Antigen stimulation of naive T cells in conjunction with strong costimulatory signals elicits the generation of effector and memory populations. Such terminal differentiation transforms naive T cells capable of differentiating along several terminal pathways in response to pertinent environmental cues into cells that have lost developmental plasticity and exhibit heightened responsiveness. Because these cells exhibit little or no need for the strong costimulatory signals required for full activation of naive T cells, it is generally considered memory and effector T cells are released from the capacity to be inactivated. Here, we show that steady-state dendritic cells constitutively presenting an endogenously expressed antigen inactivate fully differentiated memory and effector CD8+ T cells in vivo through deletion and inactivation. These findings indicate that fully differentiated effector and memory T cells exhibit a previously unappreciated level of plasticity and provide insight into how memory and effector T-cell populations may be regulated.
Introduction
Upon antigen stimulation, naive T cells rapidly undergo a program of expansion and terminal differentiation that leads to generation of effector and memory T cells. Whereas most of the clonally expanded T cells die, apparently through selective death of effector cells,1,2 a small number (5%-10%) survive to generate memory cells.2 Activation of this terminal differentiation program generates a population of cells that, compared with naive T cells, exhibit faster response kinetics,3 increased avidity,4 and reduced or no dependence on costimulation.3,5 During this programming, antigen-specific T cells are therefore transformed from a naive population, possessing a highly malleable differentiation potential, to specialized committed memory and effector populations with little or no plasticity
Because of the high degree of plasticity exhibited by naive T cells, their end-stage differentiation is readily “educated” for a variety of effector functions in response to antigen-presenting cell (APC)– or environment-derived signals. In the absence of strong costimulatory signals, they are abortively activated, and naive CD8+ T cells that interact with resting dendritic cells (DC) presenting cognate antigen are deleted or inactivated.6,7 Although this is one pathway to CD8+ T-cell inactivation, studies of chronic viral infection or of transgenically targeted antigen expression have indicated that persistent antigenic stimulation is another route through which naive CD8+ T cells are inactivated. The molecular mechanisms that lead to, or maintain, naive CD8+ T-cell inactivation appear similar for both routes, with inhibitory signaling molecules such as CD5 and programmed death 1 (PD-1) being implicated as critical regardless of whether antigen is chronically expressed or targeted to resting DC.7,,,–11
Loss of costimulation dependence and developmental plasticity implies that memory T cells will not be subject to the same regulatory checkpoints as naive T cells, and it is unclear whether traits imposed by the terminal differentiation program active in memory and effector T-cell populations render these cells impervious to cell-intrinsic inactivation. CD8+ T-cell inactivation has invariably been studied under conditions in which “inactivated” or “tolerant” CD8+ T cells have differentiated from populations of naive T cells6,12 and in many systems, when the influence of “tolerising” antigen remains present13,14 thereby preventing acquisition of memory.15,16 Consequently, our knowledge of CD8+ T-cell inactivation reflects that of naive T-cell populations, and it is unknown whether fully differentiated memory cells are susceptible to inactivation in a fashion similar to naive T cells.
Memory T cells develop rapidly after antigen challenge during viral and other microbial infections.2,17 Similarly, upon initiation of autoimmune disease, autoaggressive memory and effector T cells develop rapidly and are readily detectable early in the preclinical phase of autoimmune diseases such as type 1 diabetes.18,19 Because the immunotherapeutic goal for autoimmune disease is restoration of antigen-specific tolerance, the presence of autoaggressive memory/effector T-cell populations present in ongoing autoimmune disease could represent a significantly greater hurdle for immunotherapy than autoreactive naive T cells. Indeed, memory CD8+ T-cell populations are reported to present a potent barrier to transplant tolerance induction.20,21 Therefore, it is imperative that we gain understanding of how established populations of memory and effector T cells can be inactivated. In the present study, we directly test, by transferring fully differentiated memory and effector CD8+ T cells, the outcome of tolerogenic antigen presentation by steady-state DC to memory T cells.
Methods
Animals
Mice were bred and maintained at the Biologic Research Facility, Princess Alexandra Hospital, Woolloongabba, or purchased from Animal Resources Center, Perth. OT-I mice carry a major histocompatibility complex (MHC) class I–restricted transgenic T-cell receptor (TCR) for the ovalbumin (OVA) peptide OVA257–26422 and were crossed with CD45.1 congenic C57Bl/6.SJL-Ptprca mice to generate mice bearing CD45.1+ OT-I cells. OT-II mice carry an MHC class II–restricted transgenic TCR specific for OVA323–339.23 OT-I.bim−/− mice have been described24 and were provided by Drs P. Bouillet and W. Heath (Walter and Eliza Hall Institute). 11c.OVA mice express a membrane-bound OVA construct under the control of the CD11c promoter, which targets OVA expression to CD11chi conventional DC.7 11c.OVA mice were backcrossed to C57Bl/6.SJL-Ptprca to obtain 11c.OVA mice expressing CD45.1. Nontransgenic controls for 11c.OVA mice were C57Bl/6 or C57Bl/6.SJL-Ptprca as appropriate. Mice were sex matched within experiments, and OT-I donor mice were used at 6 to 12 weeks of age. Animal studies were approved by an institutional ethics committee.
Cell preparation and adoptive transfers
For in vivo generation of memory CD8+ T cells, OVA-specific CD45.1+ OT-I mice were injected with OT-II T cells (5 × 106) to provide “help” and immunized subcutaneously (s.c.) with OVA/QuilA (100 μg OVA [grade 5; Sigma-Aldrich, St Louis, MO], 20 μg QuilA [Soperfos Biosector DK-Vedback, Denmark]). Forty to 60 days later, CD8+/CD44hi memory T cells were recovered from spleens and lymph nodes of these animals by fluorescence activated cell sorter (FACS). For in vitro generation of effector/memory T cells, lymph node cells from CD45.1+ OT-I mice were cultured in 6-well plates (2 × 106/mL) in 3 mL of complete RPMI (RPMI 1640 supplemented with 1 mM sodium pyruvate, 0.1 mM nonessential amino acids [all from Invitrogen, Carlsbad, CA], 50 μM 2-mercaptoethanol [Sigma-Aldrich]) with 1% normal mouse serum, 0.1 μg/mL OVA257–264 (Mimotopes, Melbourne, Australia) and 10 ng/mL interleukin-2 (IL-2; PeproTech, Rocky Hill, NJ). After 3 days, cells were harvested and washed (3×) with RPMI 1640 and recultured in 6-well plates at 2 × 106/mL in the absence of antigen but with 10 ng/mL IL-15 (PeproTech) for an additional 2 days. Cells were then harvested and washed (3×) in phosphate-buffered saline (PBS) before transfer to experimental mice. In all experiments, 2 × 106 OT-I T cells were transferred intravenously (i.v.). In some experiments, CD45.2+ OT-I.bim donors were used with CD45.1+ recipient mice. Anti-CD40 (FGK-45) was administered intraperitoneally (100 μg on days 0, 5, 10, 15, and 20 after injection) where indicated.
In vitro and in vivo assays
ELISpots and intracellular cytokine staining (ICS) were performed as described.7 Cytotoxic T lymphocyte (CTL) degranulation in vitro was measured as mobilization of the lysosomal markers CD107a and CD107b to the cell surface as described.25 To assess proliferation in vivo, in vitro–generated OVA-specific effector/memory CD8+ T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) as described26 and injected i.v. into 11c.OVA or nontransgenic control mice. Three days later, recipient spleens were harvested and CFSE dilution in CD45.1+/CD8+ cells was assessed by FACS. To determine responsiveness to antigen challenge, mice were immunized s.c. at the tail base with OVA/QuilA. In vivo CTL activity was determined as described previously,7 with the modification that antigen-pulsed cells were labeled with 0.5 μM CFSE, and unpulsed cells were labeled with 2.5 μM CFSE. For retransfer studies, CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA, and 42 days later, CD8+ cells were isolated from pooled spleens and lymph nodes by magnetic cell sorting separation and transferred to 11c.OVA or nontransgenic secondary recipients, which were challenged with OVA/QuilA or left unchallenged 21 days later.
Flow cytometry
Monoclonal antibodies (mAb) against CD8 (53-6.7), CD44 (IM7), CD45.1 (A20), CD62L (MEL-14), CD69 (H1.2F3), and PD-1 (RMP1–30) were purchased from BioLegend (San Diego, CA). mAb against CD127 (B12-1), TCR Vα2 (B20.1), CD107a (1D4B), and CD107b (ABL-93) were obtained from BD Pharmingen (San Diego, CA). For cytometric analysis of OVA-specific T-cell frequency and phenotype, spleens and lymph nodes were passed through 70-μm nylon mesh cell strainers, washed in PBS/2.5% fetal calf serum (FCS) and erythrocytes (for spleen cells) lysed in NH4Cl/Tris buffer. Livers and lungs were removed from PBS-perfused mice. Livers were pressed through 100-μm nylon mesh and lymphocytes enriched over a 30% to 35% discontinuous Percoll gradient. The hepatocyte-rich supernatant was aspirated and the pellet washed with RPMI plus 10% FCS. Erythrocytes (for spleen cells) were lysed with NH4Cl/Tris buffer. Lungs were chopped finely and digested with 1 mg/mL collagenase type III (Worthington Biochemicals, Freehold, NJ) and 1000 U/mL DNase (Roche Diagnostics, Indianapolis, IN) in 10 mL RPMI/2% FCS for 1 hour at 37°C. The resulting cell suspension was passed through a 70-μm nylon mesh cell strainer and washed with PBS/2.5% FCS. Immunofluorescence staining for flow cytometry was performed as described previously.27 Cytometric data were acquired on a FACScalibur or FACScanto (BD Biosciences). Absolute cell numbers were determined by a bead-based procedure.28
Statistical analysis
Comparison of means was performed using Student t test, and multiple groups were compared using 1-way analysis of variance followed by Newman–Keuls post test (GraphPad Software, San Diego, CA).
Results
Antigen targeted to DC inactivates long-lived memory CD8+ T cells
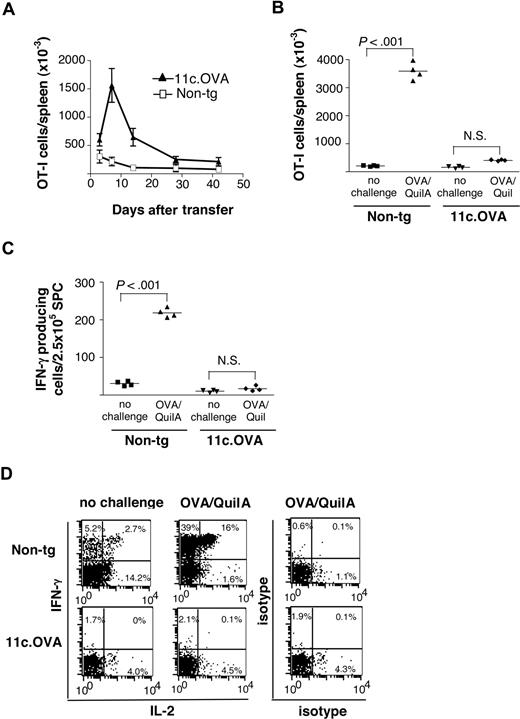
Memory T cells are largely costimulation-independent, and activation by steady-state DC might be expected to lead to expansion and acquisition of effector function. Alternatively, activation of memory CD8+ T cells by steady-state DC could lead to abortive activation, as seen for naive CD8+ T cells. To test these alternate possibilities, long-lived CD8+ memory T cells (OT-I) displaying a CD62Lhi central memory (TCM) phenotype (data not shown) specific for OVA were isolated from immunized mice and transferred to transgenic (11c.OVA) mice expressing OVA in DC under control of the CD11c promoter7 or to nontransgenic controls. After transfer, a large population of OT-I cells, which peaked in number 1 week after transfer, accumulated in the spleen of 11c.OVA recipients (Figure 1A). The number of OT-I T cells then diminished gradually, and by 28 days after transfer, a relatively stable population was established (Figure 1A). In contrast, in nontransgenic recipients, OT-I T cells showed no rapid expansion in spleen but declined in number gradually for 14 days after transfer, after which a relatively stable population was established (Figure 1A). From 28 days after transfer, the number of residual OT-I T cells remained approximately 4-fold greater in 11c.OVA than in nontransgenic recipients (Figure 1A).
Antigen-expressing DC inactivate long-lived memory CD8+ T cells. FACS-sorted CD45.1+ OT-I memory T cells (CD8+/CD44hi) were transferred to 11c.OVA or nontransgenic (non-tg) mice. (A) The total number of OT-I (CD45.1+/CD8+/Vα2+) T cells in spleens of 11c.OVA (▴) or non-tg (□) recipients was determined at 3, 7, 14, 28, and 42 days after transfer. Data (mean±SD) are pooled from 2 independent experiments of 2 mice per group at each time point. Error bars represent SD. (B,C) Thirty-five days after OT-I transfer, mice were challenged with OVA/QuilA s.c or left unchallenged, and 7 days later (B), the total number of OT-I (CD45.1+/CD8+/Vα2+) T cells in spleens was determined (C) or the frequency of OT-I cells secreting IFN-γ in response to OVA257–264 was determined by ELISpot assay. Data are pooled from 2 independent experiments with 2 mice per group. Horizontal bars represent mean values. (D) Thirty-five days after OT-I transfer, mice were challenged with OVA/QuilA s.c. or left unchallenged, and 7 days later, cytokine production by OT-I (CD45.1+/CD8+) cells was determined using ICS. Data are representative of 2 mice in each of 2 independent experiments. Numbers on plots are the percentages of cells in each quadrant (gated for CD8+, CD45.1+ cells).
Antigen-expressing DC inactivate long-lived memory CD8+ T cells. FACS-sorted CD45.1+ OT-I memory T cells (CD8+/CD44hi) were transferred to 11c.OVA or nontransgenic (non-tg) mice. (A) The total number of OT-I (CD45.1+/CD8+/Vα2+) T cells in spleens of 11c.OVA (▴) or non-tg (□) recipients was determined at 3, 7, 14, 28, and 42 days after transfer. Data (mean±SD) are pooled from 2 independent experiments of 2 mice per group at each time point. Error bars represent SD. (B,C) Thirty-five days after OT-I transfer, mice were challenged with OVA/QuilA s.c or left unchallenged, and 7 days later (B), the total number of OT-I (CD45.1+/CD8+/Vα2+) T cells in spleens was determined (C) or the frequency of OT-I cells secreting IFN-γ in response to OVA257–264 was determined by ELISpot assay. Data are pooled from 2 independent experiments with 2 mice per group. Horizontal bars represent mean values. (D) Thirty-five days after OT-I transfer, mice were challenged with OVA/QuilA s.c. or left unchallenged, and 7 days later, cytokine production by OT-I (CD45.1+/CD8+) cells was determined using ICS. Data are representative of 2 mice in each of 2 independent experiments. Numbers on plots are the percentages of cells in each quadrant (gated for CD8+, CD45.1+ cells).
Because population expansion and contraction is characteristic of T-cell responses generating either memory2 or tolerance,7 we next determined whether the residual OT-I T cells in 11c.OVA recipients comprised a population of secondary memory cells derived from the OT-I memory T cells that were initially transferred. Nontransgenic and 11c.OVA recipients were challenged with a highly immunogenic regimen of OVA/QuilA immunization 35 days after OT-I memory cell transfer and the resultant OT-I- T-cell expansion measured. In distinct contrast to extensive OT-I- T-cell expansion observed in nontransgenic recipients (18-fold; P < .001), no OT-I expansion occurred after OVA challenge of 11c.OVA recipients (Figure 1B). Furthermore, in vivo OVA/QuilA challenge failed to elicit an increase in the frequency of interferon-γ (IFN-γ)–producing OVA257–264-responsive T cells in spleens of 11c.OVA recipients in contrast to the substantial increase (P < .001) observed in nontransgenic recipients (Figure 1C). ICS showed that few OT-I T cells recovered from 11c.OVA recipients produced IFN-γ or IL-2, hallmark cytokines produced by memory CD8+ T cells, whereas a substantial proportion of those in nontransgenic recipients did, and this was substantially increased by antigen challenge (Figure 1D). Taken together, the data indicate that in contrast to nontransgenic recipients that maintained a strong memory response for at least 42 days in 11c.OVA mice, the in vivo responsiveness of OVA-specific memory CD8+ T cells to OVA challenge was ablated.
Antigen targeted to DC induces transient expansion of CD8+ effector/memory T cells
In the previous experiments, we used long-lived memory cells that displayed a TCM phenotype isolated from immunized mice. Because CD8+ memory populations normally comprise mixtures of TCM and effector memory (TEM) cells that exhibit contrasting effector and survival characteristics, we pondered whether such mixed populations would be similarly inactivated when antigen is targeted to DC. To address this question, we adapted widely used protocols29,30 and generated a mixed population of CD8+ TCM and TEM for transfer and analysis. OT-I T cells were first cultured with OVA257–264 and IL-2 for 3 days. Under these conditions, OT-I T cells expressed CD69 and high levels of CD44 but were largely CD62Llo, indicating CD8+ effector T-cell differentiation. Cells were then washed to remove antigen and IL-2 and cultured with IL-15 to promote TCM differentiation.31,32 On transfer to IL-15, OT-I T cells converted to a predominantly CD44hi/CD69−/CD62Lhi TCM phenotype within 4 days (Figure 2A). Based on these findings, we used OVA257–264/IL-2 activated OT-I cells transferred to IL-15 for 2 days, which comprised equal proportions of CD8+ TEM and TCM, for further analysis. These are referred to as OT-I effector/memory cells. After transfer to B6 mice, OT-I effector/memory cells efficiently killed syngeneic OVA257–264-pulsed targets in vivo and mounted strong recall responses to in vivo OVA challenge for at least 42 days after transfer (data not shown).
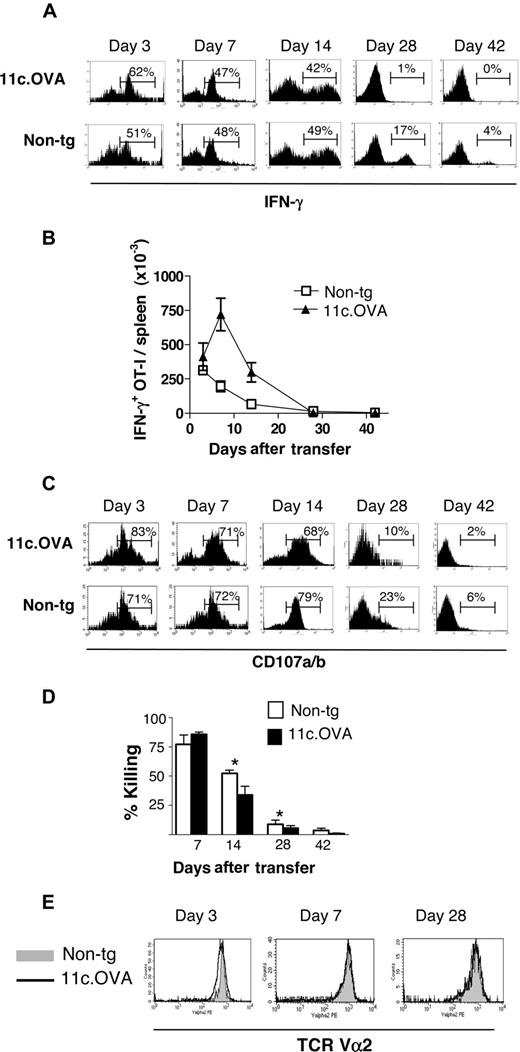
Antigen-expressing DC induce expansion of CD8+ effector/memory T cells followed by population contraction. (A) CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA (▴) or non-tg (□) recipients, and the total number of OT-I (CD45.1+/CD8+/Vα2+) cells in spleens was determined 3, 7, 14, 28, and 42 days after transfer. Data (mean±SD) are pooled from 2 independent experiments with 2 mice per group at each time point. (B) CD45.1+ OT-I effector/memory cells were labeled with CFSE and transferred i.v. to 11c.OVA or non-tg recipients. Three days later, spleen cells were analyzed by flow cytometry. Percentages indicate the proportion of OT-I T cells (CD45.1+/CD8+) that have divided. Data are representative of 2 mice per group from each of 2 independent experiments. (C) OT-I effector/memory cells (CD45.2+) generated from OT-I.bim−/− (▴,▾) or wild-type (wt) OT-I (■,□) mice were transferred to CD45.1+ 11c.OVA (open symbols [▴,□]) or non-tg littermate (closed symbols [▾,■]) recipients. Seven or 28 days after transfer, the total number of OT-I (CD45.2+/CD8+/Vα2+) T cells in spleens was determined. Data (mean±SD) are pooled from 2 independent experiments each with 2 mice per group.
Antigen-expressing DC induce expansion of CD8+ effector/memory T cells followed by population contraction. (A) CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA (▴) or non-tg (□) recipients, and the total number of OT-I (CD45.1+/CD8+/Vα2+) cells in spleens was determined 3, 7, 14, 28, and 42 days after transfer. Data (mean±SD) are pooled from 2 independent experiments with 2 mice per group at each time point. (B) CD45.1+ OT-I effector/memory cells were labeled with CFSE and transferred i.v. to 11c.OVA or non-tg recipients. Three days later, spleen cells were analyzed by flow cytometry. Percentages indicate the proportion of OT-I T cells (CD45.1+/CD8+) that have divided. Data are representative of 2 mice per group from each of 2 independent experiments. (C) OT-I effector/memory cells (CD45.2+) generated from OT-I.bim−/− (▴,▾) or wild-type (wt) OT-I (■,□) mice were transferred to CD45.1+ 11c.OVA (open symbols [▴,□]) or non-tg littermate (closed symbols [▾,■]) recipients. Seven or 28 days after transfer, the total number of OT-I (CD45.2+/CD8+/Vα2+) T cells in spleens was determined. Data (mean±SD) are pooled from 2 independent experiments each with 2 mice per group.
When OT-I effector/memory cells were transferred to 11c.OVA or nontransgenic recipients, the kinetic pattern of OT-I expansion and contraction (Figure 2A) was similar to that of transferred long-lived memory (Figure 1A). To determine whether proliferation contributed to OT-I accumulation CFSE-labeled OT-I effector/memory cells were transferred to 11c.OVA or nontransgenic mice. Three days later, when recovered from spleens of nontransgenic mice, few OT-I cells had divided, whereas almost all had undergone between one and several rounds of division in 11c.OVA recipients (Figure 2B). Therefore, we concluded that activation and expansion induced by OVA-expressing DC was followed by an extended period of population contraction in both long-lived TCM (Figure 1) and a mixed population comprised of TEM/effector cells and TCM (Figure 2).
Deletion contributes to contraction of CD8+ effector/memory T cells
Partial deletion of antigen-specific T cells after antigen-driven expansion is a normal component of immune homeostasis and tolerance induction. To ascertain the contribution of deletion to OT-I population contraction in 11c.OVA recipients, OT-I effector/memory cells derived from wild-type and deletion-resistant bim-deficient33 T-cell donors were compared. Seven days after transfer, at the peak of the expansion phase, the number of splenic OT-I T cells in 11c.OVA recipients was identical regardless of whether wild-type or bim-deficient OT-I effector/memory cells were transferred (Figure 2C). In contrast, late in the contraction phase, the number of OT-I T cells had diminished in 11c.OVA recipients of wild-type but not bim-deficient OT-I effector/memory cells (Figure 2C). Therefore, we conclude that bim-dependent deletion is induced in effector/memory T cells activated by antigen targeted to steady-state DC.
Antigen targeted to DC transiently induces effector function before CD8+ effector/memory T-cell inactivation
Because OT-I effector/memory cells were activated by antigen-expressing DC, we next tested whether this elicited effector function. Because rapid production of IFN-γ in response to antigen restimulation is a hallmark of CD8+ effector T cells, we first used ICS to track OT-I activation. Three days after transfer, the proportion of OT-I cells in 11c.OVA recipients that produced IFN-γ in vitro in response to OVA257–264 (Figure 3A) was modestly increased over that in nontransgenic recipients (62% ± 4% vs 53% ± 3%; P < .05). However, when coupled with the large increase in OT-I T cell number in 11c.OVA mice, the total number of IFN-γ–producing OT-I T cells was substantially higher in 11c.OVA than nontransgenic recipients 7 and 14 days after transfer (Figure 3B). However, 28 days after transfer, IFN-γ production by OT-I T cells had waned somewhat in nontransgenic recipients compared with early time points (3, 7, and 14 days after transfer) but was virtually undetectable in 11c.OVA recipients (Figure 3A). Similarly, the proportion of OT-I cells that degranulated in vitro in response to OVA257–264 peaked 3 days after transfer and then declined more rapidly in 11c.OVA relative to nontransgenic recipients. As a consequence, the proportion of OT-I T cells from 11c.OVA recipients that degranulated in vitro in response to OVA257–264 (69% ± 5%) was reduced relative to nontransgenic recipients (81% ± 7%; P < .05) by 14 days after transfer and remained lower at later time points.
Antigen-expressing DC inactivate CD8+ effector T cells. CD45.1+ OT-I effector/memory cells were transferred intravenously to 11c.OVA or nontransgenic (non-tg) recipients and (A-D) spleen cells were recovered 3, 7, 14, 28, or 42 days later. (A) IFN-γ production in response to OVA257–264 and (B) the total number of IFN-γ–producing OT-I cells per spleen was determined by ICS. (C) OT-I degranulation in vitro was determined at selected time points after transfer. Numbers on plots are the precentages of cells in each gate for that plot. (D) Killing of CFSE-labeled OVA257–264-pulsed targets was determined at selected time points after OT-I effector/memory transfer. *11c.OVA is significantly less than non-tg (P < .05). (E) TCR Vα2 levels on splenic OT-I cells (CD45.1+/CD8+) were determined by flow cytometry at selected time points after transfer. Data are either (A,C,E) representative of at least 2 mice in at least 2 independent experiments at each time point (B,D) or pooled (mean ± SD) from 2 independent experiments using 2 mice per group at each time point.
Antigen-expressing DC inactivate CD8+ effector T cells. CD45.1+ OT-I effector/memory cells were transferred intravenously to 11c.OVA or nontransgenic (non-tg) recipients and (A-D) spleen cells were recovered 3, 7, 14, 28, or 42 days later. (A) IFN-γ production in response to OVA257–264 and (B) the total number of IFN-γ–producing OT-I cells per spleen was determined by ICS. (C) OT-I degranulation in vitro was determined at selected time points after transfer. Numbers on plots are the precentages of cells in each gate for that plot. (D) Killing of CFSE-labeled OVA257–264-pulsed targets was determined at selected time points after OT-I effector/memory transfer. *11c.OVA is significantly less than non-tg (P < .05). (E) TCR Vα2 levels on splenic OT-I cells (CD45.1+/CD8+) were determined by flow cytometry at selected time points after transfer. Data are either (A,C,E) representative of at least 2 mice in at least 2 independent experiments at each time point (B,D) or pooled (mean ± SD) from 2 independent experiments using 2 mice per group at each time point.
To determine whether effector function was modulated in vivo, we next determined OVA257–264-specific CTL activity using a standard in vivo CTL assay. Substantial CTL activity was present in both nontransgenic and 11c.OVA recipients 7 days after OT-I effector/memory cell transfer (Figure 3D) but diminished more rapidly in 11c.OVA recipients and was significantly reduced relative to nontransgenic recipients 14 and 28 days after OT-I effector/memory transfer despite the larger number of OT-I T cells (Figure 2A), IFN-γ–producing OT-I T cells (Figure 3B) or OT-I T cells that degranulated in vitro in response to OVA257–264 (data not shown) in 11c.OVA mice at these times. However, by 42 days after transfer, CTL activity was barely detectable and did not differ between 11c.OVA and nontransgenic recipients (Figure 3D). TCR Vα2 expression on transferred effector/memory OT-I T cells did not differ between 11c.OVA and nontransgenic controls, indicating that the inability of these cells to respond to antigen was not attributable to TCR down-regulation (Figure 3E).
Collectively, the data indicate that in 11c.OVA recipients, despite substantial expansion and induction of activation-induced surrogate effector markers, no significant increase in overall CTL activity was seen, but CTL activity was significantly damped by 14 days after transfer. These paradoxical results indicate that despite activation, as indicated by IFN-γ production and in vitro degranulation in mice bearing antigen-expressing DC, the effector function of OT-I effector/memory cells was rapidly damped.
Antigen targeted to DC terminates recall responses from CD8+ memory T cells
Loss of CD8+ effector activity in 11c.OVA mice after transfer of OT-I effector/memory cells probably reflected loss of TEM or effector cells, but TCM with the ability to mount a recall response could have remained. Although the data presented in Figure 1 indicated this was unlikely, we next tested whether this was the case by challenging with OVA/QuilA. In nontransgenic recipients, OVA/QuilA challenge 21 or 35 days after OT-I effector/memory transfer elicited a substantial increase in both the frequency of IFN-γ–producing OVA257–264-responsive T cells (Figure 4A) and the total number of splenic OT-I T cells (Figure 4B) 1 week later, indicating expansion and induction of effector function. No increase in the frequency of IFN-γ–producing OVA257–264–responsive T cells was detected in 11c.OVA recipients challenged with OVA at either time point (Figure 4A). In contrast, OVA challenge of 11c.OVA recipients 21, but not 35, days after OT-I effector/memory transfer (Figure 4B) induced a small but consistent expansion of OT-I T cells, indicating that some proliferative capacity remained at this time point. To determine whether DC activation altered the outcome of OT-I effector/memory responses in 11c.OVA mice OT-I effector/memory, recipients were treated with agonistic anti-CD40 mAb or PBS (days 0, 5, 10, 15, and 20 after transfer) and left unchallenged or challenged with OVA/QuilA 21 days after transfer. In 11c.OVA recipients treated with anti-CD40, but not PBS, OVA challenge led to a substantial increase in the number of splenic OT-I T cells (Figure 4C) and IFN-γ production by these cells (data not shown) that resulted in a large, approximately 10-fold, increase in the total number of splenic IFN-γ–producing OT-I T cells compared with the approximately 90-fold increase seen in OVA-challenged untreated nontransgenic recipients (Figure 4C). Therefore, the presence of steady-state antigen-expressing DC sequentially damped the induction of effector cytokine production and proliferative capacity that would normally be characteristic components of a CD8+ memory T-cell recall response. In contrast, upon anti-CD40 activation, damping of the memory response was impaired, and memory was more sustained.
CD8+ memory T cells are inactivated by cognate antigen-expressing DC. CD45.1+ OT-I effector/memory cells were transferred intravenously to 11c.OVA or nontransgenic (non-tg) controls. Mice were left unchallenged or challenged with OVA/QuilA s.c. at 21 or 35 days after transfer. (A) The frequency of OT-I T cells in spleens secreting IFN-γ in response to OVA257–264 was determined by ELISpot assay 7 days after OVA/QuilA challenge. (B) The total number of OT-I (CD45.1+/CD8+/Vα2+) T cells in spleens of recipient mice was determined 7 days after OVA/QuilA challenge. Data are pooled from 2 independent experiments with 2 mice per group. (C) CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA or non-tg controls. Recipients were treated with anti-CD40 (FGK-45) or PBS on days 0, 5, 10, 15, and 20 after transfer and challenged or not with OVA/QuilA s.c. 21 days after transfer. The total number of OT-I (CD45.1+/CD8+/Vα2+) cells and the total number of IFN-γ–producing OT-I cells per spleen was determined by ICS and bead-based counting assays. Data are pooled from 2 independent experiments with 2 mice per group. The horizontal bars represent mean values.
CD8+ memory T cells are inactivated by cognate antigen-expressing DC. CD45.1+ OT-I effector/memory cells were transferred intravenously to 11c.OVA or nontransgenic (non-tg) controls. Mice were left unchallenged or challenged with OVA/QuilA s.c. at 21 or 35 days after transfer. (A) The frequency of OT-I T cells in spleens secreting IFN-γ in response to OVA257–264 was determined by ELISpot assay 7 days after OVA/QuilA challenge. (B) The total number of OT-I (CD45.1+/CD8+/Vα2+) T cells in spleens of recipient mice was determined 7 days after OVA/QuilA challenge. Data are pooled from 2 independent experiments with 2 mice per group. (C) CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA or non-tg controls. Recipients were treated with anti-CD40 (FGK-45) or PBS on days 0, 5, 10, 15, and 20 after transfer and challenged or not with OVA/QuilA s.c. 21 days after transfer. The total number of OT-I (CD45.1+/CD8+/Vα2+) cells and the total number of IFN-γ–producing OT-I cells per spleen was determined by ICS and bead-based counting assays. Data are pooled from 2 independent experiments with 2 mice per group. The horizontal bars represent mean values.
Antigen targeted to DC inhibits effector differentiation of residual cognate CD8+ TCM
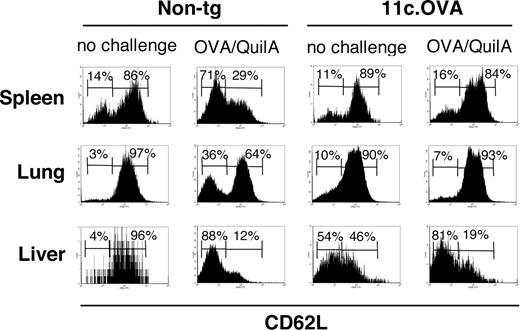
Our data suggested that after transfer of OT-I effector/memory cells, attrition of OVA-specific effector CD8+ T cells occurred rapidly in 11c.OVA mice and that a population of inactivated OT-I T cells remained. In contrast, in nontransgenic recipients, transferred effector cells persisted for longer periods in addition to the establishment of fully functional memory. Consistent with this, OT-I T cells in unchallenged nontransgenic recipients acquired predominantly CD44hiCD62Lhi phenotype by 42 days after transfer (Figure 5), indicating establishment of a long-lived TCM population. Notably, this phenotype predominated in lung and liver, indicating substantial loss of TEM (Figure 5). Similarly, in 11c.OVA recipients, despite the lack of functional recall responses, OT-I T cells in spleen and lung also exhibited a CD44hiCD62Lhi TCM phenotype. Interestingly, OT-I T cells in the livers of 11c.OVA recipients exhibited a predominantly CD62Llo phenotype, possibly reflecting clearance of apoptotic postactivated cells in this site.34 In nontransgenic recipients, OVA/QuilA challenge converted a large proportion of OT-I T cells to a CD44hiCD62Llo effector/TEM phenotype consistent with recruitment of TCM into the TEM pool (Figure 5). No change was observed in the TCM/TEM phenotype of OT-I T cells in response to OVA/QuilA challenge of 11c.OVA recipients, confirming our previous data that residual OT-I T cells in 11c.OVA mice were refractory to antigen challenge.
Inactivated CD8+ memory T cells fail to acquire an effector phenotype on antigen challenge. CD45.1+ OT-I effector/memory cells were transferred intravenously to 11c.OVA or nontransgenic (non-tg) controls. Mice were left unchallenged or challenged with OVA/QuilA s.c. at 35 days after transfer and the phenotype of OT-I T cells (CD45.1+/CD8+) in spleen, liver, and lung analyzed 7 days later. Data are representative of 2 mice per group in more than 2 independent experiments. Numbers on graphs are the percentages of cells in each gate for that plot.
Inactivated CD8+ memory T cells fail to acquire an effector phenotype on antigen challenge. CD45.1+ OT-I effector/memory cells were transferred intravenously to 11c.OVA or nontransgenic (non-tg) controls. Mice were left unchallenged or challenged with OVA/QuilA s.c. at 35 days after transfer and the phenotype of OT-I T cells (CD45.1+/CD8+) in spleen, liver, and lung analyzed 7 days later. Data are representative of 2 mice per group in more than 2 independent experiments. Numbers on graphs are the percentages of cells in each gate for that plot.
Inactivation of CD8+ memory T cells responses requires persistent antigen presentation
We next investigated whether inactivation of memory CD8+ T cells in mice bearing antigen-expressing DC was an adaptive process that required persistent antigenic stimulation to maintain the unresponsive state. We first determined whether antigen-expressing DC persisted after effector/memory T-cell transfer. Although a slight reduction in the number of splenic DC occurred in 11c.OVA mice (Figure 6A), coinciding with the peak effector response 7 days after memory T-cell transfer (Figure 3), suggestive of some CTL-mediated DC killing, DC were present in substantial numbers throughout the experimental period examined and returned to normal levels at the time of T-cell inactivation. Congenically marked (CD45.1+) OT-I effector/memory cells were then transferred to 11c.OVA mice, and 42 days later, CD8+ T cells, containing the inactivated population of OT-I cells, were isolated and a secondary transfer to 11c.OVA mice or OVA-free nontransgenic controls performed. Twenty-one days later, secondary transfer recipients were challenged with OVA/QuilA or left unchallenged, and 7 days later, the total number of splenic OT-I T cells and their ability to produce IFN-γ determined. In nontransgenic secondary transfer recipients, OVA/QuilA challenge induced a substantial expansion in the number of splenic OT-I T cells. Significantly, in 11c.OVA recipients, OVA/QuilA challenge did not lead to expansion of OT-I T cells (Figure 6B). Furthermore, OVA/QuilA challenge induced IFN-γ production detectable by ICS in OT-I T cells only in nontransgenic and not in 11c.OVA secondary recipients (Figure 6C). The data demonstrate that removal of CD8+ memory T cell from the environment containing antigen-expressing DC resulted in reinstatement of antigen responsiveness in vivo.
Persistent antigen presentation is required to maintain memory CD8+ T-cell inactivation. CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA or nontransgenic (non-tg) mice. (A) At 3, 7, and 28 days after transfer, the total number of splenic DC (CD11chi) in 11c.OVA (▴) and non-tg (■) recipients or untransferred 11c.OVA (▵) and non-tg (□) controls was determined. *11C.OVA + effector/memory OT-I is significantly reduced (P < .05) relative to 11c.OVA no transfer. (B,C) CD8+ cells were isolated from 11c.OVA mice 42 days after transfer and retransferred to 11c.OVA or non-tg secondary recipients. After 21 days, secondary recipients were challenged with OVA/QuilA or left unchallenged, and 7 days later, spleens were collected (B) and the total number of OT-I (CD45.1+/CD8+/Vα2+) T cells (C) and IFN-γ production by OT-I (CD45.1+/CD8+) T cells determined. Data (A,B) are pooled (mean±SD, or individual values) from 2 independent experiments with 2 mice per group (C) or representative of 2 individual mice for each group in each of 2 independent experiments. Horizontal bars in panel B represent mean values. Numbers on graphs in panel C are percentages of cells in each gate.
Persistent antigen presentation is required to maintain memory CD8+ T-cell inactivation. CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA or nontransgenic (non-tg) mice. (A) At 3, 7, and 28 days after transfer, the total number of splenic DC (CD11chi) in 11c.OVA (▴) and non-tg (■) recipients or untransferred 11c.OVA (▵) and non-tg (□) controls was determined. *11C.OVA + effector/memory OT-I is significantly reduced (P < .05) relative to 11c.OVA no transfer. (B,C) CD8+ cells were isolated from 11c.OVA mice 42 days after transfer and retransferred to 11c.OVA or non-tg secondary recipients. After 21 days, secondary recipients were challenged with OVA/QuilA or left unchallenged, and 7 days later, spleens were collected (B) and the total number of OT-I (CD45.1+/CD8+/Vα2+) T cells (C) and IFN-γ production by OT-I (CD45.1+/CD8+) T cells determined. Data (A,B) are pooled (mean±SD, or individual values) from 2 independent experiments with 2 mice per group (C) or representative of 2 individual mice for each group in each of 2 independent experiments. Horizontal bars in panel B represent mean values. Numbers on graphs in panel C are percentages of cells in each gate.
Discussion
After exiting the thymus, naive T cells exist as a relatively undifferentiated and highly plastic pool of cells awaiting the appropriate cues for terminal development. Individual naive T cells can follow several alternate terminal differentiation programs, including acquisition of effector function, deletion, inactivation, or conversion to regulatory or suppressive cells, depending on the accessory signals provided during activation. Naive T cells, by virtue of their highly plastic differentiation potential, are abortively activated by resting DC or other APCs that provide only weak accessory signals. Memory T cells, on the other hand, are terminally “programmed,” and it is unclear whether fully differentiated CD8+ memory T cells can be inactivated in a similar fashion. Here, using adoptive transfer of long-lived memory CD8+ T cells or a mixed population of memory and effector CD8+ T cells, we demonstrate that constitutive expression of cognate antigen by DC results in termination of memory CD8+ T-cell responses.
Although it has been shown recently that the CTL function of effector CD8+ T cells is inhibited by CD4+/CD25+ natural regulatory T cells,35,36 little is known of whether memory CD8+ T cell responses are regulated or terminated in other, cell-intrinsic ways, such as through T-cell inactivation. In fact, CD8+ T-cell inactivation has been studied almost exclusively under conditions in which “inactivated” or “tolerant” CD8+ T cells have differentiated from populations of naive T cells and mostly under conditions in which the influence of the “tolerising” antigen remains present, thereby preventing acquisition of memory.6,12,,,–16 Although in vivo administration of large doses of cognate peptide induces a program of cell death in naive and postactivated antigen-specific T cells,37,,–40 this process is unlikely to occur under normal physiologic conditions. Although evidence suggestive of deletion of postactivated CD4+ or CD8+ T cells after exposure to parenchymal antigen has been reported,40,41 our knowledge of CD8+ T-cell inactivation reflects that of naive T-cell populations, and it is unknown whether fully differentiated memory cells are susceptible to inactivation in a fashion similar to naive T cells; however, some studies conclude that memory T cells are resistant to inactivation.3,42 In our studies, when long-lived CD8+ memory T cells were activated in mice bearing steady-state DC expressing cognate antigen, they underwent a phase of expansion followed by partial deletion that left a population of residual T cells rendered unresponsive to further antigen stimulation. Thus, in response to antigen targeted to DC, CD8+ memory T cells undergo a process of abortive activation and peripheral tolerance induction remarkably similar to that described for naive CD4+ or CD8+ T cells.7,43 However, notably, inactivation of memory CD8+ T cells was somewhat slower than reported for naive CD8+ T cells.7 Strikingly, during this process, CD8+ memory T cells activated in 11c.OVA mice appeared to exert limited CTL activity relative to those in nontransgenic controls, suggesting this encounter inhibited CTL activity. Consistent with this, OT-I effector/memory cells divided more slowly than naive OT-I T cells after transfer to 11c.OVA mice (data not shown). Therefore, memory CD8+ T cells appear to undergo a limited program of effector differentiation and fail to fully acquire CTL activity before deletion and inactivation. Population contraction through deletion was a major contributor to termination of memory CD8+ T-cell responses in the present study. Consistent with previous studies on CD8+ T cells, deletion occurred via a pathway dependent on the proapoptotic bcl-2 family member bim and highlights the importance of the “intrinsic” or mitochondrial death pathway not only in contraction of primary CD8+ T cell responses after immunogenic44 or tolerogenic activation24 but also in termination of memory CD8+ T-cell responses.
Here, we found that both long-lived CD8+ TCM and a mixed population of CD8+ TCM and TEM were inactivated when antigen was targeted to DC, leading to the accumulation of a residual population of TCM-like cells with acquired unresponsiveness. These findings demonstrate that CD8+ memory T cells display a greater degree of functional plasticity than previously assumed, indeed, a level of plasticity sufficient to permit inactivation of the ability to acquire effector phenotype and function upon antigen challenge in vivo, a feature for which TCM would normally be considered “programmed.”
Chronic antigen stimulation of naive CD8+ T cells leads to functional inactivation8 and may be responsible for inactivation of virus-specific T cells resulting from chronic viral infection,45 in which the differentiation program of naive CD8+ T cells is diverted and CD8+ T cell memory fails to establish.15,16 Effector function of CD8+ T cells inactivated by chronic antigen stimulation can be restored by transfer to an antigen-free environment.8,14 Here, we show that persistent antigen presentation is required to maintain inactivation of residual antigen-specific CD8+ T cells that are derived from transferred effector/memory cells, indicating that T-cell intrinsic adaptive processes regulate not only naive but also memory CD8+ T cells. Expression of antigen genetically targeted to DC is maintained through DC renewal from differentiating hematopoietic progenitors. Under these circumstances, presentation of the targeted antigen can be maintained, even if some killing of antigen-expressing DC were to be mediated by activated antigen-specific CTL, as our data suggest, before their inactivation. However, our previous studies indicated that the presence of unresponsive OVA-specific CD8+ T cells does not prevent continued OVA presentation by DC.7 It is possible that killing of cognate antigen-expressing DC by antigen-specific CD8+ T cells contributes substantially to the inactivation of OVA-specific memory responses in 11c.OVA mice. It is also unclear whether persistent antigen presentation leads to an ongoing process of partial activation and deletion of residual antigen-specific CD8+ T cells or acts solely to maintain these cells in a globally unresponsive way. The preferential accumulation of TEM-phenotype OT-I cells in liver suggests this may be a site of clearance for postactivated apoptotic CD8+ T cells as proposed by others34 and would be consistent with a low rate of activation in the residual OVA-specific CD8+ T cells. However, it is also possible that OT-I could be locally activated in the liver,46 and this has not been ruled out here.
The relative roles of direct antigen presentation by nonhematopoietic parenchymal cells and cross-presentation by DC or other APC in CD8+ T-cell inactivation to ubiquitous self-antigens or during chronic viral infection is unclear. For CD4+ T cells, it is reasonable to assume that presentation mediated by DC or other specialized APC is required. In contrast, for CD8+ T cells, either direct presentation, for example, as shown for alloantigen presentation by keratinocytes,47 or cross-presenting APC could be responsible. Our data clearly demonstrate that expression of cognate antigen by DC alone is sufficient to terminate fully differentiated memory CD8+ T-cell responses as we have shown previously for naive CD8+ T cells;7 however, the role of costimulatory molecule-deficient parenchymal cells that might also be expected to drive CD8+ T-cell inactivation remains undetermined. Defining which cells are capable of “tolerogenic” antigen presentation will be important in understanding how CD8+ memory T-cell responses are potentially regulated.
Here, we show for the first time that constitutive expression of cognate antigen targeted to DC in the steady state inactivates fully differentiated memory CD8+ T cells in vivo through deletion and inactivation. Whereas persistent presentation of cognate antigen by steady-state DC, achieved here by genetic-targeting, inactivates memory CD8+ T cells, our data suggest other approaches used to target steady-state DC in vivo that lead to transient antigen presentation, such as antibody-antigen conjugates,6 would be unlikely to effectively silence memory CD8+ T-cell responses unless administered chronically. However, if used transiently, these approaches would be expected to boost CD8+ T-cell memory. Thus, our findings provide insight into potential approaches for therapeutic manipulation of CD8+ memory T-cell responses. Therapeutic application of antigen-expressing DC for autoimmune disease has been shown previously,48,49 and our findings suggest such an approach would be effective for targeting memory and effector T cells in established autoimmune disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Len Harrison, William Heath, Andreas Strasser, Phillipe Bouillet, Walter and Eliza Hall Institute, and Frank Carbone, University of Melbourne, for providing mice.
This work was supported by the Juvenile Diabetes Research Foundation, Princess Alexandra Hospital Foundation. R.J.S. was the recipient of a UQ Diamantina Institute for Cancer, Immunology, and Metabolic Medicine Research Fellowship.
Authorship
Contribution: T.J.K. performed experiments, collected and analyzed data, and prepared figures. R.T. provided intellectual input and advice. T.J.K. and R.J.S. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raymond J. Steptoe, Diamantina Institute for Cancer, Immunology and Metabolic Medicine, University of Queensland, Princess Alexandra Hospital, Level 4 R Wing, Building 1, Woolloongabba, 4102 Queensland, AUSTRALIA; e-mail: r.steptoe@uq.edu.au.

![Figure 2. Antigen-expressing DC induce expansion of CD8+ effector/memory T cells followed by population contraction. (A) CD45.1+ OT-I effector/memory cells were transferred i.v. to 11c.OVA (▴) or non-tg (□) recipients, and the total number of OT-I (CD45.1+/CD8+/Vα2+) cells in spleens was determined 3, 7, 14, 28, and 42 days after transfer. Data (mean±SD) are pooled from 2 independent experiments with 2 mice per group at each time point. (B) CD45.1+ OT-I effector/memory cells were labeled with CFSE and transferred i.v. to 11c.OVA or non-tg recipients. Three days later, spleen cells were analyzed by flow cytometry. Percentages indicate the proportion of OT-I T cells (CD45.1+/CD8+) that have divided. Data are representative of 2 mice per group from each of 2 independent experiments. (C) OT-I effector/memory cells (CD45.2+) generated from OT-I.bim−/− (▴,▾) or wild-type (wt) OT-I (■,□) mice were transferred to CD45.1+ 11c.OVA (open symbols [▴,□]) or non-tg littermate (closed symbols [▾,■]) recipients. Seven or 28 days after transfer, the total number of OT-I (CD45.2+/CD8+/Vα2+) T cells in spleens was determined. Data (mean±SD) are pooled from 2 independent experiments each with 2 mice per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-07-103200/3/m_zh80060813950002.jpeg?Expires=1765047149&Signature=tfb8CLBcsoasE8r3skpAZtJ2xjGKPHoYuGIHAujfQTXPE1xbDYPigushQpg2MNqJYj2FHZe2cnaN0VwXxiB~npVUbQdKDBP1Q6jWq3V2pt5U-g8mEwY~qiMqiPPIxqlFHiIhTIJMZYqQpYAQnVEiKxRTdU5-P0GQNC-Kr5b4Prh0YUUM5s-uBHyL6~kHSUwugjZ2yAIOk5in2~6~fBJCDoBjHRF7Rl~fuHXklh1H2iS3-B3NrL61cf5t0DFrZkh-REtPQLUrTMUn4El-eUHvsnIRmM8uxqAVVRCQi4fL8ExNVDAfmGmHpdBaF4yVTc3fLLnRU58X8anbRhpOXlFdug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal