar thrombin. PAR1 is activated with half maximal efficiency by a concentration of only approximately 50 pM thrombin14 and thrombin activity in vivo is rigorously controlled to prevent excessive platelet activation/fibrin formation. Although it is not known what the thrombin concentration in the endothelial cell microenvironment under conditions of inflammatory stress might be, data from Dr Coughlin's laboratory elegantly demonstrate that thrombin likely operates close to threshold levels: In murine platelets PAR4 cleavage is required for thrombin signaling and PAR3 acts as a nonsignaling cofactor that recruits thrombin and decreases the concentration required for half maximal signaling between 6- and 15-fold.40 However, PAR3- and PAR4-deficient mice showed similar degrees of protection in thrombosis models, indicating that an approximately 10-fold decrease in platelet responsiveness to thrombin has the same effect as complete unresponsiveness.41,42 Based on these findings, we expect that the range of thrombin concentrations where we obtained protective signaling and surface retention of APC-cleaved PAR1 is of physiologic relevance.
In conclusion, our results help explain how using a single receptor cell can sense proteolytic activity of thrombin and APC independently, and they support the concept that PAR1-dependent signaling might contribute to protective effects of APC in sepsis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs J. Griffin, K. Fukudome, and C. Edgell for valuable reagents.
This work was supported by National Institutes of Health grant HL 73 318 (M.R.), grants from the Swiss National Science Foundation PBBBE-108544, the Stiefel-Zangger Foundation of the University of Zurich, and the Theodor and Ida Herzog-Egli-Stiftung (R.A.S.), and an Austrian fellowship, Erwin Schrödinger-Auslandsstipendium J2413-B13 (C.F.).
National Institutes of Health
Authorship
Contribution: R.A.S., C.F., and M.R. designed and performed research and analyzed data; R.A.S and M.R. wrote the manuscript; and L.F.B contributed monoclonal antibodies against PAR1.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthias Riewald, MD, Scripps Research Institute, Department of Immunology, SP30-3040, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: riewald@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal