Abstract
Clinical observations in patients undergoing bone marrow transplantation implicate the involvement of CD8+ cells in promoting the stem-cell engraftment process. These findings are supported by mouse transplant studies, which attributed the engraftment-facilitating function to subpopulations of murine CD8+ cells, but the analogous cells in humans have not been identified. Here, we report that clinical stem-cell grafts contain a population of CD8α+CD3ϵ+ T-cell receptor– negative cells with an engraftment facilitating function, named candidate facilitating cells (cFCs). Purified cFC augmented human hematopoiesis in NOD/SCID mice receiving suboptimal doses of human CD34+ cells. In vitro, cFCs cocultured with CD34+ cells increased hematopoietic colony formation, suggesting a direct effect on clonogenic precursors. These results provide evidence for the existence of rare human CD8+CD3+TCR− cells with engraftment facilitating properties, the adoptive transfer of which could improve the therapeutic outcome of stem-cell transplantation.
Introduction
Clinical success of bone marrow (BM) transplantation is critically dependent on rapid hematopoietic and immune reconstitution.1,2 Murine studies identified rare BM cells that enhance stem-cell engraftment and induce donor-specific tolerance. These facilitating cells (FCs) were defined as CD8+TCR− plasmacytoid dendritic cell precursors,3,4 or as a subset of CD8+CD3ϵ+ cells, which lack conventional αβ– and γδ–T-cell receptor (TCR) heterodimers.5,6 With mouse CD8+TCR−CD3+ FCs, CD3 is essential for enhanced reconstitution across the allogeneic barriers.6-8 Patients receiving grafts depleted of CD3+ lymphocytes to avoid graft-versus-host disease (GVHD) are at high risk of graft failure.9 The engraftment-facilitating function associated with CD8+ but not CD4+ T-cell subsets in both clinical transplantation10,11 and the NOD/SCID mouse xenotransplantation model.12 This indicates that the human CD8+ population, harboring alloreactive T cells responsible for GvHD,1 also contains cells with graft-facilitating capacity; however these immunologic properties have not yet been dissected in either clinical or experimental studies. We identified a rare population of human CD8+TCR−CD3+ cells and used the NOD/SCID assay to show their graft-facilitating potential
Methods
Sample collection, cell purification, and culture
Granulocyte–colony stimulating factor (G-CSF)–mobilized peripheral blood (mPB), cord blood (CB), and BM collections from healthy donors were approved by the Ethical Committee of University Hospitals Basel. Informed consent was obtained in accordance with the Declaration of Helsinki. Mononuclear cells (MNCs) were isolated by Ficoll-Histopaque (Sigma-Aldrich, St Louis, MO). CB CD34+ cells were isolated by magnetic-assisted cell separation (MACS; Miltenyi Biotec, Bergish Gladbach, Germany) to a purity of more than 98%. CD8+, CD8+TCR−, and CD8+TCR−CD3+ cells were purified from mPB by sorting with MoFlo (Dako, Fort Collins, CO). Colony forming unit (CFU) assays of CD34+ cells were performed in 1% methylcellulose containing stem-cell factor (SCF), interleukin (IL)-3, G-CSF, granulocyte-macrophage–CSF (GM-CSF), and erythropoietin (EPO)13 after coculture with CD8+ cells or subsets thereof for 2 hours at 37°C, in the presence of anti-flt3 ligand (Flt3L) monoclonal antibody (mAb; M5, Immunex, Seattle, WA) where indicated. CD8+TCR−CD3+ cells were maintained in RPMI/10% fetal calf serum (FCS) and IL-7 (100 ng/mL; Novartis Basel, Basel, Switzerland) for 5 days to measure Flt3L,14 and in IL-7 on anti-CD3 mAb-coated dishes for 3 days to isolate mRNA, or 14 days to examine cell phenotype.
Flow cytometric analysis
Fluorochrome-conjugated mAbs against CD8, CD3, TCRβ, CD2, CD5, CD7, CD44, CXCR4, CD25, CD127, CD45, CD19, CD33, CD34 (Becton Dickinson, San Jose, CA), TCRαβ (Serotech, Oxford, United Kingdom), TCRγδ (Immunotech, Marseille, France), or anti-Flt3L, were used. Intracellular TCRβ was stained after fixation with 1% PAF and permeabilization with 0.1% saponin.
