The HOVON cooperative study group performed a feasibility study of escalated imatinib and intravenous cytarabine in 165 patients with early chronic-phase chronic myeloid leukemia (CML). Patients received 2 cycles of intravenous cytarabine (200 mg/m2 or 1000 mg/m2 days 1-7) in conjunction with imatinib (200 mg, 400 mg, 600 mg, or 800 mg), according to predefined, successive dose levels. All dose levels proved feasible. Seven dose-limiting toxicities (DLTs) were observed in 302 cycles of chemotherapy, which were caused by streptococcal bacteremia in 5 cases. Intermediate-dose cytarabine (1000 mg/m2) prolonged time to neutrophil recovery and platelet recovery compared with a standard dose (200 mg/m2). High-dose imatinib (600 mg or 800 mg) extended the time to platelet recovery compared with a standard dose (400 mg). More infectious complications common toxicity criteria (CTC) grade 3 or 4 were observed after intermediate-dose cytarabine compared with a standard-dose of cytarabine. Early response data after combination therapy included a complete cytogenetic response in 48% and a major molecular response in 30% of patients, which increased to 46% major molecular responses at 1 year, including 13% complete molecular responses. We conclude that combination therapy of escalating dosages of imatinib and cytarabine is feasible. This study was registered at www.kankerbestrijding.nl as no. CKTO-2001-03.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder characterized by a reciprocal translocation between the long arms of chromosomes 9 and 22, known as the Philadelphia (Ph) translocation.1,2 The molecular consequence of this translocation is the generation of a bcr-abl fusion gene, which encodes for a chimeric protein with constitutive tyrosine-kinase activity sufficient for leukemogenesis in mice.3 Imatinib is a relatively specific inhibitor of the BCR-ABL tyrosine kinase and acts by stabilizing the inactive non–ATP-binding conformation of BCR-ABL. In the International Randomized Study of Interferon and STI571 (IRIS), a complete hematologic response was obtained in 98% of the patients and a complete cytogenetic response in 87% of the patients with newly diagnosed CML after a median follow-up of 60 months.4,5 Imatinib has become the drug of choice as first-line therapy in the treatment of CML. However, the development of resistance is of concern. The estimated rate of event-free survival in the IRIS study was 83% at 60 months, while an estimated 7% of all patients progressed to accelerated phase or blast crisis. Patients who did not obtain a complete hematologic response at 3 months, a minor cytogenetic response at 6 months, a major cytogenetic response at 12 months, or a complete cytogenetic response at 18 months were at increased risk of relapse.5,6
The question has arisen whether it is possible to increase the molecular response rate and prevent resistance by combination therapy. In vitro studies have shown synergistic action between imatinib and various drugs, including cytarabine.7,8 Cytarabine is a very active drug and probably the most potent drug in acute myeloid leukemia.9,10 Low-dose cytarabine in combination with interferon alfa (IFN-α) was considered standard treatment before the introduction of imatinib,11 and higher dosages of cytarabine were associated with better response rates.12 The synergistic activity observed in vitro by combining imatinib and cytarabine was especially observed when both drugs were applied in increasing concentrations.7,8 A clear dose-response relationship has been established for imatinib monotherapy, and an increased rate of molecular remission was suggested in patients treated with 800 mg imatinib.13 These findings have evoked the question whether the combination of cytarabine and imatinib may improve response and prevent resistance. In view of the dose-dependent effects of both drugs, we explored the feasibility of the combination of imatinib and cytarabine using escalating dosages in successive dose levels.
Methods
Patients with newly diagnosed CML in first chronic phase were eligible if they were between 18 and 65 years of age and registered within 6 months of diagnosis. Other eligibility criteria included the presence of the Philadelphia chromosome or BCR-ABL rearrangement and World Health Organization performance status of 2 or less. Previous treatment for CML was not allowed with the exception of hydroxyurea. Patients with hepatic dysfunction, renal insufficiency, severe cardiac, pulmonary or neurologic disease, active uncontrolled infections, human immunodeficiency virus infection, and malignancies during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma, and pregnant or lactating women were not eligible. Patients with a human leukocyte antigen (HLA)–matched sibling donor who were scheduled to receive an allogeneic transplantation upfront were also ineligible. The study was approved by the ethics committees of the participating institutions, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Study design and treatment
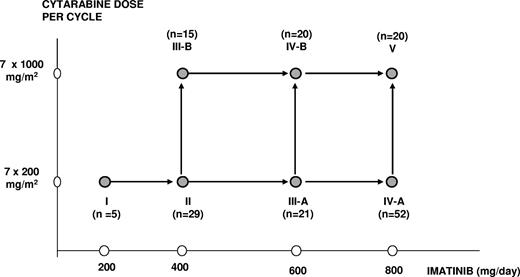
Treatment with imatinib was started, after discontinuation of hydroxyurea, at a dose of 400 mg once daily and continued for 2 to 3 weeks. This prephase of imatinib monotherapy was designed to avoid cumulative toxicity of hydroxyurea and cytarabine. Thereafter, patients were hospitalized to receive the first of 2 cycles of intravenous cytarabine in conjunction with oral imatinib. Imatinib was given once daily at a dose of either 200 mg, 400 mg, 600 mg, or 800 mg in combination with standard-dose cytarabine (200 mg/m2) in a 2-hour infusion or intermediate-dose cytarabine (1000 mg/m2) in a 3-hour infusion days 1 to 7, according to the assigned dose levels I to V (Figure 1). Patients who received standard-dose cytarabine were discharged after chemotherapy and readmitted when they became neutropenic. Patients who received intermediate-dose cytarabine were hospitalized until hematologic recovery. Prophylaxis for prevention of Gram-negative bacterial and fungal infections was mandatory until resolution of neutropenia and penicillin prophylaxis was given at days 8 to 20 of intermediate-dose cytarabine only.
Successive dose levels, by dose of cytarabine and imatinib.
Successive dose levels, by dose of cytarabine and imatinib.
Initially, 5 patients were entered in the lowest dose level (cytarabine 200 mg/m2 and imatinib 200 mg). The study was thereafter temporarily put on hold until these patients could be evaluated for dose-limiting toxicity (DLT). Patients who went off protocol before completion of cycle 1 for reasons not related to DLT were replaced. Depending on the number of patients with a DLT or patients who died of treatment-related mortality (TRM) during or after cycle 1, inclusion of patients continued in the same or in the next higher dose level, according to the decision rules specified in Table 1. In short, a subsequent dose level was open for inclusion when the criteria of acceptable toxicity and safety had been met (ie, when ≤ 5% TRM and ≤ 20% DLT [including TRM] had been observed in that dose level). In addition, inclusion in the next dose level was put on hold if evaluation of the preceding dose level was not completed, while inclusion and extension of the preceding dose level was allowed. Dose levels IIIA and IIIB were opened simultaneously after the previous dose level had met the criteria of acceptable toxicity and safety and afterwards, when both dose levels were proven feasible, dose levels IVA and IVB were also opened simultaneously. Dose level V was started after IVA and IVB had met the criteria of acceptable toxicity and safety.
Number of patients per dose level and decision rules
| No. of evaluable patients . | No. of patients with DLT . | And/Or . | No. of patients with TRM . | Action . |
|---|---|---|---|---|
| 5 | 0-1 | And | 0 | Go to dose level N+1 with entry of 5 patients |
| 5 | 5 | Or | ≥2 | STOP; dose level N not feasible* |
| 5 | 2-4 | Or | 1 | Enter 5 more patients at dose level N |
| 10 | 0-2 | And | 0 | Go to dose level N+1 with entry of 5 patients |
| 10 | ≥5 | Or | ≥2 | STOP; dose level N not feasible* |
| 10 | 3-4 | Or | 1 | Enter 10 more patients at dose level N |
| 15 | 0-3 | And | 0 | Go to dose level N+1 with entry of 5 patients |
| 15 | ≥5 | Or | ≥2 | STOP; dose level N not feasible* |
| 15 | 4 | Or | 1 | Enter 5 more patients at dose level N |
| 20 | 0-4 | And | 0-1 | Go to dose level N+1 with entry of 5 patients |
| 20 | ≥5 | Or | ≥2 | STOP; dose level N not feasible* |
| No. of evaluable patients . | No. of patients with DLT . | And/Or . | No. of patients with TRM . | Action . |
|---|---|---|---|---|
| 5 | 0-1 | And | 0 | Go to dose level N+1 with entry of 5 patients |
| 5 | 5 | Or | ≥2 | STOP; dose level N not feasible* |
| 5 | 2-4 | Or | 1 | Enter 5 more patients at dose level N |
| 10 | 0-2 | And | 0 | Go to dose level N+1 with entry of 5 patients |
| 10 | ≥5 | Or | ≥2 | STOP; dose level N not feasible* |
| 10 | 3-4 | Or | 1 | Enter 10 more patients at dose level N |
| 15 | 0-3 | And | 0 | Go to dose level N+1 with entry of 5 patients |
| 15 | ≥5 | Or | ≥2 | STOP; dose level N not feasible* |
| 15 | 4 | Or | 1 | Enter 5 more patients at dose level N |
| 20 | 0-4 | And | 0-1 | Go to dose level N+1 with entry of 5 patients |
| 20 | ≥5 | Or | ≥2 | STOP; dose level N not feasible* |
N indicates current dose level.
Enter a total of 20 patients at dose level N-1; continue entry according to the decision rules for dose level N-1.
The second cycle was given after full hematologic recovery (platelets > 100 × 109/L and white blood cell count [WBC] > 2.0 × 109/L). Cycle 2 was preferably not given before day 28 and not later than day 42 from the start of cycle 1. No dose modifications were made for cytarabine during combination therapy. Imatinib was continued after chemotherapy during the phase of neutropenia and thrombocytopenia, but withheld in case of CTC grade 4 stomatitis if this persisted for more than a week. Imatinib was also withheld in case of CTC grade 3 or 4 liver toxicity and any other CTC grade 4 toxicity except for hematologic toxicity, nausea, and vomiting. When toxicity had resolved (< grade 2), therapy was resumed at the same dose. After the second cycle of combination therapy, imatinib maintenance therapy was given at the same dose as given during cytarabine treatment. Dose adjustments were made for nonhematologic toxicity of CTC grade 2 or higher and for hematologic toxicity of CTC grade 4 or higher during maintenance therapy with imatinib. Imatinib maintenance therapy was continued until progression. Other reasons for going off protocol treatment were excessive toxicity, including toxic death, intolerance of treatment, intercurrent death, no compliance of the patient, major protocol violation, or proceeding to allogeneic stem-cell transplantation.
Definition of end points
Dose-limiting toxicities were defined as toxicities with onset within 42 days after the start of cycle 1 or 2 of the following type and grade: CTC grade 4 mucosal, hepatic enzyme, or bilirubin toxicity lasting more than 2 weeks. Any other CTC grade 4 nonhematologic toxicity and any TRM occurring after start of cycle I was also defined as DLT. Treatment-related mortality was defined as death related to the combination treatment of imatinib and cytarabine, as judged by the responsible local investigator. Feasibility was defined by TRM occurring in 5% or less of patients and DLT (including TRM) occurring in 20% or less of patients in a dose level.
Time to hematologic recovery (neutrophil count [ANC] > 0.5 × 109/L, and platelet count > 50 × 109/L) was calculated from the first day below the threshold to recovery. Criteria for a complete hematologic response were normalization of the white blood cell count to less than 10 × 109/L with no immature forms with the exception of 2% or less of myelocytes and metamyelocytes, a platelet count less than 450 × 109/L, and disappearance of all clinical symptoms and signs of disease including palpable splenomegaly. A partial hematologic response was defined as not fulfilling all the criteria for complete hematologic remission and a WBC of 20 × 109/L or less. Failure was defined as WBC of more than 20 × 109/L, or progression to accelerated phase or blast crisis. Cytogenetic response was classified as absent (100% Ph chromosome–positive metaphases), minor (35%-99% Ph chromosome–positive metaphases), partial (1%-34% Ph chromosome–positive metaphases), or complete (elimination of Ph chromosome–positive metaphases), as determined in the local cytogenetic referral center, on the basis of G-, R-, or Q-banding in at least 20 metaphase cells per sample. Cytogenetic analysis of peripheral blood was acceptable only at diagnosis. Fluorescent in situ hybridization (FISH) analysis on metaphase or interphase cells with specific BCR-ABL probesets was performed for patients with a cryptic Ph at diagnosis and follow up and, in addition, during follow-up when cytogenetic analysis failed.
Molecular response was defined as complete (≥ 4.5-log reduction of BCR-ABL mRNA detectable by real-time quantitative reverse-transcription polymerase chain reaction [RT-PCR]), major (≥ 3-log reduction of BCR-ABLmRNA), partial (≥ 1- and < 3-log reduction of BCR-ABLmRNA), or absent (< 1-log reduction of BCR-ABLmRNA). Molecular response was centrally assessed in Rotterdam using real-time quantitative PCR (RQ-PCR). Bone marrow samples for PCR analysis were required at diagnosis; immediately following combination therapy; and at regular (6 months) intervals thereafter. Patients with molecular responses were monitored by PCR of peripheral blood at 3- to 6-month intervals, and also by PCR of bone marrow once yearly.14 First, total RNA was extracted from bone marrow or peripheral blood using RNABee (Campro Scientific, Veenendaal, The Netherlands). Afterward, cDNA was synthesized from 1 μg RNA using random hexamer priming, essentially as described.15 cDNA prepared from 25 ng RNA was used for all PCR amplifications. RQ-PCR amplification was performed with the ABI PRISM 7700 or 7500 Sequence Detector (Applied Biosystems, Nieuwerkerk aan den IJssel, The Netherlands), using 25-μL mix containing 125 μM deoxyribonucleoside triphosphates (dNTPs; Amersham Pharmacia Biotech, Roosendaal, The Netherlands) and 7.5 pmol forward and 7.5 pmol reversed primer (BCR-ABL: T.BA FOR 5′-CCGCTGACCATCAATAAGGAA-3′ and T.BA REV 5′-TCAGACCCTGAGGCTCAAAGTC-3′; PBGD: PBGD FOR 5′-GGCAATGCG-GCTGCAA-3′ and PBGD REV 5′-GGTACCCACGCGAATCAC-3′); 1 mM MgCl2; 4 pmol probe for ABL (5′-AAGCCCTTCAGC-GGCCAGTAGCA-3′) and 5 pmol probe for PBGD(5′-CATCTTTGGGCTGTTTTCTTCCGCC-3′), both labeled at the 5′ end with the reporter dye molecule FAM (6-carboxy-fluorescein) and at the 3′ end with the quencher dye molecule TAMRA (6-carboxy-tetramethylrhodamine) (Eurogentec, Maastricht, The Netherlands); 1 × buffer A; 1.25 U AmpliTaq Gold with the PBGD; and 2.5 U AmpliTaq Gold with the BCR-ABL amplification (Applied Biosystems). The thermal cycling conditions for BCR-ABL and PBGD included 10 minutes at 95°C followed by 45 cycles of denaturation for 15 seconds at 95°C, annealing at 58°C for 30 seconds, and extension at 60°C for 30 seconds.
The relative expression levels of BCR-ABL were quantified using a standard curve of serial dilutions of the calibrator K562 and were normalized using the endogenous reference PBGD. The level of BCR-ABL expression of the undiluted K562 is representative for a CML patient in chronic phase at diagnosis. All RQ-PCR amplifications reached a sensitivity of at least 10−4 (K562/HL60) in duplicate and an efficiency of at least 93%.
Assessment of toxic effects and response
Complete blood counts were obtained at least every other day and biochemical analysis at least twice weekly during combination therapy. Bone marrow assessment was done after cycles 1 and 2, at 6 months, and there after at least every 6 months. Patients were evaluated for cytogenetic response after cycle 2, at 6 months, at 12 months, and once a year thereafter. Molecular analysis was done at baseline, after cycles 1 and 2, at 6 months, and at least every 6 months thereafter. Safety assessments included an evaluation of adverse events, hematologic assessment, biochemical testing, urinalysis, and physical examination. Electrocardiography and chest x-ray were done at baseline and if clinically indicated thereafter.
Statistical considerations
The primary objective of this study was to determine the feasibility of the combination of imatinib and cytarabine in a dose-escalation study of consecutive cohorts. Secondary end points were the rate and duration of complete hematologic response, the rate and duration of complete cytogenetic response, and the rate and duration of complete molecular response. Progression was defined by the first occurrence of any of the following events: the development of accelerated phase or blast crisis, complete loss of hematologic response, loss of major cytogenetic response (defined as an increase in Ph-positive cells in metaphase by at least 30 percentage points on 2 cytogenetic analyses performed at least 1 month apart), or an increasing white cell count (defined as doubling of the count to > 20 × 109/L on 2 occasions at least 1 month apart in a patient who had never had a complete hematologic response despite receiving maximally tolerated doses of therapy). Other secondary end points included side effects and infections, hematologic recovery, time to mutation of the ABL-kinase domain, progression-free survival, and overall survival. Side effects; infections; hematologic recovery; and hematologic, cytogenetic, and molecular response data after 1 or 2 cycles of combination therapy and after 1 year are shown in this report. Actuarial response rates at 1 year were calculated using competing risk analysis; patients who went off protocol treatment before the specific response had occurred were considered as competing risks. Other secondary end points will be presented separately, after having obtained sufficient follow-up. Special attention was given to nonhematologic toxicity, hematologic toxicity, and infectious complications during combination therapy. Side effects and infections were scored according to the National Cancer Institute (NCI) common toxicity criteria (CTC) version 2.0 (NCI, Bethesda, MD). Hematologic recovery was estimated by the Kaplan-Meier method. Kaplan-Meier curves were generated to illustrate differences in recovery between standard- and intermediate-dose cytarabine, as well as between low/standard-dose (200 and 400 mg) and high-dose (600 and 800 mg) imatinib and were compared using the log-rank test. All reported P values are 2 sided, and a significance level α = .05 was used.
Results
From August 2001 to November 2005, 165 patients entered the study. Five patients were assigned to dose level I; 30 patients, to dose level II; 21 patients, to dose level IIIA; 16 patients, to dose level IIIB; 52 patients, to dose level IVA; 21 patients, to dose level IVB; and 20 patients, to dose level V (Figure 1). Three patients were excluded from analysis: one was not considered because blast crisis was diagnosed shortly after registration and before start of imatinib (dose level IVB), and 2 other patients were not evaluable because they refused combination therapy (dose levels II and IIIB). The analysis reported here describes 162 patients and includes the feasibility and response of the prephase and 2 cycles of chemotherapy in combination with imatinib.
Patient baseline characteristics are presented according to the dose of cytarabine received (200 mg/m2 vs 1000 mg/m2) and are summarized in Table 2. All but 2 patients received a prephase of imatinib monotherapy 400 mg once daily for 3 weeks (median: 21 days; range: 5-84 days). All 162 patients received at least one cycle of combination therapy. Five patients in dose level I, 28 patients in dose level II, 18 patients in dose level IIIA, 11 patients in dose level IIIB, 45 patients in dose level IVA, 17 patients in dose level IVB, and 16 patients in dose level V received both scheduled courses of combination therapy. The remaining 22 patients did not receive a second course of combination therapy because of nonhematologic toxicity in 8 patients, insufficient hematologic recovery in 10 patients, and refusal in 4 patients. The dose of cytarabine was given as scheduled, except for one patient who received a mitigated dose because of central nervous system toxicity. A reduction of the scheduled dose of imatinib was performed in 31 patients during the first course and in 23 patients during the second course according to predefined dose-adaptation rules. One hundred fifty-seven patients started with imatinib maintenance therapy, including 19 patients who had received only one cycle of combination therapy. Five patients did not start with imatinib maintenance because of toxicity in 2 patients, progression in 1 patient, and intercurrent death in 2 patients.
Baseline characteristics of the patients, by dose of cytarabine
| Characteristic . | 200 mg/m2 cytarabine, dose levels I, II, IIIA, IVA; n = 107 . | 1000 mg/m2 cytarabine, dose levels IIIB, IVB, V; n = 55 . |
|---|---|---|
| Age at diagnosis, y | ||
| Median | 48 | 46 |
| Range | 20-65 | 19-62 |
| Sex, no. (%) | ||
| Male | 64 (60) | 31 (56) |
| Female | 43 (40) | 24 (44) |
| Spleen size, cm below midleft costal margin | ||
| Median | 3 | 1 |
| Range | 0-27 | 0-30 |
| Platelet count, ×109/L | ||
| Median | 412 | 357 |
| Range | 152-1908 | 92-1584 |
| Blasts in peripheral blood, % | ||
| Median | 1 | 1 |
| Range | 0-12 | 0-16 |
| Sokal risk group, no. (%) | ||
| Low, less than 0.8 | 30 (28) | 28 (51) |
| Intermediate, 0.8 to 1.2 | 40 (37) | 11 (20) |
| High, more than 1.2 | 31 (29) | 12 (22) |
| Unknown | 6 (6) | 4 (7) |
| Dose imatinib, no. (%) | ||
| 200 mg | 5 (5) | — |
| 400 mg | 29 (27) | 15 (27) |
| 600 mg | 21 (20) | 20 (36) |
| 800 mg | 52 (49) | 20 (36) |
| Characteristic . | 200 mg/m2 cytarabine, dose levels I, II, IIIA, IVA; n = 107 . | 1000 mg/m2 cytarabine, dose levels IIIB, IVB, V; n = 55 . |
|---|---|---|
| Age at diagnosis, y | ||
| Median | 48 | 46 |
| Range | 20-65 | 19-62 |
| Sex, no. (%) | ||
| Male | 64 (60) | 31 (56) |
| Female | 43 (40) | 24 (44) |
| Spleen size, cm below midleft costal margin | ||
| Median | 3 | 1 |
| Range | 0-27 | 0-30 |
| Platelet count, ×109/L | ||
| Median | 412 | 357 |
| Range | 152-1908 | 92-1584 |
| Blasts in peripheral blood, % | ||
| Median | 1 | 1 |
| Range | 0-12 | 0-16 |
| Sokal risk group, no. (%) | ||
| Low, less than 0.8 | 30 (28) | 28 (51) |
| Intermediate, 0.8 to 1.2 | 40 (37) | 11 (20) |
| High, more than 1.2 | 31 (29) | 12 (22) |
| Unknown | 6 (6) | 4 (7) |
| Dose imatinib, no. (%) | ||
| 200 mg | 5 (5) | — |
| 400 mg | 29 (27) | 15 (27) |
| 600 mg | 21 (20) | 20 (36) |
| 800 mg | 52 (49) | 20 (36) |
Percentages may not sum up to 100% due to rounding.
— indicates not applicable.
Dose-limiting toxicities and treatment-related mortality
All dose levels met predefined feasibility criteria. Dose-limiting toxicities were reported in 7 patients. Streptococcal infections associated with DLTs were diagnosed in 5 patients. Toxicities in these patients were considered a consequence of streptococcal bacteremia, including 2 patients with cerebral abscesses (Table 3). Two of these 5 patients succumbed following these septic episodes. Four of the 5 infectious DLTs occurred after intermediate-dose cytarabine. In 3 patients, these DLTs occurred after penicillin prophylaxis was stopped according to protocol. One patient experienced streptococcal septicemia during levofloxacine prophylaxis, and one other patient had not received prophylaxis according to protocol. Two other DLTs included myalgia CTC grade 4 and an anaphylactic reaction following platelet transfusion. All DLTs were observed during the first cycle.
Dose-limiting toxicity and treatment-related mortality
| Dose level . | Cycle . | Specify . | Treatment-related mortality, yes/no . |
|---|---|---|---|
| IIIA | I | Streptococcus mitis sepsis, acute respiratory distress syndrome, hypotension, and cerebral abscesses | Yes |
| IIIB | I | Streptococcus species bacteremia with transient cerebral edema | No |
| IVA | I | Anaphylactic reaction on platelet transfusion | No |
| IVA | I | Myalgia CTC grade 4 | No |
| V | I | Streptococcus viridans sepsis, cerebral abscesses, and Aspergillus fumigatuspneumonia | Yes |
| V | I | Streptococcus mitis sepsis and pneumonia, liver toxicity CTC grade 4, and renal failure (acute tubular necrosis) | No |
| V | I | Streptococcus oralis bacteremia and myalgia CTC grade 4 | No |
| Dose level . | Cycle . | Specify . | Treatment-related mortality, yes/no . |
|---|---|---|---|
| IIIA | I | Streptococcus mitis sepsis, acute respiratory distress syndrome, hypotension, and cerebral abscesses | Yes |
| IIIB | I | Streptococcus species bacteremia with transient cerebral edema | No |
| IVA | I | Anaphylactic reaction on platelet transfusion | No |
| IVA | I | Myalgia CTC grade 4 | No |
| V | I | Streptococcus viridans sepsis, cerebral abscesses, and Aspergillus fumigatuspneumonia | Yes |
| V | I | Streptococcus mitis sepsis and pneumonia, liver toxicity CTC grade 4, and renal failure (acute tubular necrosis) | No |
| V | I | Streptococcus oralis bacteremia and myalgia CTC grade 4 | No |
Side effects and infections
The CTC grade 3 and 4 nonhematologic and noninfectious toxicities are listed in Table 4. The incidence of these toxicities was comparable between patients receiving either the standard- or intermediate-dosage cytarabine. Most patients receiving combination therapy developed hematologic toxicity CTC grade 4. Infectious complications CTC grade 3 or 4 were diagnosed in 48 patients (87%) after intermediate-dose cytarabine compared with 46 patients (43%) after standard-dose cytarabine (P < .001) and are listed in Table 5. Most infectious complications occurred after the first cycle of cytarabine (Table 5). The dose of imatinib did not influence the incidence of infectious complications (data not shown).
Number of patients with nonhematologic adverse events CTC grade 3 or 4 (percentages), by daily dose cytarabine and by cycle number
| Adverse event . | Cytarabine 200 mg/m2, dose levels I, II, IIIA, IVA . | Cytarabine 1000 mg/m2, dose levels IIIB, IVB, V . | ||
|---|---|---|---|---|
| Cycle 1, n = 107 (%) . | Cycle 2, n = 96 (%) . | Cycle 1, n = 55 (%) . | Cycle 2, n = 44 (%) . | |
| Any | 23 (21) | 14 (15) | 14 (25) | 11 (25) |
| Hemorrhage | 8 (7) | 5 (5) | 3 (5) | 1 (2) |
| Neurology | 4 (4) | 1 (1) | 3 (5) | 1 (2) |
| Hepatic | 2 (2) | 2 (2) | 3 (5) | 1 (2) |
| Pain | 8 (7) | 1 (1) | 3 (5) | 2 (5) |
| Cardiovascular function | 1 (1) | 1 (1) | 2 (4) | 2 (5) |
| Constitutional symptoms | 1 (1) | 1 (1) | 1 (2) | 3 (7) |
| Dermatology/skin | 0 | 4 (4) | 2 (4) | 0 |
| Gastrointestinal | 2 (2) | 1 (1) | 3 (5) | 4 (9) |
| Metabolic | 1 (1) | 0 | 1 (2) | 0 |
| Pulmonary | 1 (1) | 0 | 1 (2) | 1 (2) |
| Allergy/immunology | 2 (2) | 0 | 1 (2) | 0 |
| Genitourinary and renal | 0 | 0 | 1 (2) | 0 |
| Adverse event . | Cytarabine 200 mg/m2, dose levels I, II, IIIA, IVA . | Cytarabine 1000 mg/m2, dose levels IIIB, IVB, V . | ||
|---|---|---|---|---|
| Cycle 1, n = 107 (%) . | Cycle 2, n = 96 (%) . | Cycle 1, n = 55 (%) . | Cycle 2, n = 44 (%) . | |
| Any | 23 (21) | 14 (15) | 14 (25) | 11 (25) |
| Hemorrhage | 8 (7) | 5 (5) | 3 (5) | 1 (2) |
| Neurology | 4 (4) | 1 (1) | 3 (5) | 1 (2) |
| Hepatic | 2 (2) | 2 (2) | 3 (5) | 1 (2) |
| Pain | 8 (7) | 1 (1) | 3 (5) | 2 (5) |
| Cardiovascular function | 1 (1) | 1 (1) | 2 (4) | 2 (5) |
| Constitutional symptoms | 1 (1) | 1 (1) | 1 (2) | 3 (7) |
| Dermatology/skin | 0 | 4 (4) | 2 (4) | 0 |
| Gastrointestinal | 2 (2) | 1 (1) | 3 (5) | 4 (9) |
| Metabolic | 1 (1) | 0 | 1 (2) | 0 |
| Pulmonary | 1 (1) | 0 | 1 (2) | 1 (2) |
| Allergy/immunology | 2 (2) | 0 | 1 (2) | 0 |
| Genitourinary and renal | 0 | 0 | 1 (2) | 0 |
CTC indicates common toxicity criteria.
Number of patients with infectious episodes CTC grade 3 or 4 (percentages), by daily dose cytarabine and by cycle number
| Infection . | Cytarabine 200 mg/m2, dose levels I, II, IIIA, IVA . | Cytarabine 1000 mg/m2, dose levels IIIB, IVB, V . | ||
|---|---|---|---|---|
| Cycle 1, n = 107 (%) . | Cycle 2, n = 96 (%) . | Cycle 1, n = 55 (%) . | Cycle 2, n = 44 (%) . | |
| Any | 36 (34) | 23 (24) | 44 (80) | 24 (55) |
| Fever of unknown origin | 17 (16) | 9 (9) | 23 (42) | 7 (16) |
| Blood | ||||
| Staphylococcus | 1 (1) | 0 | 6 (11) | 4 (9) |
| Streptococcus | 2 (2) | 0 | 4 (7) | 2 (5) |
| Pseudomonas aeruginosa | 0 | 0 | 1 (2) | 1 (2) |
| Other/unknown | 0 | 1 (1) | 2 (4) | 2 (5) |
| Gastrointestinal tract | 4 (4) | 3 (3) | 8 (15) | 8 (18) |
| Ear/nose/throat | 7 (7) | 6 (6) | 4 (7) | 2 (5) |
| Skin/subcutaneous | 5 (5) | 3 (3) | 1 (2) | 2 (5) |
| Pulmonary | ||||
| Aspergillus | 0 | 1 (1) | 3 (5) | 1 (2) |
| Streptococcus | 1 (1) | 0 | 1 (2) | 0 |
| Other/unknown | 3 (3) | 0 | 4 (7) | 0 |
| Catheter | 1 (1) | 1 (1) | 2 (4) | 5 (11) |
| Genitourinary tract | 2 (2) | 1 (1) | 2 (4) | 2 (5) |
| Other | 2 (2) | 3 (3) | 0 | 2 (5) |
| Infection . | Cytarabine 200 mg/m2, dose levels I, II, IIIA, IVA . | Cytarabine 1000 mg/m2, dose levels IIIB, IVB, V . | ||
|---|---|---|---|---|
| Cycle 1, n = 107 (%) . | Cycle 2, n = 96 (%) . | Cycle 1, n = 55 (%) . | Cycle 2, n = 44 (%) . | |
| Any | 36 (34) | 23 (24) | 44 (80) | 24 (55) |
| Fever of unknown origin | 17 (16) | 9 (9) | 23 (42) | 7 (16) |
| Blood | ||||
| Staphylococcus | 1 (1) | 0 | 6 (11) | 4 (9) |
| Streptococcus | 2 (2) | 0 | 4 (7) | 2 (5) |
| Pseudomonas aeruginosa | 0 | 0 | 1 (2) | 1 (2) |
| Other/unknown | 0 | 1 (1) | 2 (4) | 2 (5) |
| Gastrointestinal tract | 4 (4) | 3 (3) | 8 (15) | 8 (18) |
| Ear/nose/throat | 7 (7) | 6 (6) | 4 (7) | 2 (5) |
| Skin/subcutaneous | 5 (5) | 3 (3) | 1 (2) | 2 (5) |
| Pulmonary | ||||
| Aspergillus | 0 | 1 (1) | 3 (5) | 1 (2) |
| Streptococcus | 1 (1) | 0 | 1 (2) | 0 |
| Other/unknown | 3 (3) | 0 | 4 (7) | 0 |
| Catheter | 1 (1) | 1 (1) | 2 (4) | 5 (11) |
| Genitourinary tract | 2 (2) | 1 (1) | 2 (4) | 2 (5) |
| Other | 2 (2) | 3 (3) | 0 | 2 (5) |
Hematologic recovery
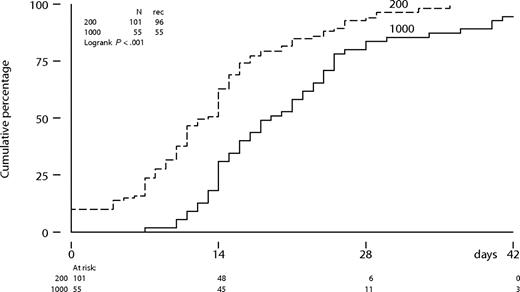
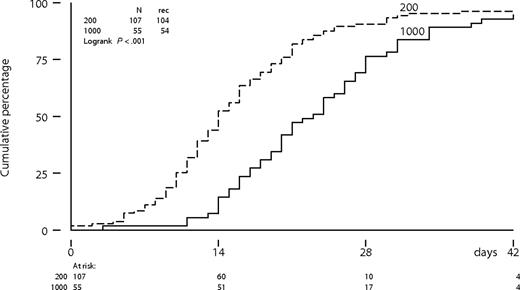
The time to neutrophil recovery to more than 0.5 × 109/L was significantly longer following intermediate-dose cytarabine compared with a standard-dose cytarabine (P < .001; Figure 2). The median number of days of neutropenia of 0.5 × 109/L or less was 13 days (range: 0-36 days) following cytarabine (200 mg/m2) compared with 19 days (range: 7-47 days) following cytarabine (1000 mg/m2) in the first cycle. Platelet recovery to more than 50 × 109/L was also significantly more protracted following intermediate-dose cytarabine (Figure 3). Time to neutrophil and platelet recovery was also significantly prolonged after intermediate-dose cytarabine in the second cycle (data not shown). Time to platelet recovery to more than 50 × 109/L was also adversely affected by a higher dose of imatinib (600 or 800 mg). The dose of imatinib did not affect the time to neutrophil recovery. Patients received a median number of 3 platelet transfusions (range: 0-16 transfusions) after standard-dose cytarabine, compared with 5 transfusions (range: 2-21 transfusions) after intermediate-dose cytarabine in the first cycle. Furthermore, patients received a median number of 3 red blood cell transfusions (range: 0-13 transfusions) after standard-dose cytarabine, compared with 4 transfusions (range: 0-24 transfusions) after intermediate-dose cytarabine. Difference in transfusion requirements was largely similar after the second cycle of combination therapy.
Neutrophil recovery from below threshold to more than 0.5 × 109/L according to dose level of cytarabine in cycle 1.
Neutrophil recovery from below threshold to more than 0.5 × 109/L according to dose level of cytarabine in cycle 1.
Platelet recovery from below threshold to more than 50 × 109/L according to dose level of cytarabine in cycle 1.
Platelet recovery from below threshold to more than 50 × 109/L according to dose level of cytarabine in cycle 1.
Hematologic, cytogenetic, and molecular responses
One hundred forty-eight patients obtained a complete hematologic response after 1 or 2 cycles of combination therapy. Eight patients obtained a partial hematologic response, one patient was unresponsive, and another patient progressed to accelerated phase. The hematologic response could not be assessed appropriately in 4 patients due to insufficient hematologic recovery in 3 patients and death before evaluation in 1 patient. The cytogenetic response was evaluated in 133 patients after combination therapy and included a complete cytogenetic response in 64 patients (48%), a partial cytogenetic response in 36 patients (27%), a minimal cytogenetic response in 29 patients (22%), and an absent cytogenetic response in 4 patients (3%) after a median of 91 days. The molecular response was evaluated after a median of 80 days in 138 patients and included a major molecular response in 42 patients (30%, including 3 patients with a complete molecular response), a partial molecular response in 79 patients (57%), and an absent molecular response in 17 patients (12%). At 12 months, actuarial probabilities of a complete hematologic response and a complete cytogenetic response were 95% (95% confidence interval [CI]: 91%-97%) and 63% (95% CI: 55%-70%), respectively. One hundred patients achieved a complete cytogenetic response within 12 months, including 71 patients with a major molecular response. Twenty-two patients achieved a complete molecular response at that time. As a result, probabilities of major and complete molecular response were 46% (95% CI: 39%-55%) and 13% (95% CI: 9%-20%), respectively, at 1 year. Six patients progressed during the first year, including 1 patient who developed accelerated phase, 4 patients who developed blast crisis, and 1 patient who lost his partial cytogenetic response. Four patients died, including 2 patients due to TRM, 1 patient due to progression of CML, and 1 patient due to death from unrelated causes.
Discussion
Given the synergistic and dose-dependent actions of imatinib and cytarabine, as was observed in in vitro studies, the HOVON-51 study was designed to investigate whether escalating doses of imatinib (200 mg, 400 mg, 600 mg, or 800 mg) combined with 2 cycles of intravenous cytarabine (200 mg/m2 or 1000 mg/m2 days 1-7) would be feasible and would induce an early molecular response in patients with first chronic phase CML. All dose levels (I-V) proved feasible. Seven DLTs were observed among 162 patients, who had received 302 cycles of combination therapy. Five of these 7 DLTs resulted from streptococcal bacteremia. More infectious complications were observed after intermediate-dose cytarabine (1000 mg/m2) compared with standard-dose cytarabine (200 mg/m2), especially after the first cycle of combination therapy. While the percentage of DLTs at dose level V was less than 20%, 3 DLTs were observed at that particular level, which was associated with TRM in one patient. The dose of imatinib did not affect the rate of infectious complications. Intermediate-dose cytarabine significantly prolonged the period of neutropenia and thrombocytopenia compared with a standard-dose of cytarabine. High-dose imatinib (600 mg or 800 mg) delayed only thrombocyte recovery. Nonhematologic and noninfectious toxicity did not differ between the different combinations of imatinib and cytarabine.
The increased frequency of infectious complications that was noted in this series of patients treated with intermediate-dose cytarabine was most likely due to the prolonged period of neutropenia. Prolonged neutropenia is clearly associated with an increased risk of infectious complications.16,17 Some additional mucosal toxicity and/or the placement of a central venous catheter may have contributed to the high number of patients with infectious complications after intermediate-dose cytarabine.18,19 The frequency of fever of unknown origin was also increased, which is often observed during prolonged neutropenia and may also be related to the dose of cytarabine.10,20
Five of the 7 DLTs were accompanied by a streptococcal bacteremia. All occurred after the first cycle and especially after intermediate-dose cytarabine. Four patients with a streptococcal bacteremia had discontinued penicillin prophylaxis, which was according to protocol, and another patient with a streptococcal bacteremia did not receive penicillin but levofloxacine. The 2 toxic deaths, both with cerebral abscesses, were considered to be related to viridans streptococci. Serious complications associated with viridans streptococcal bacteremia are well known to occur in neutropenic patients with cancer receiving high-dose chemotherapy and are associated with a high mortality rate.21 Severe oral mucositis after high-dose chemotherapy is a major risk factor for these complications. Complications including acute respiratory distress syndrome, septic shock, and renal failure are often described in these patients. Viridans streptococci are a common cause of brain abscesses in the literature, mostly occurring after otopharyngeal infections, endocarditis, or neurosurgical or dental procedures with secondary hematogenous spread.22,23 No cases of cerebral abscesses have been described in the literature after imatinib monotherapy. Cerebral edema has been reported as a rare complication of imatinib treatment.24 However, no neurologic symptoms indicating cerebral edema were present in these 2 patients prior to streptococcal septicemia, suggesting that the abscesses resulted mainly from streptococcal bacteremia.
A major molecular response was obtained in 30% of the patients shortly following combination therapy, which increased to 46% at 1 year. The initial molecular response rate obtained after combination therapy in our study seems promising. Longer follow up is, however, needed to determine whether combination therapy increases the molecular response rate, prevents resistance, and to determine which patients benefit most. Preliminary results with combination therapy of imatinib and low-dose cytarabine in 30 patients with newly diagnosed CML in first chronic phase were reported by Gardembas et al.25 At 1 year, a complete hematologic response was observed in 97% of the patients and a complete cytogenetic response in 70% of the patients.24 These results were comparable with our study and with those obtained with imatinib alone, but interestingly they also observed some early molecular responses.24 Another important observation in the study of Gardembas et al was an increased hematologic CTC grade 3 or 4 toxicity of 53% and nonhematologic CTC grade 3 or 4 toxicity of 23% compared with approximately 15% hematologic and 15% to 20% nonhematologic CTC grade 3 or 4 toxicity with imatinib alone (400 mg).4,5 In the present study, hematologic toxicity CTC grade 3 or 4 was observed in nearly all patients and nonhematologic toxicity CTC grade 3 or 4 was observed in 36% of patients, which seems slightly more than in the French study. Collectively, both combination studies have demonstrated the feasibility of combining imatinib and cytarabine, but at the expense of enhanced toxicity compared with imatinib alone. Toxicities with respect to infectious complications, and hematologic and other toxicities are acceptable only if combination therapy would be associated with enhanced efficacy. However, mature follow-up of the combination of imatinib and cytarabine (either low or intermediate dose) is currently lacking and, therefore, it is not known whether combination therapy would prevent resistance and disease progression.
The present study was designed to explore the feasibility of imatinib and intravenous cytarabine and to obtain long-term efficacy of the different dose levels. Furthermore, our aim was to select a feasible, efficacious dose level associated with a high rate of molecular response that could be explored further in a randomized study. While both dose levels of cytarabine met predefined feasibility criteria, the standard dose of cytarabine (200 mg/m2) seems preferable, because the higher dose of cytarabine (1000 mg/m2) was associated with significantly more infectious complications. Increasing the dose of imatinib did not affect the feasibility of that combination. In addition, the combination of standard-dose cytarabine and imatinib may be given on an outpatient basis. Therefore, we selected a standard dose of cytarabine (200 mg/m2) together with high-dose imatinib (800 mg) to be compared with high-dose imatinib (800 mg) monotherapy for a subsequent randomized clinical trial, which was recently started.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to our colleagues from the molecular diagnostics laboratory for all molecular analysis and for providing material for central analysis.
This work was supported by the Queen Wilhelmina Fund (KWF)–Kankerbestrijding for support of data management.
Authorship
Contribution: W.D., B.H., G.E.G.V., B.L., G.J.O., and J.C.J. were responsible for the initial design of present analysis, actual evaluation, and writing the paper; all authors were responsible for the design of HOVON study, treatment of patients, critical review of the paper, suggestions for additional analysis, and finalizing writing the paper.
Conflict-of-interest disclosure: P.S., J.J.C., and G.J.O. have received consulting fees from Novartis Oncology. The other authors declare no competing financial interests.
Correspondence: J. J. Cornelissen, Erasmus University Medical Center, Department of Hematology, Groene Hilledijk 301, 3075 EA Rotterdam, The Netherlands; e-mail: j.cornelissen@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal