Abstract
The inherited skeletal dysplasia osteogenesis imperfecta (OI) results in multiple fractures and is currently treated empirically. We transplanted human first-trimester fetal blood mesenchymal stem cells (MSCs) into homozygous oim mice in utero. This resulted in a two-thirds reduction in long bone fractures (P < .01), with fewer fractures per mouse (median 1, range 0-2 in mice that received transplants vs median 3, range 1-5 in mice that did not receive transplants by 12 weeks, P < .01). Nearly all mice that did not receive transplants had fractures (47 [97.9%] of 48), in contrast to 17 (58.6%) of 29 4- to 12-week-old mice that received transplants (P < .01). Transplantation was associated with increased bone strength (P < .01), thickness (P < .01), and length (P < .01), and normalization/reduction of growth plate height in 4- to 12-week-old oim was reduced in mice that underwent transplantion (P < .001). More donor cells were found in bone tissues compared with other organs (P < .001), with cells clustered in areas of active bone formation and remodeling, and at sites of fracture healing. Donor cells found in the bone expressed osteoblast lineage genes, and produced the extracellular bone structural protein osteopontin. Finally, MSC transplantation decreased bone hydroxyproline content. In conclusion, intrauterine transplantation of fetal MSCs markedly reduced fracture rates and skeletal abnormalities in a mouse model of the intermediate severity type III OI, suggesting a scientific basis for MSC treatment of affected human fetuses.
Introduction
Osteogenesis imperfecta (OI) is due to abnormal collagen production by osteoblasts due to mutations in the α chains of collagen type I and characterized by osteopenia and bone fragility. Severity ranges from the perinatally lethal type II to the nondeforming type I. The intermediate-severity type III is progressively deforming, with recurrent fractures from or before birth, short stature, and kyphoscoliosis predisposing to premature respiratory death.1 Current orthopedic management is empirical, relying on the use of biphosphonates to inhibit osteoclasts, and hence increase bone density and reduce fracture frequency.2 No treatment addresses the underlying collagen defect, and in pregnancy termination remains the only option for prenatal OI.
Stem cell therapy could replace defective osteoblasts with wild-type cells producing normal collagen type 1. In humans, infusion of allogeneic whole bone marrow3,4 and/or subsequent bone marrow mesenchymal stem cells (MSCs)5 was associated in 5 of 6 children with type III OI with increased bone mineral content, improved linear growth, reduced fracture frequency and improved growth velocity.3,5,6 An anecdotal report of an attempt at rescue therapy in an affected human fetus by transplanting fetal liver MSCs noted better than expected progress in childhood, although this was confounded by concomitant biphosphonate therapy.7
Such approaches have not been substantiated experimentally, although adult murine MSCs injected into neonatal oim mice have been shown to migrate to sites of bone formation.8 Oim mice (B6C3fe-a/a-oim), a model of human type III, have a G deletion at nucleotide 3983 in COL1A2 resulting in the absence of normal heterotrimeric collagen α1(I)2α2(I)1, replaced by homotrimeric α1(I)3, which accumulates in the extracellular matrix.9 Homozygous oim have reduced bone strength, multiple fractures, skeletal deformities, reduced long bone length and thickness, enlarged growth plate, and diminished bone volume.9-11
A fetal-to-fetal approach to cell therapy has several advantages. First, fetal MSCs are more primitive than adult MSCs,12,13 and potential vehicles for ex vivo gene therapy.14 Second, capitalizing on the fetuses' small size and immunologic naiveté, cell are delivered in utero into an environment which facilitates stem-cell expansion and engraftment. Third, prenatal administration may prevent organ injury before irreversible damage.15 We investigated the effect of transplanting human first-trimester fetal blood MSCs in utero on bone pathology in homozygous oim mice.
Methods
Human samples
Fetal blood collection was approved by the Research Ethics Committee of Hammersmith & Queen Charlotte's Hospital in accordance with the Declaration of Helsinki. Blood (gestation 10 weeks and 4 days) was collected by cardiocentesis under ultrasound guidance.16 Cells were selected by adherence, cultured in Dulbecco modified Eagle medium–high glucose (Invitrogen, Paisley, United Kingdom) supplemented with 10% fetal bovine serum (BioSera, Ringmer, United Kingdom), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen), and expanded at 10 000 cells/cm2 at 37°C with 5% CO2.
Immunohistochemistry
Fetal MSCs were fixed in 4% PFA in 125 mM HEPES (pH 7.6; 10 minutes at 4°C), refixed in 8% PFA (50 minutes at 4°C), and permeabilized in 0.5% Triton X-100 in phosphate-buffered saline (PBS; 30 minutes).17 Cells were blocked (1 hour) with PBS+ (PBS with 1% bovine serum albumin [BSA], 0.2% fish skin gelatin, and 0.1% casein; pH 7.6), incubated (2 hours) with primary antibodies in PBS+, washed (1.5 hours) in PBS+, incubated (1 hour) with secondary antibodies, and washed (4°C) before being mounted in DAPI-Vectashield media (Vector Laboratories, Burlingame, CA) and visualized under fluorescence (Zeiss Axioscope I microscope [Jena, Germany] with CCD camera and iPlab software (BD Biosciences, Rockville, MD). Raw TIFF images were collected without saturation of the intensity signal and analyzed in Adobe Photoshop (Adobe Systems, San Jose, CA) without further thresholding or filtering (eg, no background substraction).
Primary antibodies used were: vimentin, CD34, CD45, CD14, CD44, CD29, CD90, CD105, CD 73, STRO-1, hTERT (all from Abcam, Cambridge, United Kingdom), mouse oct-4, and rabbit nanog (both from Santa Cruz Biotechnology, Santa Cruz, CA), all at 1:10 to 1:100 dilution. Secondary antibodies were donkey anti-mouse or anti-rabbit IgG conjugated with FITC or Cy3 (1:100-1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA).
Transduction
Fetal MSCs were transduced with the bicistronic lentiviral vector (p.EF1α.RLuc.ppt.IRES.eGFP.Wpre from F. Marini, MD Anderson Cancer Center Houston, Texas) expressing the renilla luciferase gene (RLuc) as previously described.14 Transduction did not affect growth rate or osteogenic differentiation.
Animals
All experimental protocols complied with Home Office guidelines (PPL 70/5857). Heterozygous male and female (B6C3Fe a/a-Col1a2oim/Col1a2oim) mice (The Jackson Laboratory, Bar Harbor, ME) were housed in filter cages with a 12:12-hour light-dark cycle (21°C), with water and chow (Purina, St Louis, MO) ad libitum. Offspring were genotyped by sequencing the oim fragment,9 and homozygous colonies were established. Progeny were weaned at 30 plus or minus 1 days.
Intrauterine transplantation
After mating virgin oim/oim females (3-4 months old) with oim/oim males, pregnant mice underwent intrauterine transplantation (IUT) at embryonic day (E) 13.5 to E15. We performed a midline laparotomy on isoflurane-anesthetized females, and exteriorized the uterine horns one at a time moisturized with warm PBS. Each fetus was injected intraperitoneally with 106 fetal blood MSCs (passages 3-6) under direct vision using a 33-G Hamilton Microlitre syringe (Bonaduz, Switzerland). Uterine horns were replaced, and the wound was closed.
Optical bioluminescence imaging
Images were acquired on postmortem tissues. Animals were injected intraperitoneally with colenterazine substrate (100 μL of 100 mg/mL in PBS; Promega, Madison, WI). After 15 minutes, mice were culled, and tissues were harvested and imaged with the Xenogen IVIS 100 system (Xenogen, Cranbury, NJ). Images were acquired with a 15-cm field of view, a binning factor of 10, and an exposure time of 5 minutes. Negative controls included (1) oim/oim mice that received transplants without substrate injection (2) oim/oim that did not receive transplants after substrate injection; and (iii) oim/oim mice that received transplants of nontransduced cells and were given substrate injection. The photon image was superimposed on a video image of the organs with the Xenogen livingimage v2.50 (Xenogen) and IGOR Carbon v4.09A (Wavemetrics, Lake Oswego, OR) image analysis software. A region of interest (ROI) was manually placed, with the ROI size constant across all images of a given organ.
Counting of fractures
Fractures were assessed first by 1 unblinded observer, and then confirmed independently by 3 observers blinded as to whether or not each particular mouse had undergone transplantation. Fractures were delineated by callus formation, and each bone was graded visually as fractured or intact. Fracture incidence (fractured/total bones assessed) was calculated per bone, and per mouse.
Real-time quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen), and cDNA were synthesized using Pd(N)6 random hexamers (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) and 1 μL of 200 U murine Moloney leukemia virus (M-MLV) reverse transcriptase in the presence of dNTPs (10 minutes at 25°C, 60 minutes at 42°C, and 10 minutes at 75°C; Promega). Quantitative reverse transcription–polymerase chain reaction (qRT-PCR) was performed with the ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA), and all reactions were carried out in duplicate in a total volume of 25 μL. We used primers amplifying sequences of the β-actin gene (accession number: NM_001101) present in humans and not in mouse to determine the amount of human cellular cDNA in samples (primer specificity confirmed by absence of amplification of mouse cDNA) and primers common to both man and mouse to determine the total cDNA in each sample. For both sets, the absence of dimer formation was confirmed using Dissociation Curves 1.0 software (Applied Biosystems). Human-to-mouse chimerism was estimated as a ratio. Serial dilution of human in mouse cells formed the calibration curves. The primer sequences were as follows: human-specific, 5′-CTGGAACGGTGAAGGTGACA-3′ and 5′-AAGGGACTTCCTGTAACAATGCA-3′; and nonspecific, 5′-GCTCCTCCTGAGCGCAAGTA-3′ and 5′-GATGGAGGGGCCGGACT-3′.
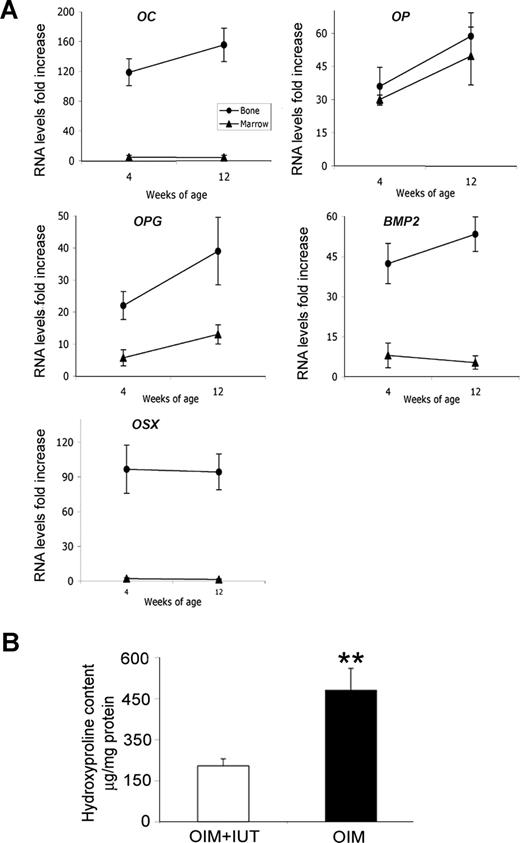
To quantify expression levels of osteoblast lineage genes in human donor cells within mouse tissues, total RNA was extracted and cDNA was synthesized as described from bone and bone marrow from 3 animals at age 4 weeks and 3 animals at age 12 weeks. Gene expression of osteocalcin (OC), osteopontin (OP), osteoprotegerin (OPG), bone morphogenic protein (BMP2), and osterix (OSX) was evaluated using primers specific for human cDNA.18 Levels of human mRNA (fold increase relative to basal level in undifferentiated cells) were normalized to human β-actin.
Skeletal preparations
Limbs were fixed for 24 to 48 hours in 10% formalin solution (Sigma, St Louis, MO) and transferred in 70% ethanol at 4°C. After dissection, bones were placed in 95% ethanol (48-72 hours at 4°C), transferred to acetone (48-72 hours at 4°C), and stained (48 to 72 hours at 37°C) in Alizarin Red (Sigma) and Alcian Blue (Sigma) containing 5% Alcian Blue (0.3% in 70% ethanol), 5% Alizarin Red (0.1% in 95% ethanol), 5% glacial acetic acid, and 85% ethanol (70% in H2O). Samples were destained for 48 to 72 hours in 1% KOH at 4°C and subsequently in 20%, 40%, 60%, and 80% glycerol/1% KOH for further periods of 48 to 72 hours before storage in 100% glycerol at 4°C. Skeletal preparations were photographed using an MZF L111 binocular microscope (Leica, Wetzlar, Germany) and an Intralux 6000-1 light source (Volpi, Auburn, NY). Bone length was measured using Mitutoyo Absolute Digital Calipers (Mitutoyo, Wednesbury, United Kingdom) and a Wild M3Z microscope (Leica). Measurements were assessed by 1 unblinded observer, and then independently by another observer blinded to transplantation status.
Histology
Bones were fixed for 48 to 72 hours in 10% neutral buffered formalin and decalcified in 10% formic acid and 10% neutral buffered formalin at room temperature. Bones were embedded in paraffin, and 3-μm sections were cut onto superfrost slides (Sigma), deparaffinized in xylene, and rehydrated.
Growth plate analysis and measurement of cortical bone
Sections were stained with hematoxylin and eosin, or van Gieson and Alcian Blue after O'Shea et al.19 The height of the tibial growth plate (GP) and its constituents, the reserve zone (RZ), proliferative zone (PZ), and hypertrophic zone (HZ), was determined by calculating the average of 4 measurements along the growth plate using a BH2 microscope (Olympus, Tokyo, Japan) and an AX0067 20.4-mm 10/100 eyepiece micrometer (Olympus). Mean RZ, PZ, HZ, and GP height were determined as the mean of measurements at each of 5 positions across the width of the growth plate using IMAGE J software (http://rsb.info.nih.gov/ij/index.html). Cortical femoral thickness was determined by calculating the mean of 5 measurements along the diaphysis. All measurements were assessed by 1 unblinded observer, and then independently by another, blinded to transplantation status.
Fluorescence microscopy
Sagittal sections (3 μm paraffin-embedded) of bones previously decalcified and fixed in neutral-buffered formalin solution (Sigma) were used. Heat-induced epitope retrieval was performed in a steamer (Dako, Glostrup, Denmark). Donor cells were visualized using a pancentromeric probe (Cambio, Cambridge, United Kingdom) or a mouse monoclonal vimentin (Abcam) primary antibody. Staining specificity was verified using appropriate negative controls (vimentin and osteopontin on nontransplanted tissues and isotype antibody controls on transplanted tissues) and positive controls.
Mechanical testing
Three-point bending tests were performed on 8-week-old unfractured tibias, fresh-frozen and thawed before testing. Bones were placed on 2 supports 10 mm apart and tested to failure in a materials testing machine (Instron 5565, High Wycombe, United Kingdom) with a loading rate of 1.2 mm/min. Loads were measured with a 100-N load cell, and data were collected every 0.1 second. A load-displacement graph was generated for each bone from which ultimate force, yield force, and stiffness values were derived.
Hydroxyproline assay
All reagents were from Sigma. Hydroxyproline levels were determined using the standard method described by Morrison et al20 on homogenized 4-week-old ulnas.
Statistical analysis
Data are expressed as means plus or minus SEM or median and range. Parametric statistics were applied after confirming normal distributions on histograms and unpaired 2-tailed Student t testing was used for comparison between groups. Nonparametric data were compared by the Mann-Whitney test, whereas the Fisher exact was used for categoric comparisons. A P value less than .05 was considered significant.
Results
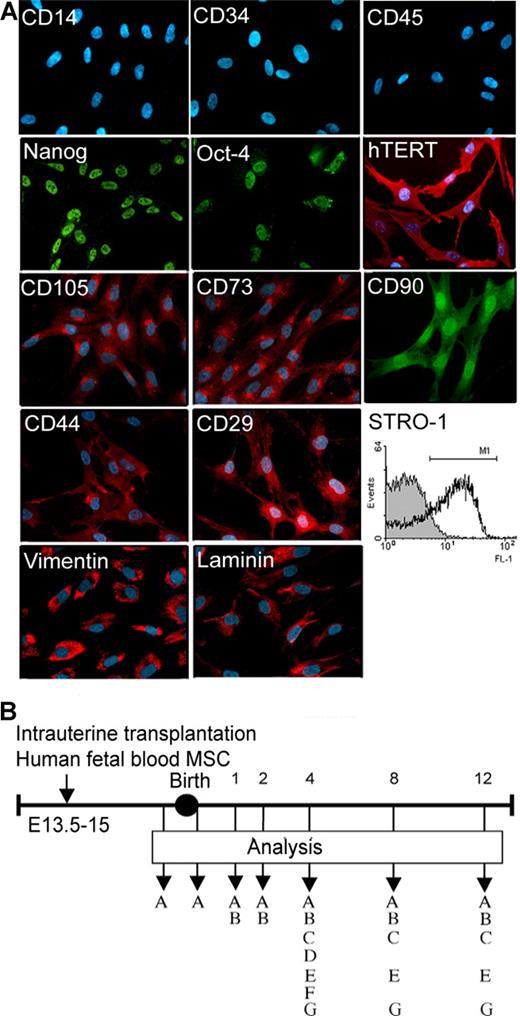
Cells were 52% STRO-1+, expressed hTERT, Oct-4, and Nanog, were depleted of haemopoietic cells and expressed intracellular matrix proteins, markers characteristic of MSC and adhesion molecules (Figure 1A).13 Offspring that underwent transplantation at E13.5 to E15 that survived neonatally (n = 70; 26 of 32 oim females that underwent IUT suckled their offspring) were analyzed in the first 12 weeks (E18, n = 7; 1 week, n = 12; 2 weeks, n = 15; 4 weeks, n = 15, 8 weeks, n = 15; and 12 weeks, n = 6; Figure 1B).
Characterization of transplanted human fetal mesenchymal stem cells and experimental design. (A) Immunostaining for hematopoietic markers (CD14, CD34, and CD45), markers associated with pluripotency and telomerase activity (Nanog, Oct-4, and hTERT), MSC-associated markers (CD105, CD73, CD90, and STRO-1), adhesion molecules (CD44, CD29), and matrix proteins (vimentin and laminin). Original magnification, 40×/0.75 NA oil objective. (B) Cells were transplanted intraperitoneally in oim fetuses and analyzed 1, 2, 4, 8, and 12 weeks after birth. MSC indicates mesenchymal stem cells; A, bioluminescence; B, engraftment; C, fractures; D, 3-point bending; E, cortical thickness; F, bone length; and G, growth plate analysis.
Characterization of transplanted human fetal mesenchymal stem cells and experimental design. (A) Immunostaining for hematopoietic markers (CD14, CD34, and CD45), markers associated with pluripotency and telomerase activity (Nanog, Oct-4, and hTERT), MSC-associated markers (CD105, CD73, CD90, and STRO-1), adhesion molecules (CD44, CD29), and matrix proteins (vimentin and laminin). Original magnification, 40×/0.75 NA oil objective. (B) Cells were transplanted intraperitoneally in oim fetuses and analyzed 1, 2, 4, 8, and 12 weeks after birth. MSC indicates mesenchymal stem cells; A, bioluminescence; B, engraftment; C, fractures; D, 3-point bending; E, cortical thickness; F, bone length; and G, growth plate analysis.
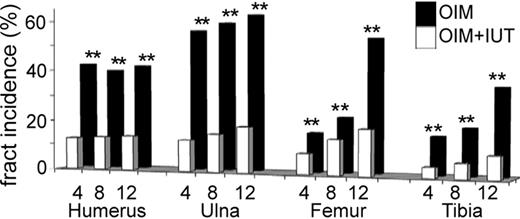
At each time point, the proportion of fractured bones was lower in oim (OIM) mice that received transplants (OIM + IUT) compared with those that did not receive transplants (all P < .01; Figure 2). In 4-week-old mice, 3 (12.5%) of 24 humeri, 3 (12.5%) of 24 ulnas, 2 (8.3%) of 24 femurs, and 1 (4.2%) of 24 tibias were fractured in OIM + IUT mice compared with 14 (43.8%) of 32 humeri, 18 (56.3%) of 32 ulnas, 5 (15.6%) of 32 femurs, and 5 (15.6%) of 32 tibias in OIM mice. In 8-week-old mice, 3 (13.6%) of 22 humeri, 3 (15%) of 20 ulnas, 3 (13.6%) of 22 femurs, and 1 (5.9%) of 17 tibias were fractured in oin plus iut mice compared with 13 (40.6%) of 32 humeri, 19 (59.4%) of 32 ulnas, 7 (21.9%) of 32 femurs, and 6 (18.8%) of 32 tibias in OIM mice. In 12-week-old mice, 1 (14.3%) of 7 humeri, 2 (18.2%) of 11 ulnas, 2 (18.2%) of 11 femurs, and 1 (8.3%) of 12 tibias were fractured in OIM + IUT mice compared with 13/31 (41.9%) humeri, 20 (62.5%) of 32 ulnas, 17 (53.1%) of 32 femurs, and 11 (34.4%) of 32 tibias in OIM mice. Overall fracture incidence (total bones fractured/total bones assessed) was decreased by 71.4% at 4 weeks (P < .01), 65.8% at 8 weeks (P < .01), and 69.3% at 12 weeks of age (P < .01); that is, approximately two-thirds fewer fractured bones in OIM + IUT mice.
IUT reduces long bone fractures. Incidence of fractured bones in humerus, ulna, femur, and tibia of 4-week-old (n = 15), 8-week-old (n = 15), and 12-week-old (n = 6) OIM + IUT and OIM mice (n = 20 for each group). **P < .01.
IUT reduces long bone fractures. Incidence of fractured bones in humerus, ulna, femur, and tibia of 4-week-old (n = 15), 8-week-old (n = 15), and 12-week-old (n = 6) OIM + IUT and OIM mice (n = 20 for each group). **P < .01.
The number of fractured bones per mouse was reduced in mice that received transplants at 4 weeks (median, 1 of 8 bones assessed; range, 0-4 bones vs median, 3 bones; range, 0-5 bones for OIM + IUT and OIM, respectively; P < .001), at 8 weeks (median, 1 bone; range, 0-2 bones vs median, 3.5 bones; range, 2-6 bones; P < .001), and at 12 weeks (median, 1 bone; range, 0-2 bones vs median, 3 bones; range, 1-5 bones; P < .01). Overall, the proportion of mice with any fractures was lower in OIM + IUT mice (17 [58.6%] of 29) compared with OIM mice (47 [97.9%] of 48; P < .001). When analyzed by time point, this reached significance at 4 weeks (5 [41.7%] of 12 OIM + IUT mice vs 15 [93.8%] of 16 OIM mice; P < .01), but fell just short at 8 weeks (8 [72.7%] of 11 OIM + IUT mice vs 16 [100%] of 16 OIM mice, respectively; P < .06) and 12 weeks (4 [66.7%] of 6 OIM + IUT mice vs 16 [100%] of 16 OIM mice; P < .06).
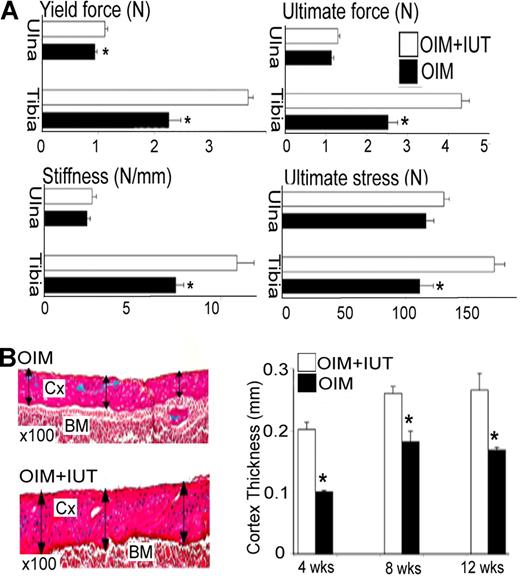
All tibial parameters were increased in OIM + IUT mice (Figure 3A); that is, structural yield force (3.7 ± 0.1 N vs 2.2 ± 0.2 N; P < .01), ultimate force (4.3 ± 0.2 N vs 2.5 ± 0.2 N; P < .01), ultimate stress (171.9 ± 8.5 N vs 111.4 ± 11.0 N; P < .01), and stiffness (10.9 ± 1.0 N/mm vs 7.4 ± 0.5 N/mm; P < .01), reflecting increased bone strength. Changes were less pronounced in the ulna, with an increase in yield force (1.12 ± 0.1 N vs 0.9 ± 0.1 N; P < .05), and trends toward an increase in other parameters not reaching significance.
IUT increases long bone strength and cortical thickness. (A) Mechanical properties (3-point bending test) of ulna and tibia in 4-week-old mice that received transplants (OIM + IUT; n = 8) and oim mice that did not receive transplants (OIM; n = 4). Yield force (N), ultimate force (N), stiffness (N/mm), and ultimate force (N). *P < .05. (B) Femoral cortical thickness in 4-week-old (n = 6), 8-week-old (n = 4), and 12-week-old (n = 3) OIM + IUT and OIM mice (n = 7, 4, and 4, respectively). Cx indicates cortex; and BM, bone marrow. Bars on graphs are means ± SE.
IUT increases long bone strength and cortical thickness. (A) Mechanical properties (3-point bending test) of ulna and tibia in 4-week-old mice that received transplants (OIM + IUT; n = 8) and oim mice that did not receive transplants (OIM; n = 4). Yield force (N), ultimate force (N), stiffness (N/mm), and ultimate force (N). *P < .05. (B) Femoral cortical thickness in 4-week-old (n = 6), 8-week-old (n = 4), and 12-week-old (n = 3) OIM + IUT and OIM mice (n = 7, 4, and 4, respectively). Cx indicates cortex; and BM, bone marrow. Bars on graphs are means ± SE.
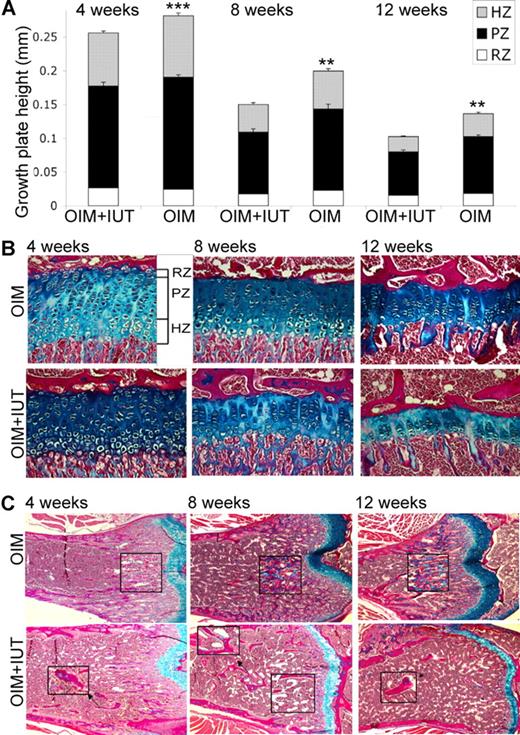
Femoral cortical thickness was increased in OIM + IUT mice by 4 weeks of age (by 0.20 ± 0.01 mm vs 0.10 ± 0.01 mm; P < .001), 8 weeks of age (0.26 ± 0.01 mm vs 0.18 ± 0.02 mm; P < .05), and 12 weeks of age (0.27 ± 0.02 mm vs 0.17 ± 0.01 mm; P < .01) compared with OIM mice alone (Figure 3B). In 4-week-old mice, the ulna, tibia, and femur lengths, respectively were greater in OIM + IUT mice than OIM mice (ulna, 14.1 ± 0.2 mm vs 11.8 ± 0.4 mm; P < .001; tibia, 16.6 ± 0.3 mm vs 14.6 ± 0.2 mm; P < .001; and femur, 14.2 ± 0.1 mm vs 12.3 ± 0.1 mm; P < .05) for males and (ulna, 12.9 ± 0.1 mm vs 11.2 ± 0.6 mm; P < .05; tibia, 15.5 ± 02 mm vs 13.9 ± 0.1 mm; P < .001; and femur, 13.5 ± 0.2 mm vs 11.2 ± 0.3 mm; P < .01) for females. In 8- to 12-week-old mice (both length time points were pooled as similar), ulna, tibia, and femur lengths were, respectively, greater in OIM + IUT mice compared with OIM mice (ulna, 14.3 ± 0.1 mm vs 13.6 ± 0.1 mm; P < .001; tibia, 19.5 ± 0.2 vs 18.7 ± 0.1; P < .01; and femur, 17.3 ± 0.3 vs 14.4 ± 0.1; P < .001) for both males and (ulna, 13.6 ± 0.3 mm vs 11.5 ± 0.3; P < .001; tibia, 18.2 ± 0.2 mm vs 16.5 ± 0.5; P < .01; and femur, 17.0 ± 0.3 mm vs 14.1 ± 0.2; P < .01) females. Compared with OIM mice that did not receive transplants, tibial growth plate height was reduced in 4-week-old OIM + IUT mice (0.26 ± 0.01 mm vs 0.28 ± 0.01; P < .001), 8-week-old OIM + IUT mice (0.15 ± 0.01 mm vs 0.20 ± 0.01; P < .01), and 12-week-old OIM + IUT mice (0.10 ± 0.01 mm vs 0.14 ± 0.01; P < .001). This decrease affected both PZ and HZ, with a decrease in PZ height (4 weeks, 0.15 ± 0.01 mm vs 0.17 ± 0.01 mm; P < .01; 8 weeks, 0.09 ± 0.01 mm vs 0.12 ± 0.01 mm; P < .01; and 12 weeks, 0.06 ± 0.01 mm vs 0.08 ± 0.01 mm; P < .01) and HZ height (4 weeks, 0.08 ± 0.01 mm vs 0.09 ± 0.01 mm; P < .05; 8 weeks, 0.04 ± 0.01 mm vs 0.06 ± 0.01 mm; P < .01; and 12 weeks, 0.02 ± 0.01 mm vs 0.03 ± 0.01 mm; P < .05; Figure 4A,B). Femoral sagittal sections from 4-, 8-, and 12-week-old mice revealed defective remodeling in the metaphyseal trabeculae, with highly mineralized cartilage in OIM mice that was reduced in OIM + IUT mice (Figure 4C).
IUT decreases growth plate height. (A) Tibial growth plate analysis in 4-week-old (OIM + IUT, n = 7; OIM, n = 12), 8-week-old (OIM + IUT, n = 9; OIM, n = 11), and 12-week-old (OIM + IUT, n = 3; OIM, n = 5) mice. The heights of the total GP, RZ, PZ and HZ are shown (± SE). **P < .01; or ***P < .001. (B) Tibial sagittal sections showing growth plates in 4-, 8- and 12-week-old OIM + IUT and OIM mice. (C) Femur sagittal sections under the growth plate showing trabeculae and calcified cartilage (magnified inserts) in OIM versus OIM + IUT mice at 4, 8, and 12 weeks of age. Magnification was 200×.
IUT decreases growth plate height. (A) Tibial growth plate analysis in 4-week-old (OIM + IUT, n = 7; OIM, n = 12), 8-week-old (OIM + IUT, n = 9; OIM, n = 11), and 12-week-old (OIM + IUT, n = 3; OIM, n = 5) mice. The heights of the total GP, RZ, PZ and HZ are shown (± SE). **P < .01; or ***P < .001. (B) Tibial sagittal sections showing growth plates in 4-, 8- and 12-week-old OIM + IUT and OIM mice. (C) Femur sagittal sections under the growth plate showing trabeculae and calcified cartilage (magnified inserts) in OIM versus OIM + IUT mice at 4, 8, and 12 weeks of age. Magnification was 200×.
Bioluminescence (BLI) showed that donor cells persisted in various organs, remaining for up to 12 weeks after birth in the bones, ribs, spine, thymus, lung, heart, liver, spleen, kidney, and skin (Figure 5A,B). Quantification of photon emission confirmed the presence of donor cells in organs from all 3 germ layers and showed a reduced signal in 12-week-old OIM + UIT mice compared with 1-week-old OIM + IUT mice (Figure 5C). Although we had optimized both sensitivity and specificity in preliminary experiments (data not shown) and validated the technique on individual organs, the possibility remains with in vivo BLI that artifact contributed to the signal quantified. Accordingly, we further quantified engraftment using qRT-PCR. This showed that the proportion of human cells (Figure 5D-G) was higher in all organs in 1-week-old mice (ribs, femur, spine, spleen, liver, kidney, heart, lung, brain, thymus, and skin), subsequently decreasing with age (Figure 5H). However, donor cell retention was greater (P < .001 at all time points) in the skeleton (femur, ribs, and spine) compared with other organs (Figure 5I), respectively 2.1, 2.5, 3.9, 4.9, and 5.3 times higher in 1-, 2-, 4-, 8-, and 12-week-old mice.
Donor cells preferentially found in bones. (A) Donor cell tracking after IUT by bioluminescence imaging showing no light emission in a control postnatal oim mouse that did not receive a transplant. (B) Imaging in a postnatal post-IUT oim mouse showing luminescence emitted by internal organs (ie tail [Ta], limbs [L], back spine [BS], thymus [T], heart [H], ribs [R], spleen [S], kidneys [K], brain [B], liver [Li], lungs [Lu], and skin [Sk]). (C) Quantification of photon emission on postmortem organs of in 1- and 12-week-old oim mice that received transplants. (D) Amplification plot showing change in normalized reporter dye fluorescence (ΔRn) against number of amplification cycles in samples containing from 1 to 10−7 human cDNA using human-specific primers. (E) Human specificity of primers. (F) Change in ΔRn versus the number of amplification cycles in samples containing from 1 to 10−6 human (red) and mouse (blue) cDNA using primers amplifying sequences common to human and mouse. (G) Standard curve generated from data in panel A showing samples from oim mice that received transplants (red dots). (H) Quantification of engraftment by qRT-PCR in bone versus nonbone organs at different time points in oim mice that received transplants. ***P < .001. (I) Summary of the quantification of engraftment by qRT-PCR in bone vs nonbone organs over 12 weeks postnatal.
Donor cells preferentially found in bones. (A) Donor cell tracking after IUT by bioluminescence imaging showing no light emission in a control postnatal oim mouse that did not receive a transplant. (B) Imaging in a postnatal post-IUT oim mouse showing luminescence emitted by internal organs (ie tail [Ta], limbs [L], back spine [BS], thymus [T], heart [H], ribs [R], spleen [S], kidneys [K], brain [B], liver [Li], lungs [Lu], and skin [Sk]). (C) Quantification of photon emission on postmortem organs of in 1- and 12-week-old oim mice that received transplants. (D) Amplification plot showing change in normalized reporter dye fluorescence (ΔRn) against number of amplification cycles in samples containing from 1 to 10−7 human cDNA using human-specific primers. (E) Human specificity of primers. (F) Change in ΔRn versus the number of amplification cycles in samples containing from 1 to 10−6 human (red) and mouse (blue) cDNA using primers amplifying sequences common to human and mouse. (G) Standard curve generated from data in panel A showing samples from oim mice that received transplants (red dots). (H) Quantification of engraftment by qRT-PCR in bone versus nonbone organs at different time points in oim mice that received transplants. ***P < .001. (I) Summary of the quantification of engraftment by qRT-PCR in bone vs nonbone organs over 12 weeks postnatal.
Donor cells were clustered in areas of active bone formation and remodeling and at healed fracture sites (Figure 6A), where human osteopontin was deposited in high amounts within the bone matrix, in the primary spongiosa, and below the growth plate (Figure 6B-D). All 10 bones examined with macroscopically evident fracture callus had human cells concentrated many-fold around the old fracture site compared with the adjacent intact bone. In the diaphysis, vimentin-positive cells were found in the bone marrow (Figure 6E,F) and cortex, in both the periosteum (Figure 6G) and the endosteum (Figure 6H). Finally, human osteopontin was identified within the trabecular bone, indicating that donor cells contributed to bone formation not only at sites of remodeling following fracture, but also in intact bones. To confirm bone differentiation of human fetal MSCs in vivo, we documented expression of OC, OP, OPG, BMP2, and OSX by qRT-PCR using human-specific markers. The levels of OC, OPG, BMP2, and OSX were increased in bones but not in bone marrow from 4- and 12-week-old mice, indicating that donor cells remained as progenitors in bone marrow but expressed a osteoblast phenotype in bones (Figure 7A). Finally, hydroxyproline was decreased in OIM + IUT mice compared with OIM mice (204.2 ± 27.1 μg/mg protein vs 480.6 ± 79.9 μg/mg protein; P < .01; Figure 7B). ColIα1 is richer in hydroxyproline than colIα2, suggesting that donor cells reduced collagen type I oim homotrimers, presumably by producing colIα2 chains to alter the imbalance in the α1(I)/α2(I) ratio.
Donor cells found at fracture and bone formation sites. (A) Visualization of donor cells by fluorescence in situ hybridization (FISH). Magnification of a single cell (small panel; 100×/0.75 NA oil objective) showing multiple chromosomal signals. Cells clustered at callus fractures and at sites of active bone formation (hematoxylin and eosin [H&E] staining; middle). (B) Human-specific OP staining in the callus area at site of new bone formation. (C) DAB staining revealing OP deposited in the primary spongiosa below the growth plate (GP; H&E staining, middle) and (D) within the matrix of the callus. (E,F) Visualization of donor cells (circled) by immunostaining using human-specific vimentin antibody and Cy3-conjugated secondary in the bone marrow cavity (BM) of the diaphysis (H&E staining, middle). (G,H) Donor cells (vimentin staining) clustered at site of bone formation in the periosteum (H&E staining, middle; G) and in the endosteum (DAB staining; H). (I,J) OP-positive staining within trabecular bone using immunofluorescence (I) and DAB staining (J).
Donor cells found at fracture and bone formation sites. (A) Visualization of donor cells by fluorescence in situ hybridization (FISH). Magnification of a single cell (small panel; 100×/0.75 NA oil objective) showing multiple chromosomal signals. Cells clustered at callus fractures and at sites of active bone formation (hematoxylin and eosin [H&E] staining; middle). (B) Human-specific OP staining in the callus area at site of new bone formation. (C) DAB staining revealing OP deposited in the primary spongiosa below the growth plate (GP; H&E staining, middle) and (D) within the matrix of the callus. (E,F) Visualization of donor cells (circled) by immunostaining using human-specific vimentin antibody and Cy3-conjugated secondary in the bone marrow cavity (BM) of the diaphysis (H&E staining, middle). (G,H) Donor cells (vimentin staining) clustered at site of bone formation in the periosteum (H&E staining, middle; G) and in the endosteum (DAB staining; H). (I,J) OP-positive staining within trabecular bone using immunofluorescence (I) and DAB staining (J).
Effect of IUT on hydroxyproline and expression of osteogenic genes. (A) qRT-PCR using markers specific for human cDNA for OC, OP, OPG, BMP, and OSX normalized to human β-actin. Results are expressed as RNA levels fold increase in bone and bone marrow relative to basal levels in undifferentiated human fetal MSCs. (B) Hydroxyproline content (μg/mg protein) in 4-week-old OIM + IUT mice (n = 11) that received transplants and OIM mice that did not receive transplants (n = 17). Results are presented as means (±SD). **P < .01.
Effect of IUT on hydroxyproline and expression of osteogenic genes. (A) qRT-PCR using markers specific for human cDNA for OC, OP, OPG, BMP, and OSX normalized to human β-actin. Results are expressed as RNA levels fold increase in bone and bone marrow relative to basal levels in undifferentiated human fetal MSCs. (B) Hydroxyproline content (μg/mg protein) in 4-week-old OIM + IUT mice (n = 11) that received transplants and OIM mice that did not receive transplants (n = 17). Results are presented as means (±SD). **P < .01.
Discussion
In utero transplantation of human fetal MSCs ameliorated the disease phenotype in a mouse model of OI, producing a clinically relevant two-thirds reduction in fracture incidence, along with increased bone strength, length, and thickness. In terms of mechanism, we observed cells preferentially clustered around sites of bone formation and repair, where the presence of human OP-positive cells suggested osteogenic differentiation. Donor cells present in bone, but not in bone marrow, expressed key oseogenic genes such as osteocalcin, OPG, OSX, and BMP2, providing additional evidence for osteogenic differentiation of donor cells and contribution to matrix formation. Our data are supported by a recent publication from Li et al suggesting that expanded retrieved donor cells, found at a comparable frequency in recipient bones of 0.3% to 28.0%, expressed osteogenic genes.21
The marked improvement in skeletal phenotype associated with MSC transplantation in our study was associated with engraftment levels in bone of only around 5%. In keeping with this, limited clinical data support phenotypic improvement at low engraftment in OI. Horwitz et al reported a 45% to 77% increase in bone mineral content in children with OI who underwent transplantation with less than 2% engraftment,3,4,6 while Leblanc et al found up to 7% engraftment following prenatal cell therapy.7 Low engraftment in these limited clinical studies reflects engraftment only at the site of biopsy, whereas whole-mouse studies enables global skeletal engraftment. Experiments in irradiated adult transgenic OI mice reported 4% to 19% donor cells in primary tissue cultures, in association with phenotypic improvement.22 Engraftment rates in our study were increased several-fold at fracture, where there is active bone formation and repair. We found donor cells clustered around all 10 fractures examined. We acknowledge that to prevent all fractures, transplanted cells need to engraft in target tissues before injury occurs. Although it is possible in our study that all the fractures analyzed for engraftment occurred before IUT, this seems unlikely, especially against the background of the temporal increase in fracture frequency with increasing postnatal age. Instead, clustering around fracture sites is consistent with donor cell mobilization from reservoirs such as the bone marrow to sites of acquired tissue injury.
The literature suggests that adult MSCs may in part evade host immune detection and have a variable degree of immunomodulatory properties, although Badillo et al showed that allogenic adult murine MSCs elicit both cellular and humoral responses in immune-competent mice.23 Human fetal MSCs also have immunomodulatory properties in vitro but take longer than adult MSCs to express HLAII in response to interferon-γ stimulation, and did not induce alloreactivity after fetal transplantation in a child with OI. We did not assess donor cell tolerance in this model, and thus cannot exclude the possibility of immune rejection of xenogenic human proteins, which could be responsible for the decrease in engraftment seen over time.
The duration of observation in this study was limited to 12 weeks to comply with strict severity limits under British law covering the use of animals in scientific procedures, limiting our ability to determine the longevity of the observed improvements in phenotype. Although the present data clearly document phenotype improvement up to 3 months of age, equivalent to the young adult stage, it would be valuable to extend this to older mice to assess the long-term effects, and in particular whether engraftment and therapeutic efficacy declines with time.
Nevertheless, the effects within the 0- to 12-week window were pronounced. There are several potential explanations. First, tissue damage facilitates engraftment and differentiation. IUT with fetal MSCs in the mdx mouse led to less engraftment than in this study, presumably due to lack of overt pathology at time of transplantation.24,25 In noninjury models, MSCs home and engraft tissues at only very low levels, but can be recruited from remote storage sites to areas of wound healing.26,27 Second, amelioration of the phenotype may be related to the nature of the donor cells. Fetal MSCs may be ontologically advantageous within the fetal environment, being less lineage-committed, less immunogenic, and faster growing than adult MSCs,13,16,28-30 as well as expressing a distinct adhesion molecule profile,31 engrafting long term after pregnancy in maternal bone,32 and having a default osteogenic predisposition in vitro and in vivo.
The question as to how human cells are capable of such therapeutic action in mice remains unclear, although several mechanisms can be postulated. First, donor cell differentiation as demonstrated herein is implicated. Next, the high level of homology between human and murine Col1α2 protein and/or other proteins involved in bone formation may explain the beneficial effect of human cells. Alternatively, the murine environment may be favorable to functional human-mice hybrid proteins. Second, human donor cells may have a paracrine effect on surrounding cells via secretion of cytokines and growth factors. Finally, improvement after IUT might reflect additional mechanisms such as paracrine effects on collagen metabolism. Lower hydroxyproline content after transplantation suggests that donor cells reduced collagen I homotrimers. As in other injury paradigms affecting the heart and nervous system, the cellular processes underpinning therapeutic effects may be more complex than simply altering donor-host cell ratios.
In humans, OI is treated empirically by administration of biphosphonates, which inhibit osteoclast function.1 The effect of IUT of MSCs we observed appears similar to that of postnatal biphophonate therapy. For example, treating 6-week-old oim mice for 8 weeks with alendronate induced a 34% decrease in fracture rate, a 45% reduction in tibial bowing, and 16% decrease in growth plate height compared with controls. Starting treatment earlier at 2 weeks of age showed a greater decrease in fracture frequency (65%), but also a decrease in growth plate height that approached that of wild-type mice, albeit with no improvement in bone length.33 However, there are also a number of negative effects of biphosphonates, including persistence of calcified cartilage and shorter femora,34 as also reported by Evans et al, who observed a reduction in humeral and ulna length and increased growth plate height in oim mice.10 Another therapeutic option, growth hormone, led in oim mice to a 2% increase in femur length with improved mechanical properties (ie, a 24% increase in stiffness and 14% increase in ultimate stress).35 Thus, our study demonstrates that fetal MSC transplantation in utero not only ameliorates OI pathology to at least the same degree as conventional therapies, but has additional benefits on bone structure such as decreased growth plate height and increased limb length.
In summary, IUT of human first-trimester fetal MSCs considerably ameliorated skeletal pathology in a mouse model of OI in which donor cells were preferentially recruited to tissues rich in mutant colI(α1)3 homotrimers and to sites of injury, where they produced OP, expressed an osteoblast phenotype, and contributed to bone formation and repair. In a recent review, Horwitz et al emphasized that MSC therapy for tissue regeneration should be able to be delivered to the relevant tissue, differentiate to the tissue of interest, and finally improve damaged or diseased tissues.36 According to these criteria, our data support the feasibility of prenatal treatment using human fetal MSCs. It establishes proof of concept for translating intrauterine fetal MSC transplantation into a therapeutic option for pregnancies affected by OI. This work is also of wider relevance both for the generic development of prenatal therapy for early-onset mesenchymal deficiency diseases, and as a useful model to test homing and engraftment in response to tissue injury.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mahrokh Nohadani for assistance with the histology.
This work was supported by a project grant from Action Medical Research (N.M.F. and J.H.D.B.), with additional infrastructural support from the Institute of Obstetrics & Gynaecology Trust (N.M.F.).
Authorship
Contribution: N.M.F., P.V.G., J.C., G.B.-G., G.R.W., and J.P. designed research; P.V.G., O.A., J.H.D.B., S.J.S., G.B.-G., J.C., and H.K. performed research; P.V.G., O.A., N.M.F., and J.H.D.B. analyzed data; and P.V.G., G.R.W., and N.M.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pascale Guillot, Institute of Reproductive and Developmental Biology, Imperial College London, Hammersmith Campus, Du Cane Rd, London W12 ONN, United Kingdom; e-mail: pascale.guillot@imperial.ac.uk.

![Figure 5. Donor cells preferentially found in bones. (A) Donor cell tracking after IUT by bioluminescence imaging showing no light emission in a control postnatal oim mouse that did not receive a transplant. (B) Imaging in a postnatal post-IUT oim mouse showing luminescence emitted by internal organs (ie tail [Ta], limbs [L], back spine [BS], thymus [T], heart [H], ribs [R], spleen [S], kidneys [K], brain [B], liver [Li], lungs [Lu], and skin [Sk]). (C) Quantification of photon emission on postmortem organs of in 1- and 12-week-old oim mice that received transplants. (D) Amplification plot showing change in normalized reporter dye fluorescence (ΔRn) against number of amplification cycles in samples containing from 1 to 10−7 human cDNA using human-specific primers. (E) Human specificity of primers. (F) Change in ΔRn versus the number of amplification cycles in samples containing from 1 to 10−6 human (red) and mouse (blue) cDNA using primers amplifying sequences common to human and mouse. (G) Standard curve generated from data in panel A showing samples from oim mice that received transplants (red dots). (H) Quantification of engraftment by qRT-PCR in bone versus nonbone organs at different time points in oim mice that received transplants. ***P < .001. (I) Summary of the quantification of engraftment by qRT-PCR in bone vs nonbone organs over 12 weeks postnatal.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/3/10.1182_blood-2007-08-105809/6/m_zh80050813230005.jpeg?Expires=1768736305&Signature=jErpkmu28buiJoTwYWwwWhjgxemzH-2fv8HY1K2ct5kybBPZMxv4oIN5BNG-ViO0F37aSAFUEYkfE2Vq0-WluG2Io7PkbnzbUeR0FJeUE98XR-n~7adhkMnuBdt2vDQ2ycbM0-~MYhDoeQZHcgDfPAfEY~FxJLsoBPhfU-wxL7NwIw-OQtTqNBCQH9fcwI19t3u0SSDoOj1l5JKemJpzcwr~hAumLXepaqHVqj8n9nLAGZz8zQZA5oSkXrTElCNwXVz56OLpxWV4zMU49LNj~fEyFjXzeqtANK1d5M1LSqPTiFYxsqvlepM0NojlZoroyB8Mch~fKFQK-yui5uIK8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Donor cells found at fracture and bone formation sites. (A) Visualization of donor cells by fluorescence in situ hybridization (FISH). Magnification of a single cell (small panel; 100×/0.75 NA oil objective) showing multiple chromosomal signals. Cells clustered at callus fractures and at sites of active bone formation (hematoxylin and eosin [H&E] staining; middle). (B) Human-specific OP staining in the callus area at site of new bone formation. (C) DAB staining revealing OP deposited in the primary spongiosa below the growth plate (GP; H&E staining, middle) and (D) within the matrix of the callus. (E,F) Visualization of donor cells (circled) by immunostaining using human-specific vimentin antibody and Cy3-conjugated secondary in the bone marrow cavity (BM) of the diaphysis (H&E staining, middle). (G,H) Donor cells (vimentin staining) clustered at site of bone formation in the periosteum (H&E staining, middle; G) and in the endosteum (DAB staining; H). (I,J) OP-positive staining within trabecular bone using immunofluorescence (I) and DAB staining (J).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/3/10.1182_blood-2007-08-105809/6/m_zh80050813230006.jpeg?Expires=1768736305&Signature=1nzZFMLcslg4PQFsOUtwsqtu~Q-77vK765bHpYXXTbUcjsX~bgqEn~-mAmDszLQH3MnQ5FTZpsaTNbUcerY6uOnygEPaLTntXI0iSTc4lVjganH~q7bpDY1t1e6LIiF1PjltzxURL1K6fLdEcYSqtycZqP83KmGhsc-WTf2HrlI2SDBnbR1i1GI8X3cV2AmDm1Ge6jx6EpLu~v~q6H6pWxuWqO~a9~9jr0trqdZq-JoZsjtAb9K7Nb4ii1FCEGwXQzExkwQkpc3qNFErgMN9wwRMZrIjMmy7sixXvoNt2SP6ZFqBIYPCFv3xkWxVT5e4mt1WexFg-gjDGf-fzY5g~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)