RNA analysis
RNA was isolated with Trizol (Invitrogen, Carlsbad, CA), and quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) for interferon (IFN)–γ and RPL19 reference transcripts was as described.15
NOD/SCID mouse repopulation
Sublethally irradiated (350 cGy) 8-week-old mice were injected intravenously16 with 3 to 5 × 104 CB CD34+ cells without or with 6 to 10 × 104 mPB CD8+, CD8+TCR−, or CD8+TCR−CD3+ cells. Population comparisons within experiments were derived from the same mPB donor.
Statistical analysis
Student t test and GraphPad Prism4 software (GraphPad QuickCalcs Software, San Diego, CA) were used.
Results and discussion
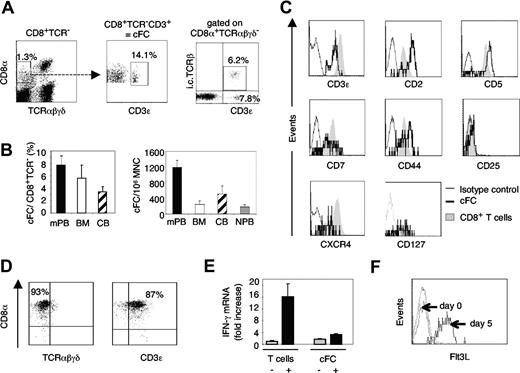
We identified a small but distinct subpopulation of human CD8α+ cells which lack cell-surface expression of TCR αβ and γδ but are CD3ϵ+, similar to murine CD8+TCR−CD3+ FCs (Figure 1A). The highest content of these cells, referred to as candidate FCs (cFCs), was in mPB, representing 7.7% (± 1.4%) of CD8+TCR− cells and amounting to 1200 (± 220) per 106 MNC; 2- to 5-fold lower cFC numbers were also present in human BM, CB, and normal PB (Figure 1B). Intracellular TCRβ-chain was detected in 40% of CD8+TCR−CD3+, but not CD8+TCR−CD3− cells (Figure 1A), which, along with the significantly lower expression of CD8α (Figure 1A) and CD3ϵ (Figure 1C) compared with conventional T cells, resembled the phenotypic features of murine FCs.6,7 Human cFCs expressed CD2, CD5, CD7, and CD44 at levels different from mature CD8+ T cells, carried CXCR4 and CD127 (IL-7 receptor α chain), but not CD25 (Figure 1C). To test if cFCs were conventional T cells that have temporarily down-regulated cell-surface TCR, cFCs were purified from mPB and cultured for 14 days on anti-CD3 antibody-coated plates with IL-7. The CD8α+TCRαβγδ−CD3ϵ+phenotype was well preserved (Figure 1D). Upon CD3 and IL-7 receptor stimulation, IFN-γ mRNA expression was not increased in cFC, unlike in mature T cells (Figure 1E), whereas cell-surface Flt3L was significantly up-regulated (Figure 1F), as described for T cells.14 Thus, human CD8+TCR−CD3+ cells share properties with conventional T cells, yet are a distinct cellular entity. Low levels of CD3ϵ expression without cell-surface TCR attributes to cFC a characteristic of immature T cells of either thymic17 or BM18 origin, a feature postulated also with murine FCs.4-6
Human cFCs are present in clinical stem-cell sources and are distinct from conventional T lymphocytes. (A) Representative FACS analysis of CD8+TCR−CD3+ cFCs in mPB, intracellular (i.c.) TCRβ chain expression by cFCs. Numbers are percentage of total MNC (left plot) or of the gated population, as indicated (middle and right panels). (B) The content of cFCs shown as % of CD8+TCR− cells or per 106 MNC (mean ± SEM) in mPB (n = 24), BM (n = 3), CB (n = 3), and normal PB (NPB, n = 8). (C) FACS analysis (using FACS Calibur and CellQuest software, Beckton Dickinson Biosciences, San Jose, CA) of cell-surface markers expressed by cFCs (solid line) compared with conventional CD8+ T cells (shaded area); broken line, staining with IgG1 isotype control mAbs. Further phenotypic analysis of cFCs showed absence of early hematopoietic and lineage markers CD34, CD56, CD10, and CD19 (data not shown). (D) FACS analysis of cFCs purified by FACS sorting and cultured for 14 days on anti-CD3 mAb-coated plates in the presence of IL-7. The CD8+TCR−CD3+ phenotype was maintained in 80% to 90% of cultured cells. Numbers on plots are percentages of total cells. (E) Quantitative real-time RT-PCR analysis of IFN-γ mRNA (mean ± SEM) in conventional T cells and cFCs, resting (−) and after 3 days culture on anti-CD3 mAb-coated plates in the presence of IL-7 (+). IFN-γ mRNA expression level in relation to resting T cells, defined as 1. IFN-γ primer sequences: forward 5′-TCAGCTCTGCATCGTTTTGG-3′, reverse 5′-GTTCCATTATCCGCTACATCTGAA-3′. Levels of mRNAs encoding Flt3L, SCF, TGF-β, SDF-1, and IFN-α in resting cFCs and conventional T cells were not significantly different (not shown). (F) FACS analysis of cell-surface Flt3L by purified cFCs cultured with IL-7 for 5 days.
Human cFCs are present in clinical stem-cell sources and are distinct from conventional T lymphocytes. (A) Representative FACS analysis of CD8+TCR−CD3+ cFCs in mPB, intracellular (i.c.) TCRβ chain expression by cFCs. Numbers are percentage of total MNC (left plot) or of the gated population, as indicated (middle and right panels). (B) The content of cFCs shown as % of CD8+TCR− cells or per 106 MNC (mean ± SEM) in mPB (n = 24), BM (n = 3), CB (n = 3), and normal PB (NPB, n = 8). (C) FACS analysis (using FACS Calibur and CellQuest software, Beckton Dickinson Biosciences, San Jose, CA) of cell-surface markers expressed by cFCs (solid line) compared with conventional CD8+ T cells (shaded area); broken line, staining with IgG1 isotype control mAbs. Further phenotypic analysis of cFCs showed absence of early hematopoietic and lineage markers CD34, CD56, CD10, and CD19 (data not shown). (D) FACS analysis of cFCs purified by FACS sorting and cultured for 14 days on anti-CD3 mAb-coated plates in the presence of IL-7. The CD8+TCR−CD3+ phenotype was maintained in 80% to 90% of cultured cells. Numbers on plots are percentages of total cells. (E) Quantitative real-time RT-PCR analysis of IFN-γ mRNA (mean ± SEM) in conventional T cells and cFCs, resting (−) and after 3 days culture on anti-CD3 mAb-coated plates in the presence of IL-7 (+). IFN-γ mRNA expression level in relation to resting T cells, defined as 1. IFN-γ primer sequences: forward 5′-TCAGCTCTGCATCGTTTTGG-3′, reverse 5′-GTTCCATTATCCGCTACATCTGAA-3′. Levels of mRNAs encoding Flt3L, SCF, TGF-β, SDF-1, and IFN-α in resting cFCs and conventional T cells were not significantly different (not shown). (F) FACS analysis of cell-surface Flt3L by purified cFCs cultured with IL-7 for 5 days.
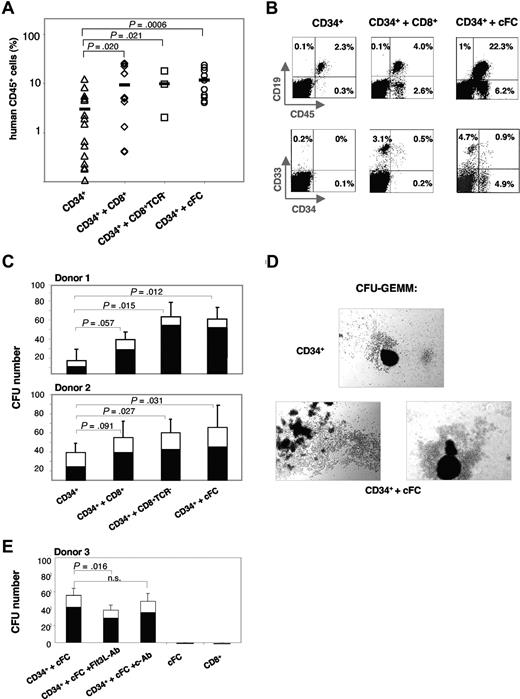
To assess the potential of cFCs to facilitate stem-cell engraftment, we used a NOD/SCID xenotransplantation model. Suboptimal dose of 3 to 5 × 104 CB CD34+ cells were transplanted alone (controls) or with 6 to 10 × 104 purified allogeneic CD8+ cells and subsets thereof (Figure 2A). At 8 weeks, the BM repopulation in control mice was highly variable with 3.0% (± 0.9%) human CD45+ cells. Cotransplantation of CD8+, CD8+TCR−, or cFCs increased the engraftment to 9.4% (± 2.7%; P = .02), 9.9% (± 4.6%; P = .02) and 11.7% (± 2.3%; P < .001), respectively. Importantly, the content of human CD45+ cells was never below the 1% threshold in mice that also received transplants of CD8+TCR− cells or cFCs (0/3 or 0/10, respectively), in contrast to 7/16 (44%) controls, or 2/12 (17%) mice that received unfractionated CD8+ cells (Figure 2A). While the percentage of human cells in the NOD/SCID BM increased with cFC cotransplantation (Figure 2B), the proportion of CD19+ B cells and CD33+ myeloid-lineage cells remained constant among human cells in all experimental groups (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Interestingly, the CD34+ hematopoietic progenitors constituted 18.8% (± 3.8%) of CD45+ cells in mice that received cFC cotransplants, which was significantly higher than in controls (P = .008; Figure S1A). NOD/SCID spleens were repopulated with human CD45+ cells at low efficiency (0.5%-1.5%) without or with cFCs (Figure S1B), indicating that cFCs preferentially promote the long-term establishment of human hematopoiesis in the BM.
Human mPB-derived cFCs enhance repopulation of hematopoietic CD34+ progenitors in NOD/SCID mice in vivo and clonogenicity in methylcellulose in vitro. (A) The content of human CD45+ cells in NOD/SCID BM at 8 weeks after transplantation with CD34+ cells alone (n = 16) or together with CD8+ cells (n = 12), CD8+TCR− cells (n = 3) or cFCs (n = 10), as indicated. Bar indicates mean repopulation efficiency. Cells are derived from 3 CB and 3 mPB donors. (B) Representative FACS analysis of human hematopoietic lineages in NOD/SCID BM. Percentages in FACS plots refer to lineage marker–positive cells of total cells in the BM. (C) CFU assay of CB CD34+ cells (250 cells) preincubated for 2 hours at 37°C alone or with CD8+ cells, CD8+TCR− cells or cFCs (750 cells each) from 2 mPB donors. CFU-GM and CFU-GEMM, in triplicates or quadruplicates, were counted after 14 days. SEM and P values refer to total colony numbers. Specifically for the CD34+ plus cFC group, P values of CFU-GEMM were less than .001 (Donor 1) and less than .01 (Donor 2), and of CFU-GM were greater than 0.5. (D) Large GEMM-CFU–derived colonies formed after coculture with cFCs are shown. Micrographs were acquired by imaging methylcellulose cultures with a Leica microscope (Leica Microsystems, Wetzlar, Germany) fitted with 100× objective, DS-5M camera head, and camera control unit DS-L1 image acquisition software (Nikon, Tokyo, Japan). (E) CFU assay of CB CD34+ cells (250 cells) preincubated for 2 hours at 37°C with cFCs (750 cells), without and with anti-Flt3L (Flt3L-Ab) or control mAb (c-Ab), and CFU assay of cFCs or CD8+ cells alone, as indicated. Preincubation with Flt3L-Ab did not inhibit growth of CD34+ cells alone (not shown). CFU-GM and CFU-GEMM, in triplicates were counted after 14 days. SEM and P values refer to total colony numbers; n.s., not significant.
Human mPB-derived cFCs enhance repopulation of hematopoietic CD34+ progenitors in NOD/SCID mice in vivo and clonogenicity in methylcellulose in vitro. (A) The content of human CD45+ cells in NOD/SCID BM at 8 weeks after transplantation with CD34+ cells alone (n = 16) or together with CD8+ cells (n = 12), CD8+TCR− cells (n = 3) or cFCs (n = 10), as indicated. Bar indicates mean repopulation efficiency. Cells are derived from 3 CB and 3 mPB donors. (B) Representative FACS analysis of human hematopoietic lineages in NOD/SCID BM. Percentages in FACS plots refer to lineage marker–positive cells of total cells in the BM. (C) CFU assay of CB CD34+ cells (250 cells) preincubated for 2 hours at 37°C alone or with CD8+ cells, CD8+TCR− cells or cFCs (750 cells each) from 2 mPB donors. CFU-GM and CFU-GEMM, in triplicates or quadruplicates, were counted after 14 days. SEM and P values refer to total colony numbers. Specifically for the CD34+ plus cFC group, P values of CFU-GEMM were less than .001 (Donor 1) and less than .01 (Donor 2), and of CFU-GM were greater than 0.5. (D) Large GEMM-CFU–derived colonies formed after coculture with cFCs are shown. Micrographs were acquired by imaging methylcellulose cultures with a Leica microscope (Leica Microsystems, Wetzlar, Germany) fitted with 100× objective, DS-5M camera head, and camera control unit DS-L1 image acquisition software (Nikon, Tokyo, Japan). (E) CFU assay of CB CD34+ cells (250 cells) preincubated for 2 hours at 37°C with cFCs (750 cells), without and with anti-Flt3L (Flt3L-Ab) or control mAb (c-Ab), and CFU assay of cFCs or CD8+ cells alone, as indicated. Preincubation with Flt3L-Ab did not inhibit growth of CD34+ cells alone (not shown). CFU-GM and CFU-GEMM, in triplicates were counted after 14 days. SEM and P values refer to total colony numbers; n.s., not significant.
Using the CFU assay, we compared the effect of CD8+, CD8+TCR−, and cFCs on clonogenicity of CB CD34+ progenitors in vitro. Colony formation increased up to 4.5-fold after preincubation with cFCs or CD8+TCR− cells (Figure 2C). Moreover, the CFU-GEMM (granulocyte-erythrocyte-monocyte-megakaryocyte) formed by early multipotent hematopoietic progenitors were increased preferentially both in numbers (Figure 2C) and size (Figure 2D). Preincubation with anti-Flt3L mAb before CFU assay reduced the effect of cFCs by 20%; CD8+ cells and cFCs cultured alone did not give rise to colonies (Figure 2E). These results show that cFCs have a direct stimulatory effect on clonogenic precursors, similarly to murine CD8+TCR− FCs.19 Taken together, our data on increased colony formation (Figure 2C-E) and enhanced numbers of human hematopoietic cells in transplanted mice (Figure 2A,B), along with a potential relevance of CXCR4, IL-7 receptor and cell-surface Flt3L expression (Figure 1C,F), support a cFC tropic effect on stem cell homing and retention in the BM environment, suggested previously with murine FCs and human CD8+ cells.12,19
To investigate the clinical relevance of cFCs, we evaluated the transplantation parameters in individual donor-recipient pairs (n = 24; Table S1). Neither engraftment nor development of GvHD correlated with the number of infused CD8+TCR− cells or cFCs. This, however, may be due to insufficient cFC numbers in clinical grafts (ratio of 1:24; cFC:CD34+ cells), whereas experimental transplantations were performed with at least 2:1 ratio of human cFCs or murine FCs4,6,7 to CD34+ cells. While culture conditions for significant expansion of murine FCs20 or human cFCs (our unpublished data) have not been established, Flt3L-driven mobilization4,19,20 might provide an alternative to adoptive transfer of ex vivo expanded cFCs, and will be important for elucidating mechanisms of their function.
This work is the first description of a specific CD8+TCR−CD3+ cell subset capable of facilitating human stem-cell engraftment in the myeloablated recipient. The unique ability of CD8+TCR−CD3+ cells to enhance donor-type reconstitution in the absence of allogeneic HLA-TCR recognition suggests that identification of human cFC can have a significant impact in optimizing BM transplantation to overcome crucial problems of engraftment failure.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. Kohler for expert cell sorting, S. Sendelov for technical assistance, A. Genitsch and G. Stüssi for statistical evaluation of clinical data, and G. De Libero and E. Palmer for helpful comments on the manuscript.
This work was supported by grants from the Swiss National Science Foundation (3100-067072 and -110511), Novartis Stiftung 03C56, and Stiftung für Hämatologische Forschung.
Authorship
Contribution: S.B., L.K., C.P.K. and E.S. performed research, analyzed data, and edited the paper. E.B.-P. performed research and collected and analyzed data. A.G. provided clinical data. A.W.-F. designed research, analyzed data, and wrote and edited the paper.
Conflict-of-interest-disclosure: The authors declare no competing financial interests.
Correspondence: Aleksandra Wodnar-Filipowicz, Department of Research, University Hospital Basel, Hebelstrasse 20, CH-4031 Basel, Switzerland; e-mail: Aleksandra.Wodnar-Filipowicz@unibas.ch.
References
Author notes
S.B., L.K., and E.B.P. made equal contributions to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal