To the editor:
Elliott and colleagues have reported in a group of 73 patients with acute myeloid leukemia (AML) that the time to clearance of peripheral blood blasts (PBB) during standard induction therapy is a strong predictor of both overall (OS) and relapse-free survival (RFS).1 We have previously shown2 in 30 AML patients that the kinetics of PBB clearance is a predictor of complete remission (CR). Thus, the 2 studies have in common the objective to obtain the maximum predictive information from the analysis of peripheral blood (ie, a much less invasive procedure than bone marrow aspiration); however, their results differ in several respects.
(1) The study reported by Elliott et al was retrospective whereas ours was prospective. (2) Elliott et al assumed that PBB clearance is a surrogate of in vivo chemosensitivity, but their study was carried out only on responder patients whose leukemic cells are, by definition, at least sufficiently chemosensitive for the patients to achieve CR; our study, instead, was carried out on unselected consecutive patients. (3) In the study by Elliott et al, PBB clearance was evaluated by differential count; in our study we identified by flow cytometry for each patient at the time of diagnosis a population of leukemic cells with aberrant immunophenotype (LAIP), and then determined absolute LAIP-positive blast counts on each of the first 5 days of treatment. Approval was obtained from Careggi Hospital institutional review board for this study. Informed consent was obtained in accordance with the Declaration of Helsinki.
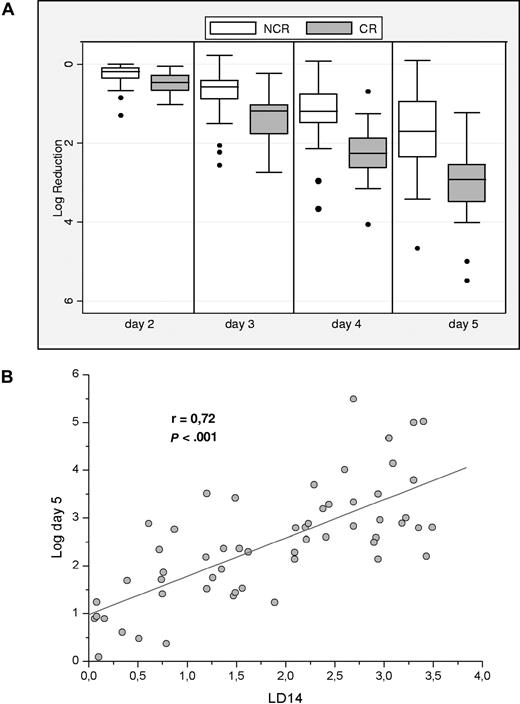
By our approach (having doubled our series2 to 61 patients), we have observed from day 2 (ie, within 24 hours from starting therapy) a clear dichotomy between responders and nonresponders (Figure 1A): indeed, the difference between the medians in the 2 groups is statistically significant from day 2. CR took place in 31 of 41 (76%) patients who had a reduction greater than 2 logs on day 5; but in only 1 of 20 (5%) patients who had a lesser reduction.
Relationship between PBB clearance and bone marrow response. (A) PBB clearance promptly resolves responders (CR) from nonresponder (NCR) patients. Log reduction is the ratio between baseline and daily absolute LAIP-positive blast count converted to a logarithmic scale. The ranges of log reduction show minimal overlap between the 2 groups. Horizontal bars are medians, boxes are 25th percentiles, and whiskers are 75th percentiles. Dots are outliers. (B) Bone marrow blast clearance correlates with PBB clearance. In this graph the log decrease in bone marrow LAIP-positive blasts (assessed by flow cytometry on day 14) is in linear relationship to log reduction of LAIP-positive blasts from peripheral blood at day 5 of induction treatment. In fact, a linear statistically significant correlation is found as from day 2.
Relationship between PBB clearance and bone marrow response. (A) PBB clearance promptly resolves responders (CR) from nonresponder (NCR) patients. Log reduction is the ratio between baseline and daily absolute LAIP-positive blast count converted to a logarithmic scale. The ranges of log reduction show minimal overlap between the 2 groups. Horizontal bars are medians, boxes are 25th percentiles, and whiskers are 75th percentiles. Dots are outliers. (B) Bone marrow blast clearance correlates with PBB clearance. In this graph the log decrease in bone marrow LAIP-positive blasts (assessed by flow cytometry on day 14) is in linear relationship to log reduction of LAIP-positive blasts from peripheral blood at day 5 of induction treatment. In fact, a linear statistically significant correlation is found as from day 2.
Unlike Elliott et al, in our series we do not yet have long-term follow-up data. However, because we found that peripheral blood LAIP-positive cell clearance correlates with bone marrow LAIP-positive residual disease (LD14: see Figure 1B), and residual disease in turn is known to correlate with RFS,3-5 it is reasonable to assume that PBB clearance will correlate with RFS. Thus, the combined data provided by Elliott et al and by our study demonstrate that from peripheral blood analysis it is possible to obtain strong predictors of both CR and RFS: in this respect the 2 studies are complementary. We concur therefore in the hope that PBB clearance could be of help also with respect to tailoring treatment.
Authorship
We thank Professor Lucio Luzzatto for his support and precious collaboration in manuscript reviewing.
Contribution: G.G. and F.M. designed research, performed cytofluorimetric analysis, analyzed the data, and wrote the paper; S.B. performed cytofluorimetric analysis and performed statistical analysis; F.L. and A.B. evaluated patients and reviewed the paper; and S.B. performed cytofluorimetric analysis.
G.G. and F.M. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Giacomo Gianfaldoni, Department of Haematology, University of Florence, Azienda Ospedaliera-Universitaria Careggi, 50134 Florence, Italy, e-mail: ggianfaldoni@libero.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal