In the past, most patients with multiple myeloma (MM) died within 5 to 10 years after diagnosis. Within the past decade, several new therapeutic interventions have been introduced, including autologous stem-cell transplantation, thalidomide, lenalidomide, and bortezomib. We estimated trends in age-specific 5- and 10-year relative survival of patients with MM in the United States from 1990-1992 to 2002-2004 from the 1973-2004 database of the Surveillance, Epidemiology, and End Results (SEER) Program. Techniques of period analysis were used to show most recent developments. Overall, 5-year relative survival increased from 28.8% to 34.7% (P < .001), and 10-year relative survival increased from 11.1% to 17.4% (P < .001) between 1990-1992 and 2002-2004. Much stronger increases were seen in the age group younger than 50 years, leading to 5- and 10-year relative survival of 56.7% and 41.3% in 2002-2004, and in the age group 50 to 59 years, leading to 5- and 10-year relative survival of 48.2% and 28.6% in 200-2004. By contrast, only moderate improvement was seen in the age group 60 to 69 years, and essentially no improvement was achieved among older patients. Our period analysis discloses a major increase in long-term survival of younger patients with MM in recent years, which most likely reflects the effect of recent advances in therapy and their dissemination in clinical practice.
Introduction
In the past, most patients with multiple myeloma (MM) died within 5 to 10 years after diagnosis. Several new therapeutic interventions have been introduced for MM during the past decade. These include autologous stem-cell transplantation (SCT) and novel agents, including thalidomide, an antiangiogenic and immunomodulatory small molecule, lenalidomide, a derivative of thalidomide, and bortezomib, a protosome inhibitor. All 3 novel agents have been shown to be active in MM, and regimens containing one of these compounds are gradually replacing chemotherapy-only regimens as standard of care in MM for patients who are not candidates for SCT.1,2 The effect of such therapeutic innovation and its dissemination on long-term prognosis should be monitored in an as timely as possible manner, but it is only disclosed with substantial delay by conventional methods of survival analysis. We aimed to disclose trends from 1990-1992 to 2002-2004 and to derive up-to-date estimates of long-term survival of patients with MM by novel techniques of period survival analysis.3,4 Because of the differential application, efficacy and tolerance of novel therapies according to age, we were specifically interested in age-specific trends of prognosis
Methods
All data presented in this study are derived from the 1973-2004 limited-use database of the Surveillance, Epidemiology, and End Results (SEER) Program of the US National Cancer Institute issued in April 2007.5 Data included in the 1973-2004 SEER database are from population-based cancer registries in Connecticut, New Mexico, Utah, Iowa, Hawaii, Atlanta, Detroit, Seattle-Puget Sound, and San Francisco-Oakland that together cover a population of approximately 30 million people. Geographic areas were selected for inclusion in the SEER Program based on their ability to operate and maintain a high-quality population-based cancer reporting system and for their epidemiologically significant population subgroups. The SEER population is comparable to the general US population for measures of poverty and education, although it tends to be more urban and has a higher proportion of foreign-born persons than the latter.
For this analysis, we selected 27 038 patients aged 15 years or older with a first diagnosis of MM (and no previous cancer diagnosis) between 1980 and 2004, who have been followed for vital status until the end of 2004. After exclusion of 58 patients (0.21%) who were reported by autopsy only and 457 patients (1.69%) who were reported by death certificate only, there remained 26 523 patients (98.10%) for the survival analysis.
Five- and 10-year survival was calculated for the calendar periods 1990-1992, 1993-1995, 1996-1998, 1999-200, and 2002-2004 with the period analysis method.3 Furthermore, we tested for statistical significance of trends in 5- and 10-year survival between 1990-1992 and 2002-2004 by a recently described modeling approach.4 All analyses were performed separately for the following 5 major age groups: younger than 50 years, 50 to 59 years, 60 to 69 years, 70 to 79 years, and 80 years and older.
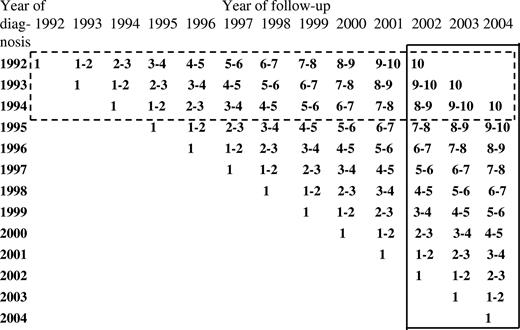
With period analysis, first proposed by Brenner and Gefeller in 1996,6 only survival experience during the period of interest is included in the analysis. This is achieved by left truncation of observations at the beginning of the period in addition to right censoring at its end. A graphical illustration of the data included to estimate 10-year relative survival for the 2002-2004 period compared with the data used to derive the most up-to-date estimate of 10-year survival from the same database using traditional “cohort analysis” is shown in Figure 1. The latter would pertain to patients whose disease was diagnosed in 1992-1994 only and would thus not capture recent progress in therapy. It has been shown by extensive empirical evaluation that period analysis provides more up-to-date long-term survival estimates than does traditional “cohort-based” survival analysis, and it quite closely predicts long-term survival expectations of patients with cancer diagnosed within the period of interest.7,8
Data used for estimating 10-year survival for the 2002-2004 period by period analysis (closed frame). For comparison, data used to derive the most up-to-date estimates of 10-year survival from the same database using traditional “cohort analysis” is shown (dashed frame).
Data used for estimating 10-year survival for the 2002-2004 period by period analysis (closed frame). For comparison, data used to derive the most up-to-date estimates of 10-year survival from the same database using traditional “cohort analysis” is shown (dashed frame).
In addition to 5-year survival from diagnosis, we calculated 5-year survival in the subsequent 5 years among patients who have already survived 1, 2, 3, 4, and 5 years since diagnosis. In this way, trends and recent achievements in late survival can be analyzed specifically. They are of particular interest for MM, given the frequency of late deaths among patients with this malignancy.
According to standard practice in population-based cancer survival analysis, relative rather than absolute survival was calculated. Relative survival reflects survival of patients with cancer compared with survival of the general population. It is calculated as the ratio of absolute survival of patients with cancer divided by the expected survival of a group of persons of the corresponding sex, age, and race in the general population.9,10 Estimates of expected survival were derived according to the so-called Ederer II method11 using US sex-, age-, and race-specific life tables.12
Results
Numbers of cases by age group and calendar period are shown in Table 1. Approximately half of the patients were 70 years or older, and less than 10% were younger than 50 years at the time of diagnosis. Overall, numbers of patients, as well as numbers of patients in the older age groups, were rather stable over time. However, case numbers increased by approximately half in the 3 youngest age groups between 1990-1992 and 2002-2004.
For all age groups combined, 5-year relative survival increased from 28.8% in 1990-1992 to 34.7% in 2002-2004, an increase of 5.9 percentage points (P for trend < .001; Table 2). More substantial increases of 11.9 and 9.4 percentage points were seen in the age groups younger than 50 years and 50 to 59 years, respectively (P < .001 in each age group). Increases were much less pronounced in the older age groups and did not reach statistical significance. In this way the age gradient in 5-year relative survival, already visible in 1990-1992, further increased over time. In 2002-2004, 5-year relative survival ranged from 56.7% in the age group younger than 50 years to 15.2% in the age group 80 years and older. For the 2 youngest age groups (younger than 50 years, 50-59 years), the increase in 10-year relative survival from 1990-1992 to 2002-2004 was even more pronounced (16.8 and 11.4 percentage points; P < .001). For the age groups 60 to 69 years and 70 to 79 years some modest increase in 10-year relative survival could still be seen, which was statistically significant for the former only. Nevertheless, 10-year relative survival remained as low as 15% and 10%, respectively, in these age groups in 2002-2004. No improvement at all could be seen for age group 80 years and older.
Numbers of patients with MM by age group and calendar period
| . | 1990 to 1992 . | 1993 to 1995 . | 1996 to 1998 . | 1999 to 2001 . | 2002 to 2004 . | Total . |
|---|---|---|---|---|---|---|
| All ages | 3254 | 3289 | 3553 | 3616 | 3618 | 17 330 |
| Younger than 50 y | 236 | 273 | 293 | 287 | 325 | 1 414 |
| 50 to 54 y | 176 | 226 | 251 | 299 | 273 | 1 225 |
| 55 to 59 y | 251 | 260 | 316 | 343 | 389 | 1 559 |
| 60 to 64 y | 382 | 352 | 331 | 390 | 429 | 1 884 |
| 65 to 69 y | 524 | 506 | 487 | 445 | 476 | 2 438 |
| 70 to 74 y | 569 | 535 | 557 | 568 | 490 | 2 719 |
| 75 to 79 y | 462 | 484 | 580 | 558 | 514 | 2 598 |
| 80 to 84 y | 367 | 346 | 417 | 389 | 409 | 1 928 |
| 85 y and older | 287 | 307 | 321 | 337 | 313 | 1 565 |
| . | 1990 to 1992 . | 1993 to 1995 . | 1996 to 1998 . | 1999 to 2001 . | 2002 to 2004 . | Total . |
|---|---|---|---|---|---|---|
| All ages | 3254 | 3289 | 3553 | 3616 | 3618 | 17 330 |
| Younger than 50 y | 236 | 273 | 293 | 287 | 325 | 1 414 |
| 50 to 54 y | 176 | 226 | 251 | 299 | 273 | 1 225 |
| 55 to 59 y | 251 | 260 | 316 | 343 | 389 | 1 559 |
| 60 to 64 y | 382 | 352 | 331 | 390 | 429 | 1 884 |
| 65 to 69 y | 524 | 506 | 487 | 445 | 476 | 2 438 |
| 70 to 74 y | 569 | 535 | 557 | 568 | 490 | 2 719 |
| 75 to 79 y | 462 | 484 | 580 | 558 | 514 | 2 598 |
| 80 to 84 y | 367 | 346 | 417 | 389 | 409 | 1 928 |
| 85 y and older | 287 | 307 | 321 | 337 | 313 | 1 565 |
Five- and 10-year estimates of relative survival of patients with MM by age group and calendar period
| . | 1990 to 1992 . | 2002 to 2004 . | Increase* . | P† . | ||
|---|---|---|---|---|---|---|
| PE . | SE . | PE . | SE . | |||
| 5-y relative survival | ||||||
| All ages | 28.8 | 0.9 | 34.7 | 0.9 | 5.9 | <.001 |
| Younger than 50 y | 44.8 | 3.5 | 56.7 | 3.0 | 11.9 | .001 |
| 50 to 59 y | 38.8 | 2.5 | 48.2 | 2.1 | 9.4 | .001 |
| 60 to 69 | 30.6 | 1.8 | 36.3 | 1.8 | 5.7 | .09 |
| 70 to 79 | 27.1 | 1.7 | 28.7 | 1.6 | 1.6 | .21 |
| 80 and older | 13.8 | 2.0 | 15.2 | 1.9 | 1.4 | .96 |
| 10-y relative survival | ||||||
| All ages | 11.1 | 0.8 | 17.4 | 0.8 | 6.3 | <.001 |
| Younger than 50 y | 24.5 | 3.4 | 41.3 | 3.2 | 16.8 | <.001 |
| 50 to 59 y | 17.2 | 2.1 | 28.6 | 2.2 | 11.4 | <.001 |
| 60 to 69 y | 10.8 | 1.3 | 15.4 | 1.5 | 4.6 | .03 |
| 70 to 79 y | 7.4 | 1.4 | 10.4 | 1.4 | 3.0 | .09 |
| 80 y and older | 7.1 | 2.3 | 5.7 | 1.7 | −1.4 | .94 |
| . | 1990 to 1992 . | 2002 to 2004 . | Increase* . | P† . | ||
|---|---|---|---|---|---|---|
| PE . | SE . | PE . | SE . | |||
| 5-y relative survival | ||||||
| All ages | 28.8 | 0.9 | 34.7 | 0.9 | 5.9 | <.001 |
| Younger than 50 y | 44.8 | 3.5 | 56.7 | 3.0 | 11.9 | .001 |
| 50 to 59 y | 38.8 | 2.5 | 48.2 | 2.1 | 9.4 | .001 |
| 60 to 69 | 30.6 | 1.8 | 36.3 | 1.8 | 5.7 | .09 |
| 70 to 79 | 27.1 | 1.7 | 28.7 | 1.6 | 1.6 | .21 |
| 80 and older | 13.8 | 2.0 | 15.2 | 1.9 | 1.4 | .96 |
| 10-y relative survival | ||||||
| All ages | 11.1 | 0.8 | 17.4 | 0.8 | 6.3 | <.001 |
| Younger than 50 y | 24.5 | 3.4 | 41.3 | 3.2 | 16.8 | <.001 |
| 50 to 59 y | 17.2 | 2.1 | 28.6 | 2.2 | 11.4 | <.001 |
| 60 to 69 y | 10.8 | 1.3 | 15.4 | 1.5 | 4.6 | .03 |
| 70 to 79 y | 7.4 | 1.4 | 10.4 | 1.4 | 3.0 | .09 |
| 80 y and older | 7.1 | 2.3 | 5.7 | 1.7 | −1.4 | .94 |
PE indicates point estimate; and SE, standard error.
Increase from the first period 1990 to 1992 to the last period 2002 to 2004 in percentage points.
P for trend from the first period 1990 to 1992 to the last period 2002 to 2004.
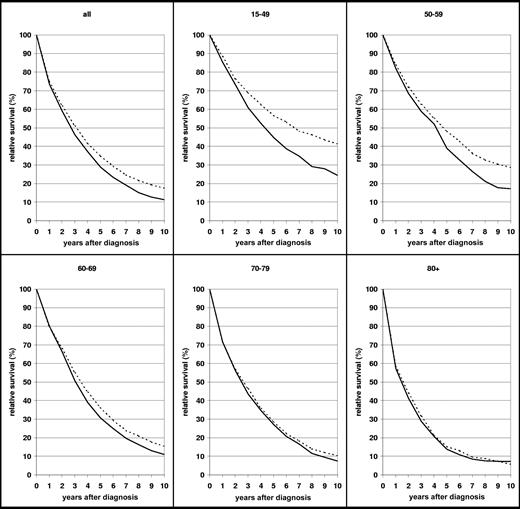
A more comprehensive picture of the survival curves by age groups and their development between 1990-1992 and 2002-2004 is given in Figure 2. In contrast to most other malignancies, the relative survival curves of patients with MM do not flatten within 10 years after diagnosis (ie, substantial excess mortality compared with the general population of the same age persists in the long run). This pattern is seen in all age groups, and it persists in all age groups even in 2002-2004, despite the major improvement in survival in the younger age groups between 1990-1992 and 2002-2004. Nevertheless, median relative survival increased from little more than 4 years in the age group younger than 50 years in 1990-1992 to almost 7 years in 2002-2004. For the age group 50 to 59 years, the increase in survival from 1990-1992 to 2002-2004 was mainly seen in years 5 to 10 after diagnosis.
Ten-year relative survival curves of patients with MM by major age groups. Period estimates for 1990-1992 (—) and 2002-2004 ( ).
).
Ten-year relative survival curves of patients with MM by major age groups. Period estimates for 1990-1992 (—) and 2002-2004 ( ).
).
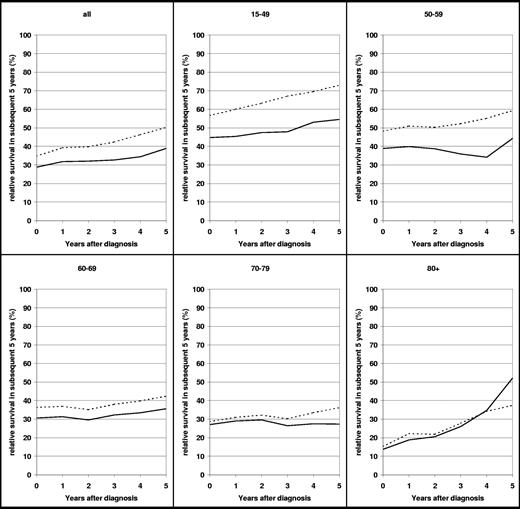
Despite the high proportion of late deaths, conditional relative survival in subsequent 5 years increased gradually in all age groups with increasing time since diagnosis in 2002-2004 (Figure 3). For example, for patients who survived 1 year after diagnosis, the probability of surviving another 5 years was approximately 40% in 2002-2004, compared with just greater than 30% in 1990-1992. Patients who survived 5 years after diagnosis had a 5-year relative survival of 50% in 2002-2004, compared with just less than 40% in 1990-1992. Thus, overall, the probability of long-term survival increased in 2002-2004 compared with 1990-1992. In the age groups younger than 50 years and 50 to 59 years, not only the overall level of conditional survival in subsequent 5 years but also their increase with time from diagnosis were substantially higher in 2002-2004 than in 1990-1992, which indicates that reduction of excess mortality was particularly pronounced among patients who survived the first years after diagnosis.
Conditional relative survival of patients with MM, all ages and age group younger than 50 years, within subsequent years after diagnosis. Period estimates for 1990-1992 (—) and 2002-2004 ( ).
).
Conditional relative survival of patients with MM, all ages and age group younger than 50 years, within subsequent years after diagnosis. Period estimates for 1990-1992 (—) and 2002-2004 ( ).
).
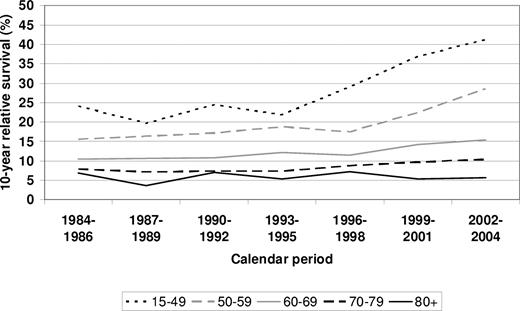
To address the question of the timing of onset in survival improvement among the various age groups, 10-year relative survival is shown for each of the 5 calendar periods under investigation in Figure 4. Results for the preceding time periods 1984-1986 and 1987-1989 are given for comparison. Between 1984-1986 and 1993-1995, no major improvement was seen in any of the age groups. The strong increase in 10-year relative survival in the age group younger than 50 years was first seen between 1993-1995 and 1996-1998 and steadily continued thereafter. The major increase in 10-year relative survival in the age group 50 to 59 years was first seen between 1996-1998 and 1999-2001 only.
Period estimates of 10-year survival of patients with MM by major age groups in defined calendar periods from 1984-1986 to 2002-2004.
Period estimates of 10-year survival of patients with MM by major age groups in defined calendar periods from 1984-1986 to 2002-2004.
Discussion
This application of period analysis to age-specific long-term survival of patients with MM discloses recent major improvements in younger age groups (younger than 60 years) which were starting in the middle and late 1990s and are ongoing since then. Not only early deaths, but especially late deaths years after initial treatment are reduced in these age groups. This way, 10-year relative survival of approximately 40% and 30% was achieved in the age groups younger than 50 years and 50 to 59 yeas in 2002-2004. Improvements remained much more modest and mostly nonsignificant in older age groups, and no improvement at all was seen in patients older than 80 years.
To the best of our knowledge, this is the first in-depth population-based analysis of long-term survival of patients with MM by age that used the period analysis method. The relative survival figures for the 2002-2004 period disclosed by this approach are higher than previously available figures,13,14 and this encouraging development should be disclosed to patients, clinicians, and researchers in an as timely as possible manner. Although the period estimates of long-term survival are more up to date than estimates obtained by traditional cohort analysis, even the period estimates may still be somewhat too pessimistic because they still partly reflect the survival experience of patients whose disease was first diagnosed and treated in earlier years. Therefore, survival expectations of patients whose disease was diagnosed in 2002-2004 may even be somewhat higher. This may be particularly true for older patients who may benefit from treatment with novel agents, the earliest of which were introduced in the late 1990s, more than from SCT.
Before the late 1990s, treatment for MM consisted of chemotherapy with or without autologous SCT rescue. Patients not eligible for SCT were classically treated with vincristine, adriamycin, and dexamethasone (VAD) or melphalan and prednisone (MP) chemotherapy.1,15 Although chemotherapy with these drugs can produce responses in MM, cures are essentially unheard of and 5-year survival using chemotherapy alone is poor.
High-dose melphalan with or without total body irradiation followed by autologous SCT improves survival in patients with MM.16 Before the early 1990s, SCT was limited to patients younger than 40 years of age. As improvements in SCT protocols decreased the risk of SCT, its use was expanded to more patients, with some patients as old as 75 years being eligible under some current protocols.17 The combination of improvements in SCT protocols and better supportive care leading to lower treatment-related mortality may account for much of the improvement in survival seen, starting in the mid-1990s for patients aged younger than 50 years and in the mid- to late-1990s for patients aged 50 to 59 years and aged 60 to 69 years. However, even with the expansion of SCT to older and less robust patients, not all patients are eligible for SCT. Patients who have relapsed after SCT have few further treatment options if they are ineligible for further SCT. In addition, SCT is rarely curative in MM, so that even patients who achieve remission with SCT may expect to need further treatment in the future. Therefore, further advances in therapy are highly desirable.
The development of novel agents for the treatment of MM in the late 1990s opened new possibilities for the patients not eligible for SCT or who had relapsed after SCT and, later, for more effective conditioning regimens for SCT. The first of these agents to undergo clinical trials was thalidomide, an antiangiogenic and immunomodulatory small molecule. Initial clinical trials conducted in 1997-1998 showed thalidomide to have activity in patients with refractory or relapsed disease, with a median event-free survival of 3 months, and overall survival of more than 12 months for patients treated with thalidomide.18 Other studies of thalidomide in relapsed and refractory melanoma showed similar results.19,20 Later studies showed that the addition of thalidomide to treatment protocols, both with and without SCT, could improve outcomes for patients with newly diagnosed MM and that the combination of thalidomide and dexamethasone may be superior to VAD chemotherapy.21,–23
After the approval of thalidomide for the treatment of MM, 2 further treatment options have appeared. A thalidomide analog known as lenalidomide, which is effective in vitro at lower concentrations than thalidomide and is potentially less toxic, was developed. It has been shown to have efficacy in the treatment of MM, including MM previously treated with thalidomide.24,25 Bortezomib, a proteosome inhibitor, was developed in the early 21st century. It was initially investigated in a number of malignancies and has been shown to be effective in MM.2,26,27 Currently, no single therapeutic option for patients not eligible for SCT has been shown to be clearly superior to the others, although the addition of any of the novel agents to a chemotherapeutic regimen can improve outcomes, and the inclusion of one of these agents in initial treatment of MM is recommended by many in the field. Further clinical trials to clarify the use of these agents as well as to determine the best use of SCT in MM may further improve survival in this disease.
Little progress was made in the treatment of MM for patients older than 70 years, particularly for those older than 80 years, and only marginal progress was made for patients 60 to 69 years during the period between 1990-1992 and 2002-2004. This is concerning given that the average age of diagnosis of MM is approximately 70 years. Elderly patients are more likely to have comorbid medical conditions that limit their treatment options and are more likely to have a poor performance status.28 In addition, elderly patients are underrepresented in clinical trials; therefore, less is known about the natural history and response to treatment of MM in this population than in younger patients.28,29 There is some evidence that MM may be undertreated in the elderly, even after accounting for comorbid conditions. One cohort study of patients aged 75 years and older showed that treatment was given to only 72% of patients and further chemotherapy was given to only 25% of patients on relapse.30 SCT, which is still the therapy that offers patients with MM the best chance of long-term survival, is offered to elderly patients only rarely. In addition, the prothrombotic effects of thalidomide may have led clinicians to be reluctant to use it in older patients. However, recent studies of thalidomide and bortezomib in older patients suggest that they can be used safely and effectively in this population.31,32 The combination of thalidomide with MP produced 3-year survival rates of 80% in patients aged 60 to 85 years in one study.31 Notably, cardiac disease, respiratory disease, and abnormal liver or kidney function were not exclusion criteria in that study, suggesting that the results may be applicable to a broad range of older adults with MM. Therefore, progress in the treatment of older patients with MM may be seen in the next few years, as treatment with newer agents plus chemotherapy becomes more common and clinicians become more comfortable using them in older patients. Further trials that include older patients with MM are still needed to delineate the best use of SCT, chemotherapy, and novel agents in this population.
In the interpretation of our results, a number of limitations requires careful consideration. Because the SEER database does not contain information on chemotherapeutic or other medical treatment nor on the location of treatment, the role of these factors cannot be assessed directly. Likewise, the SEER database does not contain stage information for myeloma which precludes assessment of a potential role of earlier diagnosis in improved survival. A major effect of earlier diagnosis seems unlikely though, given that no routine screening tests for MM have been introduced between the 1980s and the early 21st century.
In summary, our population-based period analysis discloses significant, major progress in the survival of patients with MM since the early1990s. Most likely, improvements in SCT and consequent extension of its use to older and less healthy patients account for the substantially improved survival in patients younger than 60 years during the 1990s and the early 21st century. The introduction of new agents in the treatment of MM provides new treatment options both for patients who cannot receive SCT or have relapsed after transplantation and for improved transplantation protocols. With the exception of thalidomide, the newer agents were developed and approved for use in the early 2000s. Therefore, the effects novel agents may have on 5- and 10-year survival in MM is not yet maximized, and further improvements in survival rates may well be seen during the next several years, particularly because the use of these agents spreads and their optimal role in the treatment of MM is established. Further research into the best treatment for older patients, including greater involvement of older patients in clinical trials, improved tolerability of SCT, and increased understanding of how best to use the newer agents in older patients, is needed.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Faculty Research Visit Grant from the German Academic Exchange Service (DAAD) and a Merit Review Grant from the US Department of Veterans Affairs (D.P.).
Authorship
Contribution: H.B. designed and performed the analysis; H.B. and D.P. wrote the paper; and A.G. critically reviewed and contributed to finalizing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hermann Brenner, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Bergheimer Str 20, D-69115 Heidelberg, Germany; e-mail: h.brenner@dkfz-heidelberg.de.