In blood-stage infection by the human malaria parasite Plasmodium falciparum, export of proteins from the intracellular parasite to the erythrocyte is key to virulence. This export is mediated by a host-targeting (HT) signal present on a “secretome” of hundreds of parasite proteins engaged in remodeling the erythrocyte. However, the route of HT-mediated export is poorly understood. Here we show that minimal soluble and membrane protein reporters that contain the HT motif and mimic export of endogenous P falciparum proteins are detected in the lumen of “cleft” structures synthesized by the pathogen. Clefts are efficiently targeted by the HT signal. Furthermore, the HT signal does not directly translocate across the parasitophorous vacuolar membrane (PVM) surrounding the parasite to deliver protein to the erythrocyte cytoplasm, as suggested by current models of parasite protein trafficking to the erythrocyte. Rather, it is a lumenal signal that sorts protein into clefts, which then are exported beyond the PVM. These data suggest that Maurer's clefts, which are unique to the virulent P falciparum species, are pathogen-induced secretory organelles that concentrate HT-containing soluble and membrane parasite proteins in their lumen for delivery to the host erythrocyte.
Introduction
Plasmodium falciparum causes the most virulent form of human malaria, a disease that afflicts 200 to 300 million people and kills more than a million children every year.1 Blood-stage parasites that infect mature erythrocytes export proteins to dramatically remodel the host cell surface and cause all of the major disease-related pathologic conditions, including death.1 Furthermore, several hundred parasite secretory proteins carry a host targeting (HT) motif (also known as Plasmodium Export Element [PEXEL]), which is required for protein export past the parasitophorous vacuolar membrane (PVM) surrounding the parasite, to the erythrocyte.2,3 But how the HT motif functions in exporting both soluble and membrane proteins beyond the PVM remains poorly understood
Prior studies have shown that both soluble and membrane bound antigens and virulence proteins containing the HT motif associate with flattened, lamellar intraerythrocytic structures called the Maurer's clefts.2,–4 Over the past decade, clefts have been suggested as possible intermediates of protein transport to the erythrocyte surface.5,6 A recent model suggests that an HT-containing protein exported to the erythrocyte membrane is translocated across the PVM as a soluble complex and delivered to the cytoplasmic face of clefts before membrane insertion and subsequent vesicular export from clefts to the erythrocyte surface.7 However, unequivocal biochemical evidence for the presence of soluble or cytoplasmic forms of these proteins in the erythrocyte is lacking. This model also fails to explain the mechanism by which soluble proteins containing the HT signal are released into the cytoplasm and associate with the cytoplasmic face of clefts.
We constructed minimal soluble and membrane reporters and examined their export to the erythrocyte as well as their membrane association. Our studies reveal that a minimal soluble reporter detected in the erythrocyte cytoplasm is also found in the cleft lumen, but shows no association with the cytoplasmic face of cleft membranes. Attachment of a transmembrane domain (derived from the surface adhesin P falciparum erythrocyte membrane protein 1, PfEMP1) to this soluble reporter blocked its release into the erythrocyte cytoplasm and enabled its accumulation anchored in the cleft lumen. Subsequent replacement of the HT signal did not prevent membrane anchoring but resulted in reporter accumulation at the PVM. These data establish that the HT motif does not mediate protein translocation across the PVM bilayer into erythrocyte cytoplasm. Rather, the HT seems to function as a lumenal signal that sorts secretory parasite proteins into clefts, which are then exported beyond the PVM. Thus clefts may be major intermediates of transport across the erythrocyte cytoplasm for both soluble and membrane proteins and thus central to host remodeling.
Methods
Construct production and transgene expression using piggyBac
All constructs used for transfections were first assembled in pBluescript SK+. Constructs for transfections using piggyBac strategy were further subcloned into a modified piggyBac integration vector pBacII, derived from pXL-BACII-DHFR.8
The plasmid pHTsolGFP was derived from pDCHRPIIDomainImin.his.GFP.9 Construction of pBacII(HT-GFPmembmyc), pBacII(Δ-GFPmembmyc) and pBacII(HT-GFPmembC-term) was carried out as follows. pDC110 was digested with NarI and SapI and the 3746-base-pair (bp) fragment containing the dhfr, 5′ cam, and 3′hsp86 was treated with DNA polymerase I Klenow fragment to form blunt ends. In parallel, pXL-BACII-DHFR8 was digested with EcoRI and XhoI to release the 3961-bp fragment containing oriC, the ampicillin resistance gene, and the inverted terminal repeats, which was then treated with DNA polymerase I Klenow fragment to form blunt ends and ligated to the earlier obtained fragment to form pBacII. The construct pBacII(HT-GFPmembmyc) was generated as follows. First, the entire HRPIIDomainImin.his.GFPmyc was amplified by polymerase chain reaction (PCR) using HRPIIPstIF (CGGCTGCAGATGGTTTCCTTCTCAAAAAATAATAAAGTATTATCC) and MycXhoIR (CCGCTCGAGGTCGACGGTATCGATAAGCTTATAAATCTTCTTC) primers. The PCR product was cloned at PstI and XhoI site of pBluescript SK+ to generate pBSHRPIImin.his.GFPmyc. The gfp-vartm region was amplified by PCR from pBSGFPvarTM2 using GFPKpnIF (CGGGGTACCATGCATAGATCTAAAGGAGAA) and varTMBglIIR (CCGAGATCTTTATTACTTTAGATAAAAATAAGTGAATGTAGC), digested with BglII and inserted into similar digested pBSHRPIImyc11 to generate pBSHRPIIGFPvarTMmyc. Subsequently, pBSHRPIImin.his.GFP was digested with NcoI and XhoI to replace its gfp-myc with gfp-vartm-myc derived from a similar digestion of pBSHRPIIGFPvarTMmyc. Finally, the HRPIImin.his.GFPvarTMmyc fragment was cloned into pBacII to generate pBacII(HT-GFPmembmyc). Plasmid pBacII(Δ-GFPmembmyc) was generated by a 3-step PCR. PCR 1 was performed using HRPIIPstIF and HTmutR (CGGGATATCGCATCAACAATCCATGTAGATGATGCCCATCATGC); PCR 2 used HTmutF (CGGGATATCGCATCAACAATCCATGTAGATGATGCCCATCATGC) and MycXhoIR primers. The products of PCR 1 and 2 were used to constitute the entire Δ-GFPmembmyc, which was finally cloned into pBacII at the XhoI site. For the generation of HT-GFPmembC-term, the region containing gfpvartmexon2 was amplified from Glm42GFPvarTMexon22 using GFPKpnIF and Exon2XhoIR (CCGCTCGAGTTATATATCCCATAAATCTGCTATTG). The PCR product was digested with NcoI and XhoI and cloned into pBSHRPIImin.his.GFP to replace its gfp-myc with gfpvartmexon2. Finally, the HRPIImin.his.GFPvarTMexon2 fragment was cloned into pBacII to generate pBacII(HT-GFPmembC-term).
Synchronous P falciparum 3D7 parasites in culture were transfected with indicated plasmids by standard procedures.12 Forty-eight hours after transfection, the cultures were selected with 2.5 nmol/L WR99210 (Jacobus Pharmaceuticals, Princeton, NJ), and stable cells lines were cloned by limiting dilution. Genomic DNA was isolated from respective clonal populations and digested with EcoRV. Chromosomal integration in respective piggyBac clones was detected by Southern hybridization using labeled hdhfr probe and sequencing. For each transgene, a single site of insertion was identified in 3 independent clones, suggesting that clones with specific insertions may dominate in a population. In all the 3 clones of parasites expressing HT-GFPmembmyc (named PfHT-GFPmembmyc), the piggyBac strategy resulted in insertion at TTAA target site in the 5′ UTR of pff1505w (chromosome 6). The common insertion site for each of the 3 parasite clones expressing Δ-GFPmembmyc (called PfΔ-GFPmembmyc) was in the 5′UTR of pf14_0096 (chromosome 14). Both pff1505w and pf14_0096 are hypothetical genes of unknown function. They are not predicted to be secretory proteins and are thus not expected to influence parasite protein export to the erythrocyte. Exhaustive clonal analysis has not been undertaken to determine whether these are indeed the dominant sites of insertion. Neither transgenic parasite line showed any growth defects relative to parent 3D7 (data not shown).
Generation of parasites with deletion in the putative Hsp40 substrate-binding domain in PFE0055c (Δctermpfe0055c)
P falciparum pfe0055c codes for a putative protein of 413 amino acids. DNA sequence encoding for amino acid 109-212 of pfe0055c was amplified from genomic DNA using PFE0055cXhoIF (CGGCTCGAGGATAAACACAACCAATCATTTGGAAATGAAATATTTAAAAATACAAAAG) and PFE0055cSpeIR (CCGACTAGTTGATCTGCTTGATCTAGGTCTTCTTGAATTCATACTTGCGAAACC) primers and cloned upstream of neomycin phosphotransferase gene in the vector pGTPneo4613 to generate pHsp40neo46. Parasites were transfected with DNA by loading,12 selected for bsd expression with 1 μg/mL Blasticidin hydrochloride followed by selection with 400 μg/mL G418 for single crossover recombination. Successful replacement of pfe0055c with pfe0055c fragment-neo fusion, which is deleted in region encoding for putative 40-kDa heat-shock protein (HSP40) substrate-binding domain at the chromosomal locus, was analyzed by PCR using primers 1 (CGGCTCGAGATGTCCATTTTAAATAAATACGAAGGAAAGAAAAATAAAATC), 2 (CTTTACATTCATTATGAAGAATGTGTATGATTTTTCCC), 3 (CGGCTCGAGGATAAACACAACCAATCATTTGGAAATGAAATATTTAAAAATACAAAAG), and 4 (TC-AGAAGAACTCGTCAAGAAGGCGATAGAAGGC).
Construction of Δctermsbp mutant parasites
P falciparum parasites with chromosomal deletion in pfsbp1 (Δctermsbp) were generated as follows. In brief, the region in pfsbp1 between nucleotides 109 and 744 was amplified using SBP1KOXhoIF (CGGCTCGAGTCGGATGCAGCAACAAATGTTACTGATGCAGTAAG) and SBP1KOSpeIR (CCGACTAGTTACTATTTTTTTTATTATTACTCTTTTTCCTAAGTTG) and cloned at corresponding sites in pGTPneo4613 to generate pSBP1neo46. Parasites were transfected with pSBP1neo46 by DNA loading,12 selected for bsd expression with 1 μg/mL Blasticidin hydrochloride and, after establishment of a resistant population, selected with 400 μg/mL G418 for single-crossover recombination. Successful deletion in pfsbp1 was confirmed by isolation of genomic DNA from G418-resistant population and analysis by PCR using primers 1 (CCGGGCTAGCATGTGTAGCGCAGCCCGAGCATTTGATTTTTTTACTGATTTAGCC), 2 (GGCGACCGGTCGGGCAGCAGCGGTTTCTCTAGCAACTGTTTTTGTTGTGG), 3 (CGGCTCGAGTCGGATGCAGCAACAAATGTTACTGATGCAGTAAG), and 4 (TCAGAAGAACTCGTCAAGAAGGCGATAGAAGGC).
Expression-purification of rHT-GFP and loading into erythrocyte ghosts
The region encoding HT-GFP fusion was cloned into pET29b (EMD Biosciences, Darmstadt, Germany) as follows. The HT-GFP fusion was amplified from plasmid HRPIIDomIminHisGFP9 with primers HRPII-4 (TGGCATTTAATAATAACTTGTGTAGCAAAAATG) and GFP3′-3 (GGACctcgagTTTGTATAGTTCATCCATGCCATGTG) and digested with XhoI. The expression vector pET29b was digested with NdeI, blunt-ended using T4 polymerase, and ligated with XhoI-digested PCR product to form pET29b(rHT-GFP). Escherichia coli BL21 (DE3; Invitrogen, Carlsbad, CA) cells were transformed with pET29b(rHT-GFP). An overnight grown culture was used to prime a 500-mL culture. At exponential phase (OD600nm of 0.5-0.6) cells were induced with 1 mM isopropyl-β-D-thiogalactoside for 4 hours. Recombinant HT-GFP was purified from cell pellet under native conditions using ProBond nickel purification resin (Invitrogen) according to the manufacturer's instructions. Eluted fractions were pooled, dialyzed extensively against phosphate-buffered saline (PBS), and concentrated using Centricon concentrators (Millipore, Billerica, MA). The purity of the eluted fractions was checked by SDS-PAGE, and protein concentration was estimated by Bradford reagent (Bio-Rad Laboratories, Hercules, CA).
Erythrocyte ghosts were loaded with 1 mg/mL of purified recombinant HT (rHT)-green fluorescent protein (GFP) according to published protocol.14 As a control, nonloaded erythrocyte ghosts were also prepared. Preparations of rHT-GFP–loaded and nonloaded erythrocyte ghosts were infected with purified P falciparum schizonts, and the percentage parasitemia compared with invasion in intact human erythrocytes. Cells were also harvested and processed for live cell fluorescence imaging, indirect immunofluorescence assay, or immunoelectron microscopy.
Immunofluorescence assay and immunoblotting
Immunofluorescence assay of erythrocytes infected with parasite-expressing HTsol-GFP (PfHTsol-GFP) and permeabilized with tetanolysin was performed using antibodies to GFP and PfStomatin as described previously.15 Indirect immunofluorescence assay of rHT-GFP loaded/nonloaded erythrocyte ghosts infected with nontransfected parasites or erythrocytes infected with parasite expressing HT-GFPmembmyc and Δ-GFPmembmyc (PfHT-GFPmembmyc and PfΔ-GFPmembmyc, respectively) was performed according to a published protocol.9 For quantitative colocalization between green fluorescing GFP signal and red fluorescing PfSBP1 signal, optical images from 200 sections were analyzed and result expressed in percentage.
Under specific instances, erythrocytes infected with PfHT-GFPmembmyc parasites were labeled with 0.5 μM Texas-red ceramide (Invitrogen) to view the body of parasite as well as erythrocyte membrane. In brief, cells were harvested from culture, washed with serum-free RPMI medium, and labeled with 0.5 μmol/L Texas-red ceramide for 30 minutes at 4°C. Samples were washed with serum-free RPMI, stained with Hoechst 33342 and imaged live using fluorescence microscope.
Erythrocyte ghosts were also prepared in the presence anti-GFP antibodies conjugated to Alexa Fluor 594 and infected with Percoll-purified schizonts of PfHT-GFPmembmyc or PfΔ-GFPmembmyc parasites. At trophozoite stages, cells were harvested and processed for live cell imaging as described. A fraction of erythrocyte ghosts, not loaded with Alexa Fluor 594-conjugated anti-GFP antibodies and infected with parasite expressing HT-GFPmembmyc or Δ-GFPmembmyc, was harvested, adhered to poly-L-lysine–coated coverslips, and permeabilized with 0.01% saponin. Saponin-permeabilized cells were probed with anti-GFP antibodies conjugated to Alexa Fluor 594. Parasite nucleus was stained with 5 μg/mL Hoechst 33342, and the cells were viewed under deconvolution fluorescence microscope.
Western blotting of the pellet and supernatant fractions from tetanolysin-permeabilized erythrocytes infected with parasite expressing HT-GFPmembmyc, Δ-GFPmembmyc, or HT-GFPmembC-term was performed as follows. In brief, 10% cell suspension of infected erythrocytes were permeabilized with 100 U/mL tetanolysin for 30 minutes at 37°C and separated into pellet and supernatant fractions after a brief centrifugation at 3200 rpm for 10 minutes. Samples were subsequently analyzed by Western blotting using antibodies to GFP, PfStomatin, and parasite cytosolic marker PfFKBP.
Detection of neomycin phosphotransferase (NPT) fusion products for parasite lines expressing a C-terminal deletion of PfSBP1 (Δctermsbp) or a deletion in putative Hsp40 substrate-binding region in PFE0055c (Δctermpfe0055c) was performed using antibodies to NPT.
All other Western blots were performed by directly solubilizing the infected red blood cells in Laemmli sample buffer, separating by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), immunoblotting with respective antibodies.
Immunoelectron microscopy
Immunoelectron microscopy of erythrocyte ghosts, either nonloaded and infected with parasite expressing HTsol-GFP or loaded with rHT-GFP and infected with untransfected parasites, as well as erythrocytes infected with parasite expressing HTsol-GFP, HT-GFPmembmyc, or Δ-GFP membmyc, was performed as described previously.9
A comparison between the numbers of gold particles associated with Maurer's clefts in rHT-GFP loaded (and infected) erythrocyte ghosts and nonloaded erythrocyte ghosts infected with parasite expressing HTsol-GFP was performed by manual counting in 20 cells and then averaging the number of cleft-associated gold particles per cell.
Protease treatment of tetanolysin- or saponin-permeabilized erythrocytes
Infected erythrocytes, intact or tetanolysin-permeabilized (as described “Immunofluorescence assay and immunoblotting”), were treated with 1 mg/mL trypsin (Sigma-Aldrich, St Louis, MO) on ice for 30 minutes. Trypsin inactivation was carried out by adding an equal volume of PBS containing 2 mg/mL soybean trypsin inhibitor (Sigma-Aldrich) and protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) at 4°C. Samples were analyzed in Western blots with antibodies to GFP, c-myc, and human spectrin.
Deconvolution fluorescence microscopy and image acquisition
Fluorescence microscopy and digital image collection of both fixed and live infected erythrocytes were performed on an Olympus IX inverted fluorescence microscope with a temperature-controlled stage and a Photometrix cooled charge-coupled device camera (CH350/LCCD) driven by DeltaVision software from Applied Precision (Seattle, WA). In brief, DeltaVision software (softWoRx) was used to deconvolve the bright-field and fluorescent optical images taken through cells and processed using Adobe Photoshop version 10 (San Jose, CA). For quantitative projections, a minimum of 200 optical images containing fluorescent parasites was subjected to the “additive” method of data collection. Fluorescence quantification was carried out with a 60×/1.4 NA objective. Use of a constitutive cam promoter resulted in continuous GFP chimera expression associated with the parasite.
Results
An exported, minimal soluble reporter was detected in the lumen of Maurer's clefts
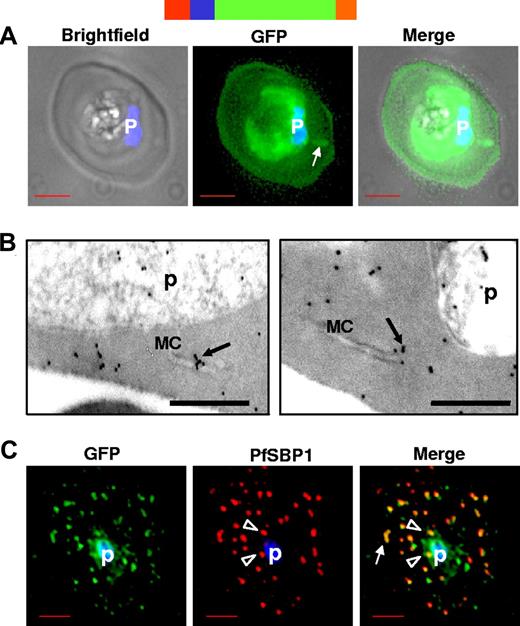
Parasite proteins exported to the erythrocyte cytoplasm via the HT motif vary greatly in size, amino acid composition, and sequence. Thus, to understand the role of HT motif in all soluble proteins, we followed a minimal reporter composed of a cleavable endoplasmic reticulum (ER)-type signal sequence (SS), HT motif, and GFP, known to result in the delivery of green fluorescence in the erythrocyte cytoplasm9 (Figure 1A). In these cells, green fluorescence is also detected in association with the parasite, probably because of constitutive action of the promoter that leads to overproduction of the protein and its accumulation in the parasite during blood stage growth.9 In addition, structures in the erythrocyte cytoplasm also seem to be labeled by the fluorescent reporter (Figure 1A arrows). Immunoelectron microscopy confirmed the presence of gold particles in the erythrocyte cytosol as well as in flattened lamellar structures called Maurer's clefts (Figure 1B arrow) in the host cell. When the infected erythrocyte membrane was permeabilized with tetanolysin and cells were subsequently fixed, permeabilized, and probed with antibodies, GFP was largely associated with punctate structures that colocalized with a resident protein of Maurer's clefts (Figure 1C). This cleft-associated GFP was detected both proximal to the parasite (Figure 1C arrowheads) and in the periphery of the erythrocyte (Figure 1C arrow). Previous studies have shown that full-length parasite proteins exported to the erythrocyte cytoplasm have also been located to the Maurer's clefts,9 suggesting that a minimal secretory reporter mimics this feature of parasite protein export to the erythrocyte.
HTsol-GFP, a minimal soluble reporter exported to the erythrocyte cytoplasm and detected in clefts. (A) 0° projection of an erythrocyte infected with transgenic parasites expressing HTsol-GFP. Arrow indicates GFP-labeled intraerythrocytic structure, possibly a cleft. (B) Immunoelectron micrographs of trophozoite parasite (p)-infected cells expressing HTsol-GFP. Ultrathin sections were probed with antibodies to GFP and secondary antibody gold (10 nm) conjugate. Arrows indicate gold particles at intraerythrocytic Maurer's clefts (MC). No gold labeling was detected in absence of primary antibody or when a nonspecific primary was used (not shown). Bar, 200 nm. (C) Single optical section of an infected erythrocyte expressing HTsol-GFP. Samples were treated to release soluble GFP, fixed, and probed with antibodies to GFP (green) and P falciparum Skeletal Binding Protein1 (PfSBP1, red). Arrow, GFP labeled cleft structures at the periphery of infected erythrocyte; arrowheads, clefts proximal to the parasite. In fluorescence micrographs, p denotes parasite nucleus stained with Hoechst 33342; bar, 2 μm. Schematic representation of the construct is indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) fused to GFP (green) and myc (orange).
HTsol-GFP, a minimal soluble reporter exported to the erythrocyte cytoplasm and detected in clefts. (A) 0° projection of an erythrocyte infected with transgenic parasites expressing HTsol-GFP. Arrow indicates GFP-labeled intraerythrocytic structure, possibly a cleft. (B) Immunoelectron micrographs of trophozoite parasite (p)-infected cells expressing HTsol-GFP. Ultrathin sections were probed with antibodies to GFP and secondary antibody gold (10 nm) conjugate. Arrows indicate gold particles at intraerythrocytic Maurer's clefts (MC). No gold labeling was detected in absence of primary antibody or when a nonspecific primary was used (not shown). Bar, 200 nm. (C) Single optical section of an infected erythrocyte expressing HTsol-GFP. Samples were treated to release soluble GFP, fixed, and probed with antibodies to GFP (green) and P falciparum Skeletal Binding Protein1 (PfSBP1, red). Arrow, GFP labeled cleft structures at the periphery of infected erythrocyte; arrowheads, clefts proximal to the parasite. In fluorescence micrographs, p denotes parasite nucleus stained with Hoechst 33342; bar, 2 μm. Schematic representation of the construct is indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) fused to GFP (green) and myc (orange).
To investigate whether protein exported to the erythrocyte cytoplasm adhering to the cytoplasmic face of intraerythrocytic structures caused localization of GFP in clefts, we first permeabilized erythrocyte membrane to release protein freely diffusible in the erythrocyte cytoplasm. Antibodies introduced into these permeabilized cells failed to detect GFP on the cytoplasmic face of intraerythrocytic membranes, although Pfstomatin, known to be in the cytoplasmic face of the vacuole, was readily detected (Figure 2A). GFP was readily detected when saponin was used to permeabilize infected cells (Figure 2A), suggesting that reporter was in the lumen of punctate intraerythrocytic clefts.
Lumenal association of HTsol-GFP at Maurer's clefts. (A) Schematic representation of the infected erythrocyte (left) and its permeabilization (dotted lines) after treatment with tetanolysin (top) or saponin (bottom). Panels of fluorescent images show infected erythrocyte expressing HTsol-GFP, permeabilized with tetanolysin (top) or saponin (bottom), and probed with antibodies to GFP (green) and PfStomatin (red). Respective merged images are also shown. Dotted lines indicate erythrocyte periphery. Arrows show intraerythrocytic clefts. (B) 0° projections of an rHT-GFP–loaded erythrocyte ghost infected with 3D7 P falciparum (top) or a mock-loaded erythrocyte ghost infected with transgenic parasite expressing HTsol-GFP (bottom). Empty arrowhead, cleft structure not labeled with intraerythrocytic rHT-GFP; solid arrowhead, GFP labeled cleft. (C) Cells in panel B fixed, permeabilized, and probed with antibodies to GFP (green) and resident cleft protein PfSBP1 (red). Arrows show clefts. (D) Immunoelectron microscopy of cells in panel B showing distribution of GFP associated with Maurer's clefts (MC). Bar indicates 500 nm. (E) Bar graph showing the percentage colocalization between GFP and Maurer's cleft in indicated samples by fluorescence microscopy. (F) Quantitation for the number of gold particles (measuring GFP) associated with clefts by immunoelectron microscopy over 20 infected erythrocytes. In all fluorescence micrographs: p, parasite (nucleus stained with Hoechst 33342; blue); ec, erythrocyte cytosol; bar, 2 μm.
Lumenal association of HTsol-GFP at Maurer's clefts. (A) Schematic representation of the infected erythrocyte (left) and its permeabilization (dotted lines) after treatment with tetanolysin (top) or saponin (bottom). Panels of fluorescent images show infected erythrocyte expressing HTsol-GFP, permeabilized with tetanolysin (top) or saponin (bottom), and probed with antibodies to GFP (green) and PfStomatin (red). Respective merged images are also shown. Dotted lines indicate erythrocyte periphery. Arrows show intraerythrocytic clefts. (B) 0° projections of an rHT-GFP–loaded erythrocyte ghost infected with 3D7 P falciparum (top) or a mock-loaded erythrocyte ghost infected with transgenic parasite expressing HTsol-GFP (bottom). Empty arrowhead, cleft structure not labeled with intraerythrocytic rHT-GFP; solid arrowhead, GFP labeled cleft. (C) Cells in panel B fixed, permeabilized, and probed with antibodies to GFP (green) and resident cleft protein PfSBP1 (red). Arrows show clefts. (D) Immunoelectron microscopy of cells in panel B showing distribution of GFP associated with Maurer's clefts (MC). Bar indicates 500 nm. (E) Bar graph showing the percentage colocalization between GFP and Maurer's cleft in indicated samples by fluorescence microscopy. (F) Quantitation for the number of gold particles (measuring GFP) associated with clefts by immunoelectron microscopy over 20 infected erythrocytes. In all fluorescence micrographs: p, parasite (nucleus stained with Hoechst 33342; blue); ec, erythrocyte cytosol; bar, 2 μm.
To examine whether GFP in the cleft lumen could be obtained by uptake of protein from the erythrocyte cytoplasm, we expressed rHT-GFP using a bacterial expression system. We purified rHT-GFP, loaded into resealed erythrocyte ghosts, and subsequently infected those with P falciparum (also see Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As shown by live cell image in Figure 2B (top), rHT-GFP was found to be uniformly distributed in the infected erythrocyte cytoplasm as well as the food vacuole of parasite (presumably via cytostome-mediated uptake of host cytoplasm). Areas devoid of green fluorescence in the erythrocyte cytoplasm were visible in the live cell image (Figure 2B top, arrowhead), which were identified as cleft structures by indirect immunofluorescence assay (Figure 2C top panel, E) as well as immunoelectron microscopy (Figure 2D top panel, F). In contrast, when resealed ghosts were infected with parasites that biosynthetically expressed secretory HTsol-GFP, green fluorescence was quantitatively associated with the clefts (Figure 2B-D bottom panels, E,F). Together, the data in Figures 1 and 2 suggest that the exported minimal soluble GFP reporter associated with Maurer's clefts is found in the lumenal and not the cytoplasmic face of these structures.
The malarial HT motif is a lumenal signal that sorts protein into Maurer's clefts without translocating soluble complexes into the erythrocyte cytoplasm
We next investigated the effect of the presence of transmembrane domain in the minimal soluble reporter and thus synthesized the chimera HT-GFPmembmyc by adding the transmem-brane domain of P falciparum erythrocyte membrane protein 1 (PfEMP1; also see Figure S2). Remarkably, this seemed to deplete green fluorescence from erythrocyte cytoplasm and to limit its detection in punctate structures dispersed in the erythrocyte (compare Figures 1A and 3A top panel). These punctate structures colocalized with Maurer's clefts, as confirmed by both indirect immunofluorescence and immunoelectron microscopy (Figure 3B,C top). This suggested that addition of the transmembrane domain to the soluble reporter blocked its efficient release to the erythrocyte cytoplasm. Because the membrane chimera accumulated in clefts rather than PVM, clefts may be the preferred site of soluble protein translocation into the erythrocyte cytoplasm.
The HT motif targets proteins to Maurer's clefts without translocation into the erythrocyte cytoplasm. (A) Live infected erythrocytes expressing HT-GFPmembmyc (top) and Δ-GFPmembmyc (bottom) viewed under bright-field image, GFP fluorescence, and merged optics. Western blot using antibodies to GFP indicating the detection of 39-kDa fusion product for HT-GFPmembmyc-expressing cells (lane 1), 39/41-kDa doublet fusion product for Δ-GFPmembmyc-expressing cells (lane 2), or no signal for untransfected cells (lane 3) is shown at the right. In lane 2, the 41-kDa band is a precursor that in pulse chase experiments can be chased into the 39-kDa band (data not shown). Vertical lines have been inserted to indicate repositioned gel lanes. (B) Indirect immunofluorescence assay showing distribution of GFP (green), associated with HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel) relative to the Maurer's cleft protein PfSBP1 (red). Parasite nucleus (p) is stained with Hoechst 33342. (C) Localization of HT-GFPmembmyc in Maurer's clefts (MC, top) and Δ-GFPmembmyc in parasitophorous vacuolar membrane (PVM, bottom) by immunoelectron microscopy. Empty arrowheads indicate gold particles showing distribution of GFP chimeras. Bar, 500 nm; p, parasite. Micrograph at the bottom has been magnified twice compared with that at the top to distinguish the parasite plasma membrane (PPM) from the PVM. (D) Western blots of tetanolysin-released infected erythrocyte cytoplasm supernatant (S) and pellet (P) fractions of cells expressing HT-GFPmembmyc (left panel), Δ-GFPmembmyc (middle panel), and HT-GFPmembC-term (right panel) probed for GFP, PVM marker PfStomatin, and parasite cytoplasmic protein PfFKBP. Hemoglobin (Hb) released (in S) by tetanolysin is expressed as a percentage of the total Hb detected by hypotonic lysis. (E) Live infected erythrocytes expressing HT-GFPmembCterm viewed under bright-field image, GFP fluorescence, and merged optics. Schematic representation for all constructs are indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) or its replacement (solid black triangle) fused to GFP (green), transmembrane region (black), and myc (orange). C-terminal region (derived from PfEMP1) is depicted in yellow.
The HT motif targets proteins to Maurer's clefts without translocation into the erythrocyte cytoplasm. (A) Live infected erythrocytes expressing HT-GFPmembmyc (top) and Δ-GFPmembmyc (bottom) viewed under bright-field image, GFP fluorescence, and merged optics. Western blot using antibodies to GFP indicating the detection of 39-kDa fusion product for HT-GFPmembmyc-expressing cells (lane 1), 39/41-kDa doublet fusion product for Δ-GFPmembmyc-expressing cells (lane 2), or no signal for untransfected cells (lane 3) is shown at the right. In lane 2, the 41-kDa band is a precursor that in pulse chase experiments can be chased into the 39-kDa band (data not shown). Vertical lines have been inserted to indicate repositioned gel lanes. (B) Indirect immunofluorescence assay showing distribution of GFP (green), associated with HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel) relative to the Maurer's cleft protein PfSBP1 (red). Parasite nucleus (p) is stained with Hoechst 33342. (C) Localization of HT-GFPmembmyc in Maurer's clefts (MC, top) and Δ-GFPmembmyc in parasitophorous vacuolar membrane (PVM, bottom) by immunoelectron microscopy. Empty arrowheads indicate gold particles showing distribution of GFP chimeras. Bar, 500 nm; p, parasite. Micrograph at the bottom has been magnified twice compared with that at the top to distinguish the parasite plasma membrane (PPM) from the PVM. (D) Western blots of tetanolysin-released infected erythrocyte cytoplasm supernatant (S) and pellet (P) fractions of cells expressing HT-GFPmembmyc (left panel), Δ-GFPmembmyc (middle panel), and HT-GFPmembC-term (right panel) probed for GFP, PVM marker PfStomatin, and parasite cytoplasmic protein PfFKBP. Hemoglobin (Hb) released (in S) by tetanolysin is expressed as a percentage of the total Hb detected by hypotonic lysis. (E) Live infected erythrocytes expressing HT-GFPmembCterm viewed under bright-field image, GFP fluorescence, and merged optics. Schematic representation for all constructs are indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) or its replacement (solid black triangle) fused to GFP (green), transmembrane region (black), and myc (orange). C-terminal region (derived from PfEMP1) is depicted in yellow.
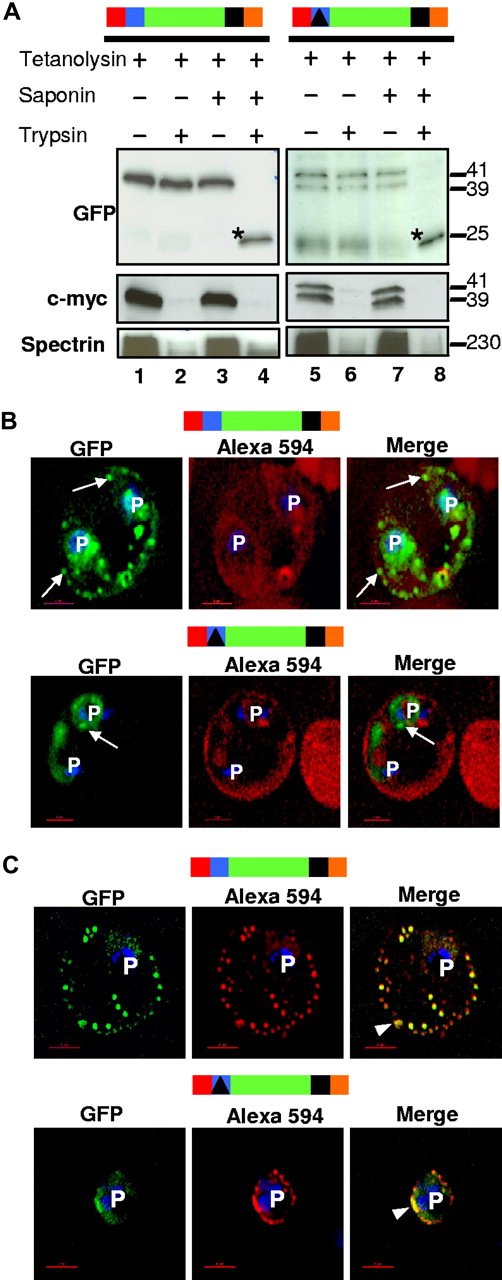
Replacing the HT motif (LNKRLLYETQA) in HT-GFPmembmyc with a nonspecific sequence (ISAATDIASTI) blocked export of green fluorescence to clefts (Figure 3A,B bottom panels). Instead, we detected GFP accumulation in the periphery of the parasite (Figure 3A bottom panel). Finally, immunoelectron microscopy suggested that Δ-GFPmembmyc product was localized in PVM (Figure 3C bottom). Together, these data suggested that the transmembrane region enabled protein insertion into the membrane, whereas the HT motif directly enables export of GFP to the clefts. To determine whether this export involves soluble intermediates translocated to the erythrocyte cytoplasm, we undertook biochemical analyses of infected erythrocytes permeabilized with tetanolysin. We found that although this treatment quantitatively released hemoglobin (Figure 3D), HT-GFPmembmyc, Δ-GFPmembmyc, and HT-GFPmembC-term (containing the cytoplasmic tail of PfEMP1 fused to HT-GFPmembmyc and exported to the clefts; Figure 3E) remained cell-associated, indicating that none of these HT-containing proteins existed as freely diffusible soluble complexes across the PVM. Further, protease treatments conducted in these permeabilized cells revealed that the c-myc tag on both cleft-associated HT-GFPmembmyc and PVM-associated Δ-GFPmembmyc faced the erythrocyte cytoplasm (Figure 4A). In contrast, GFP was lumenal. This topology was independently confirmed by demonstrating that anti-GFP antibodies in the erythrocyte cytoplasm could not access GFP of either HT-GFPmembmyc or Δ-GFPmembmyc (Figure 4B,C).
The HT motif sorts protein into Maurer's clefts without translocation across the PVM. (A) For both HT-GFPmembmyc and Δ-GFPmembmyc, Western blots show protection of GFP but quantitative degradation of myc and erythrocyte spectrin after addition of trypsin to cells where the infected erythrocyte membrane was permeabilized with tetanolysin (lanes 2 and 6). Saponin (which additionally permeabilizes PVM and clefts, lanes 4 and 8) renders GFP susceptible to protease. *, trypsin digested GFP product of 25-kDa. Molecular mass markers are expressed in kilodaltons (kDa). (B) Single optical sections of ghosts resealed with Alexa Fluor 594 anti-GFP antibodies infected with parasites expressing HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel). Cells were viewed live using optics for GFP (green), Alexa Fluor 594/Rhodamine (red), and the merged image is shown in the right panel. Arrows, GFP labeled clefts not labeled with anti-GFP Alexa Fluor 594 conjugate. (C) Immunofluorescence assay of resealed ghosts infected with parasites expressing HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel) permeabilized with saponin and treated with anti-GFP Alexa Fluor 594-conjugated antibodies. Images under GFP (green) and Alexa 594 (red) optics and their respective merge are shown. Arrowhead, region of colocalization (in yellow) between GFP and Alexa 594. In all cells, the parasite (p) nuclei were stained with Hoechst 33342 (blue); bar, 2 μm. Schematic representation of the construct is indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) or its replacement (filled triangle in black) fused to GFP (green), transmembrane region (black), and myc (orange).
The HT motif sorts protein into Maurer's clefts without translocation across the PVM. (A) For both HT-GFPmembmyc and Δ-GFPmembmyc, Western blots show protection of GFP but quantitative degradation of myc and erythrocyte spectrin after addition of trypsin to cells where the infected erythrocyte membrane was permeabilized with tetanolysin (lanes 2 and 6). Saponin (which additionally permeabilizes PVM and clefts, lanes 4 and 8) renders GFP susceptible to protease. *, trypsin digested GFP product of 25-kDa. Molecular mass markers are expressed in kilodaltons (kDa). (B) Single optical sections of ghosts resealed with Alexa Fluor 594 anti-GFP antibodies infected with parasites expressing HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel). Cells were viewed live using optics for GFP (green), Alexa Fluor 594/Rhodamine (red), and the merged image is shown in the right panel. Arrows, GFP labeled clefts not labeled with anti-GFP Alexa Fluor 594 conjugate. (C) Immunofluorescence assay of resealed ghosts infected with parasites expressing HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel) permeabilized with saponin and treated with anti-GFP Alexa Fluor 594-conjugated antibodies. Images under GFP (green) and Alexa 594 (red) optics and their respective merge are shown. Arrowhead, region of colocalization (in yellow) between GFP and Alexa 594. In all cells, the parasite (p) nuclei were stained with Hoechst 33342 (blue); bar, 2 μm. Schematic representation of the construct is indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) or its replacement (filled triangle in black) fused to GFP (green), transmembrane region (black), and myc (orange).
Together, the data in Figures 3 and 4 strongly support the idea that HT-GFPmembmyc and Δ-GFPmembmyc, respectively, spanned the cleft membrane and PVM via their PfEMP1 transmembrane domain and were not translocated across the PVM, via the HT signal. Given these considerations, because the HT clearly drives protein accumulation in Maurer's clefts, it must accomplish this as a lumenal signal that sorts protein into these secretory clefts.
HT-driven protein accumulation in clefts may occur before cleft export to the periphery of the erythrocyte and was apparently not influenced by predicted functional changes in cleft cytoplasmic protein PfSBP1 or a putative heat-shock protein, PFE0055c
Our examination of live-infected erythrocytes (Figures 1,3) suggested that punctate structures characteristic of Maurer's clefts are found in the periphery of the erythrocyte (arrows), in the intraerythrocytic space, at the edge of the parasite (arrowheads), and some may even reside within the parasite.16 Indeed, as shown in Figure 5A, single optical sections of cells labeled with lipid marker Texas-red (TR)-ceramide used to mark the body of the parasite reveal green punctate spots that are tightly apposed to or even within the periphery of the vacuolar parasite. Hence, it is possible that the HT may sort proteins into clefts before these structures are exported from the parasite to the erythrocyte.
HT-dependent protein sorting into clefts may occur at parasite periphery and is not influenced by deletion of the C-terminal domain of PfSBP1. (A) Three-dimensional projections of a live infected erythrocyte expressing HT-GFPmembmyc and stained with TR-ceramide. Clefts at the periphery of the infected erythrocyte (arrows) as well as at or within the perimeter of the vacuolar parasite (empty arrowheads) are visible. (B) 0° projection of live infected erythrocyte expressing HT-GFPmembmyc in 3D7 strains with parental (top) or chromosomal deletion of pfsbp1 (bottom), viewed under GFP optics and merged with bright field. Arrows indicate that the export of HT-GFPmembmyc to cleft structures in parental 3D7 strain is not altered in parasite line with a C-terminal deletion in PfSBP1. Parasite (p) nucleus is stained with Hoechst 33342. Bar, 2 μm.
HT-dependent protein sorting into clefts may occur at parasite periphery and is not influenced by deletion of the C-terminal domain of PfSBP1. (A) Three-dimensional projections of a live infected erythrocyte expressing HT-GFPmembmyc and stained with TR-ceramide. Clefts at the periphery of the infected erythrocyte (arrows) as well as at or within the perimeter of the vacuolar parasite (empty arrowheads) are visible. (B) 0° projection of live infected erythrocyte expressing HT-GFPmembmyc in 3D7 strains with parental (top) or chromosomal deletion of pfsbp1 (bottom), viewed under GFP optics and merged with bright field. Arrows indicate that the export of HT-GFPmembmyc to cleft structures in parental 3D7 strain is not altered in parasite line with a C-terminal deletion in PfSBP1. Parasite (p) nucleus is stained with Hoechst 33342. Bar, 2 μm.
Because our data suggest that clefts carry export cargo to the erythrocyte, we investigated the effects of truncating their major resident protein, PfSBP1, on sorting HT-GFPmembmyc to clefts or cleft export to the erythrocyte (Figure S3). Cooke et al17 show that knockout in PfSBP1 does not block export of proteins from the parasite to the Maurer's cleft. However, studies by Maier et al18 suggest that removal of the C-terminal domain of PfSBP1, which is expected to interact with the erythrocyte membrane, blocks protein export from the parasite to the clefts. We found that HT-mediated sorting to clefts and movement of clefts to the erythrocyte periphery were not blocked by truncation of PfSBP1 (Figure 5B; see also Figure S4). This is consistent with studies by Cooke et al17 but contradicts studies by Maier et al.18 Because our work focuses on minimal lumenal export signal, it is more likely that HT-mediated targeting to clefts does not depend on cytoplasmic interactions of PfSBP1. However, definitive resolution of discrepancies of the data from us and Cooke et al17 with those of Maier et al18 must await further analyses (note: all 3 studies have been carried out in the 3D7 strain).
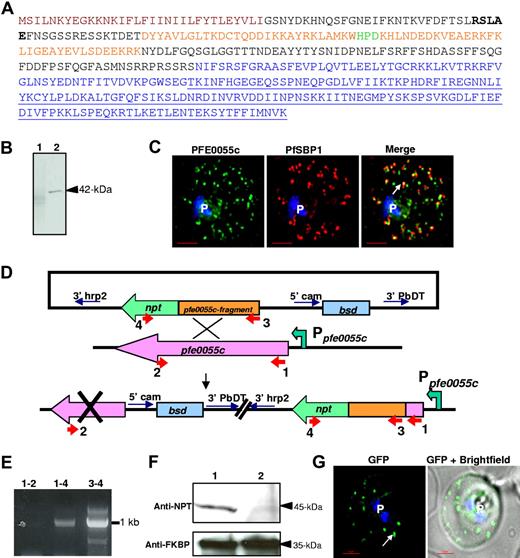
Factors that regulate targeting of the HT to clefts or mediate cleft export beyond the PVM and across the erythrocyte cytoplasm remain poorly understood. Multiple secretome predictions suggest that parasite-encoded chaperones, including heat shock proteins, may be involved in protein export to the erythrocyte and host remodeling.2,19 PFE0055c, a putative HSP40 (Figure 6A), and our previous data suggested that a GFP chimera of PFE0055c was exported to the host erythrocyte.2 Because the PFE0055c was also an HT-containing protein, it is expected to package into clefts en route to the erythrocyte. PFE0055c could have functional consequences for targeting to the clefts as well as additional chaperone activities in the erythrocyte. Indeed, we show that endogenous PFE0055c, a protein of 42 kDa (Figure 6B), localizes primarily to Maurer's clefts (Figure 6C). This provides the first definitive evidence for the presence of a parasite-encoded putative heat shock protein at the clefts. We disrupted the predicted substrate binding domain of PFE0055c by single crossover recombination13 (Figure 6D) and confirmed the replacement of chromosomal pfe0055c with pfe0055c fragment-npt fusion (Figure 6E). In Western blots, using antibodies to NPT, we detected a 45-kDa fusion product in transgenic parasites but not in parental wild-type 3D7 (Figure 6F). However, on transfecting parasites expressing PFE0055c (Δctermpfe0055c, with a deletion in substrate binding region and fused to NPT), we found that disruption of its predicted substrate binding domain, thought to be essential for HSP40 function,20 does not block accumulation of HT-GFPmembmyc in Maurer's clefts (Figure 6G). Nor does this truncation prevent cleft export from the parasite to the erythrocyte periphery (Figure 6G). These data suggest that the substrate-binding domain of PFE0055c heat-shock protein does not significantly regulate HT-mediated protein sorting to clefts. It is possible that PFE0055c acts at a later step of transport in the erythrocyte.
HT-dependent protein sorting into clefts was not influenced by deletion of putative substrate binding domain in PFE0055c. (A) Deduced amino acid sequence of PFE0055c with an N-terminal ER-type signal sequence (brown), HT motif (bold), followed by sequences containing DnaJ region (orange) with the characteristic HPD (green) motif. Further downstream region include a glycine/phenylalanine-rich stretch and C-terminal substrate-binding domain (underlined). Sequences in blue indicate region deleted in parasite line generated by single crossover recombination as shown in panels D-F. (B) Western blot, using anti-PFE0055c antibodies, detecting the presence of a 42-kDa protein in infected erythrocyte (arrowhead, lane 2) but not uninfected erythrocyte (lane 1). (C) Single optical section of a trophozoite-infected erythrocyte fixed and probed with peptide antibodies to PFE0055c (green) and the cleft protein SBP1 (red). Arrow in merge image shows proximal location of PFE0055c to clefts. (D) Strategy for deletion in the C-terminal substrate-binding region of PFE0055c by single crossover recombination with the chromosomal copy of pfe0055c. P falciparum parasites were transfected with plasmids containing an in-frame fusion of the neomycin resistance gene (npt, green) to an internal fragment of pfe0055c (orange) without sequences encoding for C-terminal substrate-binding domain. Only chromosomal integration of the vector by single crossover with the native pfe0055c (pink) drives npt expression under the control of pfe0055c promoter (Ppfe0055c), thus conferring resistance of antibiotic G418. (E) PCR-based detection for the loss of chromosomal copy of pfe0055c. Positions for primer pairs used for amplification analyses of single crossover recombination are highlighted in panel D. (F) Western blot analysis showing the detection of PFE0055c-NPT fusion protein of 45-kDa in transfected line (arrowhead, lane 1) but in not parental line (lane 2) using antibodies to NPT (top). Parasite protein PfFKBP serves as a loading control (bottom). (G) 0° projection of live infected erythrocyte expressing HT-GFPmembmyc in 3D7 strain with chromosomal deletion of pfe0055c viewed under GFP optics and merged with bright field. Arrow indicates that the export of HT-GFPmembmyc to cleft is not altered by truncation in PFE0055c. Parasite nucleus (p) in all cases is stained with Hoechst 33342 (blue). Bar represents 2 μm.
HT-dependent protein sorting into clefts was not influenced by deletion of putative substrate binding domain in PFE0055c. (A) Deduced amino acid sequence of PFE0055c with an N-terminal ER-type signal sequence (brown), HT motif (bold), followed by sequences containing DnaJ region (orange) with the characteristic HPD (green) motif. Further downstream region include a glycine/phenylalanine-rich stretch and C-terminal substrate-binding domain (underlined). Sequences in blue indicate region deleted in parasite line generated by single crossover recombination as shown in panels D-F. (B) Western blot, using anti-PFE0055c antibodies, detecting the presence of a 42-kDa protein in infected erythrocyte (arrowhead, lane 2) but not uninfected erythrocyte (lane 1). (C) Single optical section of a trophozoite-infected erythrocyte fixed and probed with peptide antibodies to PFE0055c (green) and the cleft protein SBP1 (red). Arrow in merge image shows proximal location of PFE0055c to clefts. (D) Strategy for deletion in the C-terminal substrate-binding region of PFE0055c by single crossover recombination with the chromosomal copy of pfe0055c. P falciparum parasites were transfected with plasmids containing an in-frame fusion of the neomycin resistance gene (npt, green) to an internal fragment of pfe0055c (orange) without sequences encoding for C-terminal substrate-binding domain. Only chromosomal integration of the vector by single crossover with the native pfe0055c (pink) drives npt expression under the control of pfe0055c promoter (Ppfe0055c), thus conferring resistance of antibiotic G418. (E) PCR-based detection for the loss of chromosomal copy of pfe0055c. Positions for primer pairs used for amplification analyses of single crossover recombination are highlighted in panel D. (F) Western blot analysis showing the detection of PFE0055c-NPT fusion protein of 45-kDa in transfected line (arrowhead, lane 1) but in not parental line (lane 2) using antibodies to NPT (top). Parasite protein PfFKBP serves as a loading control (bottom). (G) 0° projection of live infected erythrocyte expressing HT-GFPmembmyc in 3D7 strain with chromosomal deletion of pfe0055c viewed under GFP optics and merged with bright field. Arrow indicates that the export of HT-GFPmembmyc to cleft is not altered by truncation in PFE0055c. Parasite nucleus (p) in all cases is stained with Hoechst 33342 (blue). Bar represents 2 μm.
Discussion
Proteins of the malarial secretome exported to the erythrocyte underlie major virulence and antigenic functions. Thus, it is important to understand the transport signals underlying these events of erythrocyte remodeling. How the HT motif functions in exporting both soluble and membrane proteins beyond the PVM has been debated extensively since its discovery.6 Our data showing that the HT motif is a sorting signal that mediates protein concentration in clefts that are exported to the erythrocyte provides an explanation of how this export signal can be shared by both soluble and membrane proteins destined for the host cell (Figure 7). Our evidence that the HT motif does not translocate across the PVM bilayer conflicts with prior suggestions that HT-containing chimeras, such as HT-GFPmembC-term, diffuse as soluble complexes across the erythrocyte cytoplasm.21 However, these conclusions were based on measurements of rapid rates of photobleaching of GFP-protein chimeras,21 lacked unequivocal biochemical evidence of membrane association and protein topology, and do not preclude rapid diffusion of membrane proteins. Studies by Papakrivos et al22 suggest that there may be a soluble form of native PfEMP1 protein within the parasite. However, Papakrivos et al22 as well as Kriek et al23 do not report soluble PfEMP1 in the erythrocyte cytoplasm.
Schematic for HT-mediated cleft targeting events in erythrocyte infected with the malaria parasite P falciparum. In secretory proteins, a cleavable N-terminal ER-type signal sequence (SS) delivers proteins to the PV (step 1). The HT motif enables protein accumulation in the Maurer's clefts (MC, step 2). Clefts can either bud from the PVM (also step 2) packed with proteins exported to the red cell and function as protein reservoirs underneath the erythrocyte membrane (step 3). The HT motif may also move protein from the lumen of the PVM to lumen of clefts either within the parasite (step 2′) or at the proximity to the parasite plasma membrane (PPM, step 3′). Both steps 2 and 2′ implicate recognition of the HT motif by a putative receptor located at the Maurer's clefts (not shown).
Schematic for HT-mediated cleft targeting events in erythrocyte infected with the malaria parasite P falciparum. In secretory proteins, a cleavable N-terminal ER-type signal sequence (SS) delivers proteins to the PV (step 1). The HT motif enables protein accumulation in the Maurer's clefts (MC, step 2). Clefts can either bud from the PVM (also step 2) packed with proteins exported to the red cell and function as protein reservoirs underneath the erythrocyte membrane (step 3). The HT motif may also move protein from the lumen of the PVM to lumen of clefts either within the parasite (step 2′) or at the proximity to the parasite plasma membrane (PPM, step 3′). Both steps 2 and 2′ implicate recognition of the HT motif by a putative receptor located at the Maurer's clefts (not shown).
Recent studies on the biogenesis of clefts suggest that they may bud from the PVM.16 However, it is also possible that they undergo hitherto unknown steps of biogenesis earlier within the parasite and mature into well-developed clefts at the PVM, where they disassociate and move to the erythrocyte periphery (Figure 7). Alternatively, the HT motif may also sort soluble and membrane proteins from the lumen of the PVM to the lumen of clefts that assemble at this vacuole (Figure 7). Regardless of whether sorting occurs inside the parasite or in the PV, as a lumen sorting signal, the HT motif may enable soluble proteins to be packaged within clefts, and efficient delivery into the host cytoplasm when clefts dock at the erythrocyte skeleton, a major target of these soluble secretome determinants. This may explain why even proteins such as PfHRPII that have no clear binding partner at the host skeleton nonetheless seem to be restricted to the periphery of the infected erythrocyte.11 Parasite membrane proteins sorted by a lumenal HT signal into clefts can be brought proximal to the erythrocyte membrane as is seen for the major membrane bound antigenic families of P falciparum.
Because the HT is present on 300 to 400 secretory proteins,2,19,24 clefts may thus provide a major conduit for parasite soluble and membrane protein export across the host cytoplasm. However, little is known about the cleft movement from the parasite to the erythrocyte periphery. This is expected to occur in absence of coat protein (COP)-coated vesicles that drive classic secretion in higher eukaryotes25 (consistent with the most recent evidence that parasite-encoded components of eukaryotic secretion are not exported to the erythrocyte26 ). Our data also suggest that cleft interactions with the erythrocyte skeleton via PfSBP1 or with putative substrates of PFE0055c in the erythrocyte may not play a significant role in cleft export across the host cytoplasm. In addition, these interactions have no effect on HT-mediated protein accumulation in clefts. In the case of PFE0055c, it is possible that redundant chaperone functions may exist,24 but for the present, it is the only putative parasite HSP40 shown to be exported to the erythrocyte.
Finally, although the HT signal mediates efficient transport of GFP from PVM to clefts, we find that few or no minimal reporters are delivered to the erythrocyte surface, suggesting that additional signals and transport structures may be needed for quantitative protein export from clefts to the host membrane. We would like to indicate that Knuepfer et al,21 like us, used minimal reporters. However, they reported surface exposure only in knobby strains (Figure 4A in Knuepfer et al21 ). In knobless strains (Figure 4C in Knuepfer et al21 ), they failed to detect surface exposure of the reporter. We do not understand why this should be the case, but all of our studies reported here were carried out in knobless parasites. Thus, with respect to surface exposure, there is no discrepancy between our findings and those of Knuepfer et al.21 It should also be noted that in their export to the erythrocyte and subsequent delivery of protein to the surface, clefts may associate with a variety of intraerythrocytic structures. This may provide one explanation for the range of intraerythrocytic protein export structures reported in the literature.27,28 Nonetheless, our present data strongly support the idea that sorting into clefts before crossing the PVM may be the critical secretory decision underlying HT-mediated export of hundreds of putative effectors across the cytoplasm of the mature erythrocyte infected by this major human pathogen.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Anjen Chenn and the Haldar laboratory for editorial advice.
This work was supported by grants from the National Institutes of Health (R01-HL69630 and R01-AI39071 to K.H. and R01-AI033656 and R12-AI070888 to J.H.A.) and the Burroughs Wellcome Fund (to J.H.A.).
National Institutes of Health
Authorship
Contribution: S.B. and K.H. designed the experiments, analyzed the data, and drafted the manuscript. S.B., C.v.O., and B.B. performed experiments and contributed to data analysis. B.B. and J.H.A. contributed new reagents and carried out insertion site analyses.
The present address for B.B. and J.H.A. is Global Health Infectious Diseases Research (GHIDR) Program, University of South Florida, College of Public Health, 3720 Spectrum Blvd, Interdisciplinary Research Building Suite 304, Tampa, FL 33612.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kasturi Haldar, Ward 3-240, Northwestern University, Feinberg School of Medicine, 303 East Chicago Ave, Chicago, IL 60611; e-mail: k-haldar@northwestern.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal