Abstract
It is generally thought that mast cells influence T-cell activation nonspecifically through the release of inflammatory mediators. In this report, we provide evidence that mast cells may also affect antigen-specific T-cell responses by internalizing immunoglobulin E–bound antigens for presentation to antigen-specific T cells. Surprisingly, T-cell activation did not require that mast cells express major histocompatibility complex class II, indicating that mast cells were not involved in the direct presentation of the internalized antigens. Rather, the antigen captured by mast cells is presented by other major histocompatibility complex class II+ antigen-presenting cells. To explore how this may occur, we investigated the fate of mast cells stimulated by antigen and found that FcϵRI crosslinking enhances mast cell apoptosis. Cell death by antigen-captured mast cells was required for efficient presentation because protection of mast cell death significantly decreased T-cell activation. These results suggest that mast cells may be involved in antigen presentation by acting as an antigen reservoir after antigen capture through specific immunoglobulin E molecules bound to their FcϵRI. This mechanism may contribute to how mast cells impact the development of T-cell responses.
Introduction
Mast cells are considered “sentinels” of the immune system because of their strategic anatomic localization at the host-environment interface, including the mucosal and submucosal barriers of the host. This property grants mast cells the unique ability to respond rapidly to environmental stimuli.1 Mast cells play a pivotal role in allergic hypersensitivity reaction, where much of mast-cell activation is mediated through FcϵRI, a high-affinity receptor that binds to monomeric immunoglobulin E (IgE). Crosslinking the IgE-bound FcϵRI with cognate antigen elicits the immediate release of vasoactive amines, arachidonic acid metabolites, cytokines, and chemokines. The release of vasoactive substances such as histamine and serotonin causes increased vascular permeability, which allows the flow of inflammatory mediators and cells into the antigen-encountered site. Chemokines, cytokines, and arachidonic acid metabolites further recruit inflammatory cells.2 Together, these mediators contribute to development of both the acute and chronic symptoms of allergic reactions.
The importance of mast cells in immune responses is not confined to allergic disease, as recent studies have demonstrated that mast cells play a far broader role in the initiation and propagation of various immune responses. For example, mast cells are vital for protection against parasitic infections such as Leishmania major,3 Giardia lambia,4 and intestinal helminthes.5,6 Moreover, they provide defense against certain bacterial infections by recruiting neutrophils to the site of infection.7,8 Mast cells are also involved in the development of T cell–mediated hypersensitivity disorders such as delayed type contact hypersensitivity9,10 and asthma.11,12 More recently, they have been implicated in the induction of disease in several autoimmune mouse models, including those for rheumatoid arthritis,13,14 inflammatory bowel disease,15 and multiple sclerosis (experimental autoimmune encephalomyelitis).16,17 In at least some of these disease models, a direct correlation between the activation of T cells and the presence of mast cells has been established,3,18 as diminished activation of T cells was observed in mast cell–deficient mice compared with wild-type controls.
One means by which mast cells affect T-cell responses is through the elaboration of inflammatory mediators. One might speculate that this can occur at multiple levels of the antigen-specific T-cell activation process. Chemoattractants such as CCL119,20 and LTB421 that are released by activated mast cells can help recruit T cells to sites of inflammation. Once recruited, an abundant amount of prestored tumor necrosis factor-α (TNF-α) that is released by mast cells can directly enhance the proliferation of T cells.22,23 In addition to the direct effects of mast cell–derived products on T cells, mast cells can also promote T-cell activation indirectly through stimulation of antigen-presenting cells (APCs). Mast cell–derived TNF-α and histamine can induce migration of dendritic cells (DCs)24 and Langerhans cells,25 respectively, to draining lymph nodes, where antigen presentation to T cells can take place. Furthermore, mast cell–derived histamine26,27 as well as direct mast cell/DC interactions27 induce DC-mediated T-cell polarization into a Th2 phenotype.
Through the mechanisms described above, it is likely that the inflammatory milieu created by activated mast cells significantly contributes to nonspecific T-cell activation. However, we speculate that mast cells may also participate in antigen-specific T-cell responses through FcϵRI, which may provide a unique means by which IgE-bound antigens could be incorporated into mast cells. In the present report, we demonstrate that antigens incorporated into mast cells via FcϵRI can activate antigen-specific T cells by providing antigens to APCs.
Methods
Mice
C57BL/6 (B6), major histocompatibility complex (MHC) class II−/−, and B6.SJL (CD45.1 congenic) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in the animal care facility at the University of Pennsylvania. The following mouse was obtained through the NIAID Exchange Program: OT-II.2a/Rag1 (OT-II) mice, Mouse Line 4234 (Taconic Emerging Model).28,29 OT-II.SJL mice were generated by breeding OT-II mice with B6.SJL mice. All animal care and work was in accordance with national and institutional guidelines and the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Flow cytometry
All antibodies used for flow cytometry were purchased from BD Biosciences (Franklin Lakes, NJ) except for anti-FcϵRI (eBiosciences, San Diego, CA). Cells were blocked with anti–CD16/32 antibody, followed by staining with specified antibodies (anti–CD117-allophycocyanin or -phycoerythrin (PE), anti–FcϵRI-fluorescein isothiocyanate (FITC) or -biotin, anti–IAb-biotin (KH74), anti–IgE-biotin, anti–CD4-allophycocyanin, anti–CD69-PE, anti–CD45.1-PE, streptavidin-PE). Cells were washed with phosphate-buffered saline (PBS) between staining steps and the fluorescence intensity was measured on a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using Cell Quest (BD Biosciences) or FlowJo (FlowJo, Ashland, OR) computer software.
Generation of bone marrow–derived mast cells (BMMCs) and bone marrow–derived DCs (BMDCs)
Bone marrow–derived mast cells (BMMCs)30 and DCs (BMDCs)31 were generated as previously described. Briefly, bone marrow (BM) cells were obtained from the femurs and tibias of B6 or MHC class II−/− mice. BMMCs were obtained by culturing the BM cells in mast cell medium (MCM; RPMI1640, 15% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, 2.9 mg/mL glutamine, 50 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 1× nonessential amino acids, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; HEPES) containing interleukin-3 (IL-3) (10 ng/mL) and stem cell factor (SCF; 12.5 ng/mL) for 6 to 8 weeks, replenishing with fresh media twice weekly, and used when more than 95% of cells expressed high homogeneous levels of FcϵRI and CD117. DCs were generated by culturing BM cells in DC medium (Dulbecco modified Eagle medium; DMEM, 15% FCS, penicillin, streptomycin, glutamine) containing IL-4 (10 ng/mL) and granulocyte-macrophage colony-stimulating factor (10 ng/mL) for 7 days, and purified by magnetic cell sorting (MACS) using CD11c beads (Miltenyi Biotec, Auburn, CA). All cytokines and cell culture reagents were purchased from PeproTech (Rocky Hill, NJ) and Invitrogen (Carlsbad, CA), respectively.
Preparation of peritoneal mast cells
Peritoneal lavage was performed on MHC class II−/− mice. The peritoneal cells were allowed to adhere to tissue culture Petri dishes for 2 hours at 37°C. The nonadherent cells were further sorted for mast cells by MACS with anti-CD117 beads (Miltenyi Biotec).
Retroviral infection of BMMCs
BCL-XL cloned into the murine stem cell virus-based retroviral MigR1 vector was a kind gift of Dr Laurence A. Turka (University of Pennsylvania) and was generated as previously described.32 Freshly isolated BM cells from MHC class II−/− mice were cultured overnight in MCM supplemented with IL-3 (10 ng/mL), IL-6 (10 ng/mL), and SCF (50 ng/mL). Retroviral supernatant (MigR1 vector or BCL-XL) was added, and cells were spin-infected at 1280g for 90 minutes at room temperature with 8 μg/mL polybrene (Sigma-Aldrich, St Louis, MO). Cells were incubated overnight at 37°C and spin-infected the following day. After an overnight incubation, cells were cultured as described above to generate BMMCs. After 3 to 4 weeks, green fluorescence protein (GFP)–expressing cells were sorted using a MoFlo high-performance cell sorter (Dako, Carpinteria, CA). Sorted BMMCs were further cultured and were used when more than 95% of cells expressed high homogeneous levels of FcϵRI and CD117.
Antigen incorporation by mast cells
BMMCs or peritoneal mast cells were cultured in MCM containing IL-3 (10 ng/mL), with or without anti-dinitrophenol (DNP) IgE (Clone SPE7; Sigma-Aldrich), or anti-ovalbumin (OVA) IgE (AbD Serotec, Raleigh, NC) (1 μg/mL) for 1 to 3 days. Mast cells were washed thoroughly with MCM and cultured for 1, 24, or 48 hours with grade V OVA (Sigma-Aldrich), DNP-conjugated human serum albumin (DNP-HSA; Sigma-Aldrich), Alexa Fluor 488-conjugated OVA (Invitrogen), or DNP-conjugated OVA (DNP-OVA; Biosearch Technologies, Novato, CA) with or without IL-3 (10 ng/mL). After various incubation periods, the cells were thoroughly washed with MCM and were used further for flow cytometric analysis, microscopy, mast cell/T cell/DC coculture, or in vivo transfer experiments.
Confocal microscopy
Microscope coverslips coated with 0.1% poly-L-lysine (Sigma-Aldrich) were washed twice with PBS and deposited with Alexa Fluor 488-labeled OVA-incorporated BMMCs. The slides were fixed for 10 minutes in 4% paraformaldehye at room temperature, quenched with 50 mM NH4Cl for 15 minutes at room temperature, blocked with 10% bovine serum albumin in PBS for 1 hour on ice, stained with 1:500 choleratoxin-Alexa Fluor 594 (Invitrogen) for 10 minutes on ice, washed 3 times with PBS, and mounted with Vectashield hardset mounting media (Vector Labs, Burlingame CA). Cells were visualized using a Perkin-Elmer 5-wavelength laser UltraView LCI spinning disk confocal (Yokogawa) that was attached to a Nikon TE-300 inverted microscope equipped with a 100× objective and a z-axis controller (Physik Instrumente, Palmbach, Germany). Samples were excited by an argon laser emitting a 488 nm laser line and an argon-krypton laser emitting a 647 nm laser line in conjunction with a 488/568 RGB dichroic mirror. A Hamamatsu Orca-ER charge-coupled device camera was used to record images, and analysis of representative images was performed using IPLab version 3.9.3 r4 (BD Biosciences) software.
Live cell imaging of DC/BMMC cocultures
BMDCs were labeled with PKH26 (Sigma-Aldrich) dye and incubated in a 24-well plate overnight at 37°C. Anti-OVA IgE pretreated BMMCs were allowed to incorporate Alexa Fluor 647-labeled OVA for 2 to 3 days in the presence or absence of IL-3 (10 ng/mL) and were cocultured with the PKH26-labeled BMDCs. The 24-well plates were placed in a 37°C, 5% CO2 incubator attached to a Nikon TE2000-U inverted microscope outfitted with a motorized stage. Live images at 400× magnification were captured every 7 minutes for a period of 24 hours using a CoolSNAP HQ2 camera (Photometrics, Tucson, AZ) and QEDInVivo software (Media Cybernetics, Bethesda, MD). Completed movies were analyzed using Image-Pro (Media Cybernetics) software. The number of phagocytosed mast cells was counted and divided by the total number of mast cells observed in each field. Statistical analysis was performed by ANOVA using Microsoft Excel computer software (Microsoft, Redmond, WA).
Mast-cell, DC, and T-cell cocultures
A total of 105 antigen-incorporated BMMCs or peritoneal mast cells were cocultured in 96-well round-bottom plates with 105 MACS-sorted T cells from OT-II mice using Thy1.2 beads (Miltenyi Biotec). In some experiments, unsorted 4 × 105 OT-II splenocytes, 4 × 105 CD11c+ cell-depeleted OT-II splenocytes, or 105 MoFlo-sorted Thy1.2+ OT-II T cells were cocultured with the BMMCs. CD11c+ cell depletion was performed by MACS depletion using anti-CD11c beads (Mltenyi Biotec). CD11c+ cell depletion was confirmed by flow cytometric analysis. In experiments involving DCs, 5 × 104 BMDCs were added to the T cell/BMMC cocultures. The cocultures were incubated at 37°C for 48 hours, and CD69 expression was measured on CD4+ T cells using flow cytometry.
BMMC death assays
Untransduced, MigR1-transduced, or BCL-XL-transduced BMMCs were cultured in MCM containing IL-3 (10 ng/mL) with or without anti-DNP IgE or anti-OVA IgE (1 μg/mL) for 1 to 3 days. The cells were washed thoroughly with MCM, and 1 × 105 cells were placed in cytokine-free MCM with or without OVA or DNP-OVA. In some experiments, IL-3 (10 ng/mL) was added. After various incubation times, the cells were surface-stained with anti-CD117 and annexin V and analyzed by flow cytometry.
T-cell proliferation in mice injected with antigen-incorporated mast cells
T cells were purified from OT-II.SJL mice using Thy1.2 beads, washed with PBS, and mixed with an equal volume of PBS containing 5 μM carboxy fluorescein diacetate succinimide ester (CFSE; Sigma-Aldrich) at a concentration of 1 × 107 cells/mL. The cells were mixed gently for 9 minutes at room temperature, and the reaction was immediately quenched by addition of FCS and washed. 3 × 106 CFSE-labeled T cells were injected intravenously into B6 mice. The next day, 2 × 106 OVA-incorporated MHC class II−/− BMMCs were injected intravenously or subcutaneously into these mice. Five days later, the spleen and inguinal lymph nodes were harvested, and proliferation (CFSE dilution) of transferred T cells was measured by flow cytometry.
Results
IgE enhances the incorporation of specific antigens by mast cells
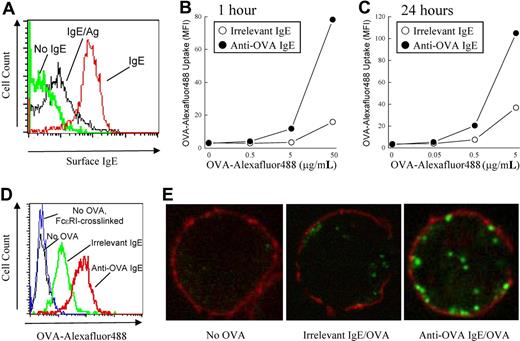
To test whether mast cells could potentially be involved in antigen-specific T-cell activation, we first examined the ability of mast cells to capture antigens via their FcϵRI. The FcϵRI on BMMCs grown in culture are initially unoccupied and can be loaded with monomeric IgE molecules of a given specificity. This IgE occupancy on the mast-cell surface can be detected by staining cells for surface IgE and analysis by flow cytometry. After crosslinking of surface IgE molecules, we found that the amount of surface IgE was rapidly down-regulated, suggesting that FcϵRI-crosslinking results in endocytosis of IgE/antigen complexes (Figure 1A).
Enhancement of antigen internalization by antigen-specific IgE. (A) BMMCs were pretreated with or without (green line) anti-DNP IgE (IgE; red line), washed, and crosslinked with DNP-OVA (IgE/Ag; black line) for 1 hour. Surface IgE was detected by flow cytometry. (B) BMMCs were pretreated with anti-DNP IgE (irrelevant IgE) or anti-OVA IgE, washed, and incubated with the indicated concentrations of Alexa Fluor 488-conjugated OVA for 1 hour or (C) 24 hours. (D) Anti-DNP IgE (green line) or anti-OVA IgE (red line) pretreated BMMCs were incubated with or without (black line) Alexa Fluor 488-conjugated OVA (5 μg/mL) or with unlabeled OVA (5 μg/mL; blue line) for 1 hour. (E) Anti-OVA (right) or anti-DNP (middle) pretreated BMMCs were incubated for 24 hours with 0.5 μg/mL Alexa Fluor 488-conjugated OVA (green fluorescence). BMMCs that received no OVA were used as a control (left). BMMCs were fixed and stained for choleratoxin to mark the cell surface (red fluorescence) and analyzed by confocal microscopy.
Enhancement of antigen internalization by antigen-specific IgE. (A) BMMCs were pretreated with or without (green line) anti-DNP IgE (IgE; red line), washed, and crosslinked with DNP-OVA (IgE/Ag; black line) for 1 hour. Surface IgE was detected by flow cytometry. (B) BMMCs were pretreated with anti-DNP IgE (irrelevant IgE) or anti-OVA IgE, washed, and incubated with the indicated concentrations of Alexa Fluor 488-conjugated OVA for 1 hour or (C) 24 hours. (D) Anti-DNP IgE (green line) or anti-OVA IgE (red line) pretreated BMMCs were incubated with or without (black line) Alexa Fluor 488-conjugated OVA (5 μg/mL) or with unlabeled OVA (5 μg/mL; blue line) for 1 hour. (E) Anti-OVA (right) or anti-DNP (middle) pretreated BMMCs were incubated for 24 hours with 0.5 μg/mL Alexa Fluor 488-conjugated OVA (green fluorescence). BMMCs that received no OVA were used as a control (left). BMMCs were fixed and stained for choleratoxin to mark the cell surface (red fluorescence) and analyzed by confocal microscopy.
To test whether this process could allow mast cells to capture specific antigens, BMMCs were pretreated with IgE against OVA or an irrelevant antigen (DNP) and were incubated with fluorescently labeled OVA protein. After a 1-hour incubation, some nonspecific uptake of fluorescent OVA was observed in mast cells pretreated with anti-DNP IgE, as measured by flow cytometry. However, in BMMCs pretreated with anti-OVA IgE, a 5- to 10-fold increase in OVA uptake was seen after a 1-hour (Figure 1B,D) or 24-hour (Figure 1C) incubation with fluorescent OVA. The increase in fluorescence intensity was not the result of increased autofluorescence from FcϵRI crosslinking because mast cells that were crosslinked with unlabeled OVA protein did not exhibit increased fluorescence (Figure 1D). These results suggest that IgE molecules can enhance the uptake of cognate but not irrelevant antigen into mast cells.
To investigate whether the IgE-bound fluorescent OVA was internalized or was just merely bound at the cell surface, we visualized the incorporated OVA by confocal microscopy. BMMCs pretreated with anti-DNP IgE followed by incubation with fluorescent OVA showed a low-intensity diffuse scattered pattern of fluorescence, which was difficult to discern from background fluorescence (Figure 1E). In contrast, large aggregates of fluorescent OVA molecules were observed in BMMCs pretreated with anti-OVA IgE. Moreover, a cross-sectional view of the intracellular compartment of the BMMCs revealed that the OVA was found intracellularly and not at the cell surface, as OVA did not colocalize with the membrane-associated choleratoxin (Figure 1E). These results suggest that IgE molecules bound to FcϵRI of mast cells can facilitate the intracellular uptake of cognate antigens.
Antigen-specific activation of CD4+ T cells by antigen-incorporated mast cells
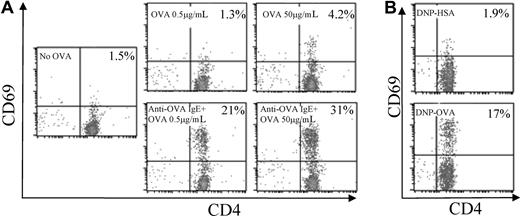
We next tested the ability of antigen-incorporated mast cells to activate CD4+ T cells. Untreated or anti-OVA IgE-treated BMMCs were incubated with OVA to allow for incorporation of OVA. The BMMCs were washed to remove any unincorporated free OVA and were cocultured with MACS-purified CD4+ T cells from OT-II OVA323-339–specific TCR transgenic mice. 2 days later, CD69 expression on CD4+ T cells was measured in the cocultures as a marker for activation. In the absence of OVA, minimal CD69 expression was observed on the CD4+ T cells. CD4+ T cells cocultured with BMMCs treated with OVA alone showed only modest up-regulation of CD69 expression. In contrast, CD4+ T cells cocultured with BMMCs that incorporated OVA through anti-OVA IgE showed dramatic up-regulation of CD69 expression (Figure 2A). These data suggest that OVA-incorporated BMMCs activate OVA-specific CD4+ T cells.
Antigen-incorporated BMMCs activate antigen-specific CD4+ T cells. (A) Anti-OVA IgE pretreated (bottom) or untreated (top) BMMCs were incubated for 48 hours with low-dose (0.5 μg/mL; middle) or high-dose (50 μg/mL; right) OVA. BMMCs that received no OVA were used as a control (left). (B) Anti-DNP IgE pretreated BMMCs were incubated for 48 hours with DNP-HSA (50 μg/mL; top) or DNP-OVA (50 μg/mL; bottom). The BMMCs were washed and cocultured with MACS-purified CD4+ T cells from OT-II mice. Forty-eight hours later, CD69 was detected on CD4+ T cells by flow cytometry. The number in the upper right corner represents the percentage of CD69+CD4+ T cells of total CD4+ T cells.
Antigen-incorporated BMMCs activate antigen-specific CD4+ T cells. (A) Anti-OVA IgE pretreated (bottom) or untreated (top) BMMCs were incubated for 48 hours with low-dose (0.5 μg/mL; middle) or high-dose (50 μg/mL; right) OVA. BMMCs that received no OVA were used as a control (left). (B) Anti-DNP IgE pretreated BMMCs were incubated for 48 hours with DNP-HSA (50 μg/mL; top) or DNP-OVA (50 μg/mL; bottom). The BMMCs were washed and cocultured with MACS-purified CD4+ T cells from OT-II mice. Forty-eight hours later, CD69 was detected on CD4+ T cells by flow cytometry. The number in the upper right corner represents the percentage of CD69+CD4+ T cells of total CD4+ T cells.
Crosslinking of FcϵRI on mast cells results in release of multiple inflammatory mediators that can potentially activate T cells in a nonspecific manner. To exclude the possibility that OVA-incorporated BMMCs activated T cells nonspecifically, anti-DNP IgE pretreated BMMCs were given DNP-OVA or DNP-HSA. CD69 up-regulation was observed only when OT-II CD4+ T cells were cocultured with BMMCs treated with DNP-OVA but not with DNP-HSA (Figure 2B). Because crosslinking of FcϵRI by both DNP-conjugated proteins caused a similar degree of degranulation and cytokine production by BMMCs (data not shown), this finding suggests that BMMC-mediated CD4+ T-cell activation occurred in an antigen-specific fashion.
Antigen-specific activation of CD4+ T cells does not require MHC class II expression by BMMCs
Mast cells have been previously reported to constitutively express MHC class II intracellularly33 and on the cell surface.34 However, a recent study examining T-cell activation by mast cells reported that cell-surface MHC class II was not detectable on either resting or activated mast cells.23 In agreement with the latter report, we did not find MHC class II molecules on the cell surface of resting or FcϵRI-activated BMMCs (Figure 3A). Furthermore, we did not detect any intracellular MHC class II molecules in our BMMC preparations. In contrast, intracellular MHC class II was detected in wild-type but not MHC class II−/− B cells (Figure 3B, data not shown), suggesting that the antibody that we used was capable of detecting intracellular MHC class II molecules. These data suggest that expression of MHC class II by mast cells was not required for antigen-specific activation of CD4+ T cells.
Activation of CD4+ T cells by antigen-incorporated BMMCs is mediated by other APCs. (A) Anti-DNP IgE pretreated BMMCs were incubated with (bottom) or without (top) DNP-OVA (50 μg/mL) for 48 hours. Cell surface and (B) intracellular MHC class II expression was measured on CD117+ BMMCs by flow cytometry. (C) Anti-OVA IgE pretreated BMMCs from wild-type (top) or MHC class II−/− (bottom) mice were incubated for 48 hours with OVA (50 μg/mL). The BMMCs were washed and cocultured with MACS-purified CD4+ T cells from OT-II mice. (D) Anti-OVA IgE pretreated MHC class II−/− BMMCs were incubated for 48 hours with OVA (50 μg/mL), washed, and cocultured with FACS-purified CD4+ T cells (top left), MACS-purified CD4+ T cells (top right), unsorted splenocytes (bottom left), or MACS-purified CD4+ T cells mixed with BMDCs (bottom right) from OT-II mice. (E) OVA-incorporated BMMCs were prepared as described here and cocultured with unsorted OT-II splenocytes (top) or CD11c+ cell-depleted OT-II splenocytes (bottom). CD69 was detected 48 hours later on CD4+ T cells by flow cytometry. The number in the upper right corner indicates the percentage of CD69+CD4+ T cells of total CD4+ T cells.
Activation of CD4+ T cells by antigen-incorporated BMMCs is mediated by other APCs. (A) Anti-DNP IgE pretreated BMMCs were incubated with (bottom) or without (top) DNP-OVA (50 μg/mL) for 48 hours. Cell surface and (B) intracellular MHC class II expression was measured on CD117+ BMMCs by flow cytometry. (C) Anti-OVA IgE pretreated BMMCs from wild-type (top) or MHC class II−/− (bottom) mice were incubated for 48 hours with OVA (50 μg/mL). The BMMCs were washed and cocultured with MACS-purified CD4+ T cells from OT-II mice. (D) Anti-OVA IgE pretreated MHC class II−/− BMMCs were incubated for 48 hours with OVA (50 μg/mL), washed, and cocultured with FACS-purified CD4+ T cells (top left), MACS-purified CD4+ T cells (top right), unsorted splenocytes (bottom left), or MACS-purified CD4+ T cells mixed with BMDCs (bottom right) from OT-II mice. (E) OVA-incorporated BMMCs were prepared as described here and cocultured with unsorted OT-II splenocytes (top) or CD11c+ cell-depleted OT-II splenocytes (bottom). CD69 was detected 48 hours later on CD4+ T cells by flow cytometry. The number in the upper right corner indicates the percentage of CD69+CD4+ T cells of total CD4+ T cells.
Although below the detectable limit by flow cytometry, we reasoned it was still possible that sufficient numbers of peptide-loaded MHC class II molecules were present at the cell surface to be responsible for activation of the CD4+ T cells. To test more rigorously whether MHC class II expression by BMMCs was necessary for antigen-specific activation of CD4+ T cells, OVA-incorporated wild-type and MHC class II−/− BMMCs were cocultured with OVA-specific CD4+ T cells. Wild-type and MHC class II−/− BMMCs were equally effective in activating OVA-specific CD4+ T cells (Figure 3C), indicating that MHC class II expression by BMMCs was not required for activation of CD4+ T cells.
Because antigen presentation to CD4+ T cells must occur through MHC class II, this finding suggested that another MHC class II–positive cell type was contaminating our BMMC/T cell cocultures. This contamination was possible given that the CD4+ T cells used in these cultures were purified by MACS, which generally resulted in 90% to 95% purity, compared with more than 98% by FACS (data not shown). Furthermore because the OT-II mice were on a RAG−/− background and devoid of B cells, the contaminating cell populations were likely to be enriched in more potent APCs such as DCs. Indeed, when OVA-incorporated BMMCs were cocultured with FACS-purified OVA-specific CD4+ T cells, CD69 up-regulation was not observed. In contrast, the same OVA-incorporated BMMCs induced CD69 up-regulation on MACS-purified CD4+ T cells; CD69 was even further enhanced when cocultured with unsorted OT-II splenocytes (Figure 3D). Collectively, these results suggested that the antigen taken up by mast cells was presented to antigen-specific T cells by other MHC class II+ APCs such as DCs. Consistent with this notion, purified BMDCs were capable of augmenting CD69 expression by T cells when added to the BMMC/T cell cocultures (Figure 3D). Furthermore, CD69 up-regulation was dramatically reduced when OVA-incorporated BMMCs were cocultured with CD11c+ cell-depleted compared with unsorted OT-II splenocytes (Figure 3E). These results suggest that CD11c+ DCs are largely responsible for presenting mast cell–incorporated OVA.
To test whether another source of mast cells could also mediate antigen-specific activation of T cells, we harvested peritoneal mast cells from MHC class II−/− mice. Similar to BMMCs, OVA-incorporated peritoneal mast cells up-regulated CD69 on OVA-specific T cells, which was enhanced by pretreatment of mast cells with anti-OVA IgE (Figure 4A).
OVA-incorporated BMMCs and peritoneal mast cells are a potent source of antigen for presentation to T cells. (A) Peritoneal mast cells from MHC class II−/− mice were pretreated with (bottom) or without (top) anti-OVA IgE, washed, and incubated with OVA (50 μg/mL) for 2 days. The peritoneal mast cells were then washed and cocultured with OT-II splenocytes. (B) OT-II splenocytes were treated without (top left) or with OVA at 5 μg/mL (bottom left) or 0.5 μg/mL (top right). Anti-OVA IgE-pretreated MHC class II−/− BMMCs were incubated with OVA (0.5 μg/mL) for 2 days, washed, and cocultured with the same OT-II splenocytes (bottom right). CD69 was detected 48 hours later on CD4+ T cells by flow cytometry. The number in the upper right corner indicates the percentage of CD69+CD4+ T cells of total CD4+ T cells.
OVA-incorporated BMMCs and peritoneal mast cells are a potent source of antigen for presentation to T cells. (A) Peritoneal mast cells from MHC class II−/− mice were pretreated with (bottom) or without (top) anti-OVA IgE, washed, and incubated with OVA (50 μg/mL) for 2 days. The peritoneal mast cells were then washed and cocultured with OT-II splenocytes. (B) OT-II splenocytes were treated without (top left) or with OVA at 5 μg/mL (bottom left) or 0.5 μg/mL (top right). Anti-OVA IgE-pretreated MHC class II−/− BMMCs were incubated with OVA (0.5 μg/mL) for 2 days, washed, and cocultured with the same OT-II splenocytes (bottom right). CD69 was detected 48 hours later on CD4+ T cells by flow cytometry. The number in the upper right corner indicates the percentage of CD69+CD4+ T cells of total CD4+ T cells.
We next compared the potency of OVA-incorporated mast cells to soluble OVA protein as an antigen source for DC-mediated presentation to T cells. Antigen-specific T-cell activation was observed when OT-II splenocytes were given OVA at 5 μg/mL but not at 0.5 μg/mL (Figure 4B). In contrast, anti-OVA IgE-pretreated mast cells incubated with 0.5 μg/mL OVA for 2 days and washed thoroughly were capable of inducing activation of OT-II T cells (Figure 4B). Because only a fraction of soluble OVA is endocytosed into mast cells, this finding suggests that OVA-incorporated mast cells are a much more potent antigen source than soluble OVA protein for DC-mediated presentation to T cells.
Antigen-specific proliferation of CD4+ T cells by mast cells in vitro and in vivo
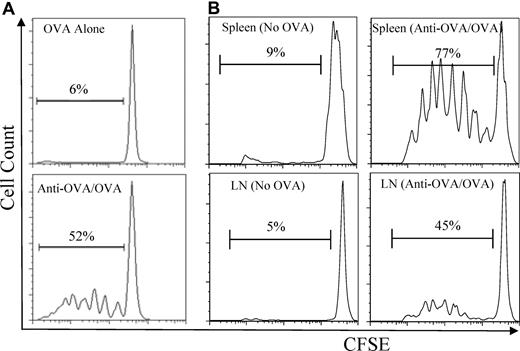
We next tested the ability of the antigen-incorporated MHC class II−/− BMMCs to prime naive antigen-specific T-cell proliferation in vitro and in vivo. Similar to CD69 up-regulation, the addition of OVA-incorporated mast cells induced OVA-specific T-cell proliferation when added to OT-II splenocyte cultures (Figure 5A). To test this in vivo, B6 mice were adoptively transferred with CFSE-labeled OT-II CD4+ T cells and were injected intravenously or subcutaneously with MHC class II−/− BMMCs that incorporated OVA through anti-OVA IgE. 5 days later, CFSE dilution of CD4+ T cells in secondary lymphoid organs was detected by flow cytometry as a measure of cell proliferation. Proliferation of CD4+ T cells was observed in the spleen or inguinal lymph nodes of mice injected with OVA-incorporated BMMCs intravenously or subcutaneously, respectively (Figure 5B). In contrast, CD4+ T-cell proliferation was not observed in mice injected with BMMCs treated without OVA (Figure 5B). These results suggest that antigen-incorporated BMMCs can serve as a source of antigen for the proliferation of naive T cells in vivo.
OVA-incorporated BMMCs induce T-cell proliferation in vitro and in vivo. (A) MHC class II−/− BMMCs were pretreated with (bottom) or without (top) anti-OVA IgE, washed, and incubated with OVA (0.5 μg/mL) for 2 days. The BMMCs were then washed and cocultured with CFSE-labeled OT-II splenocytes. (B) 3 × 106 CFSE-labeled CD4+ T cells from OT-II.SJL mice were injected intravenously into wild-type B6 mice. MHC class II−/− BMMCs were anti-OVA IgE pretreated and incubated with (right) or without (left) OVA (50 μg/mL) for 48 hours and injected intravenously into mice 1 day after T-cell transfer. Five days later, the spleen (top) and inguinal lymph nodes (LN; bottom) of mice previously injected with intravenous or subcutaneous BMMCs, respectively, were harvested, and CFSE dilution of transferred CD4+ T cells was analyzed by flow cytometry. The cells represented in the histograms are gated on CD45.1+CD4+ T cells.
OVA-incorporated BMMCs induce T-cell proliferation in vitro and in vivo. (A) MHC class II−/− BMMCs were pretreated with (bottom) or without (top) anti-OVA IgE, washed, and incubated with OVA (0.5 μg/mL) for 2 days. The BMMCs were then washed and cocultured with CFSE-labeled OT-II splenocytes. (B) 3 × 106 CFSE-labeled CD4+ T cells from OT-II.SJL mice were injected intravenously into wild-type B6 mice. MHC class II−/− BMMCs were anti-OVA IgE pretreated and incubated with (right) or without (left) OVA (50 μg/mL) for 48 hours and injected intravenously into mice 1 day after T-cell transfer. Five days later, the spleen (top) and inguinal lymph nodes (LN; bottom) of mice previously injected with intravenous or subcutaneous BMMCs, respectively, were harvested, and CFSE dilution of transferred CD4+ T cells was analyzed by flow cytometry. The cells represented in the histograms are gated on CD45.1+CD4+ T cells.
FcϵRI activation induces cell death and is required for antigen-specific activation of T cells
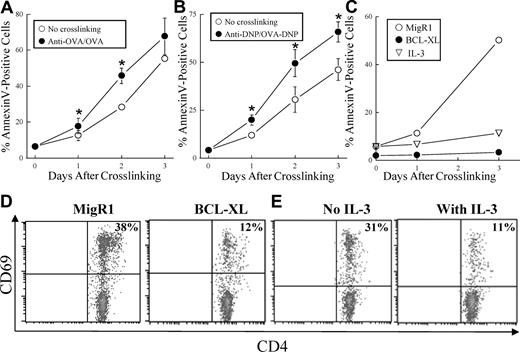
To explore how the presentation of OVA-internalized BMMCs may occur, we investigated the fate of mast cells stimulated by IgE and antigen. In our experimental setup, a proportion of the mast cells undergoes apoptosis during antigen incorporation because IL-3 is removed from the mast-cell cultures during this process. We noted that T-cell activation in the BMMC/T-cell cocultures positively correlated with an increased proportion of dead mast cells based on forward and side scatter characteristics by flow cytometry (data not shown). This observation led us to hypothesize that FcϵRI activation may enhance BMMC death in the absence of IL-3. Indeed, engagement of FcϵRI with either anti-OVA IgE/OVA or anti-DNP IgE/DNP-OVA resulted in significantly increased apoptosis of BMMCs (Figure 6A,B).
FcϵRI activation enhances BMMC apoptosis and is required for optimal induction of T-cell activation. (A) Anti-OVA IgE or (B) anti-DNP IgE pretreated BMMCs were incubated with or without OVA (50 μg/mL) or DNP-OVA (0.5 μg/mL), respectively, for the indicated time periods. Data are represented as mean plus or minus SEM of 3 independent experiments (*P < .05 between the 2 groups by paired t test). (C) Anti-OVA IgE pretreated MigR1- (vector) or BCL-XL–transduced BMMCs were incubated with OVA (50 μg/mL) with or without IL-3 (10 ng/mL) for the indicated time periods. BMMC death was measured by examining the proportion of annexin V–positive cells by flow cytometry. (D) Anti-OVA IgE pretreated MigR1- (left) or BCL-XL–transduced (right) MHC class II−/− BMMCs were incubated for 48 hours with OVA (50 μg/mL), washed, and cocultured with unsorted splenocytes from OT-II mice. (E) Anti-OVA IgE pretreated MHC class II−/− BMMCs were incubated for 48 hours with OVA (50 μg/mL), washed, and cocultured with unsorted splenocytes from OT-II mice in the presence (right) or absence (left) of IL-3 (10 ng/mL). CD69 was detected 48 hours later on CD4+ T cells by flow cytometry. The number in the upper right corner indicates the percentage of CD69+CD4+ T cells.
FcϵRI activation enhances BMMC apoptosis and is required for optimal induction of T-cell activation. (A) Anti-OVA IgE or (B) anti-DNP IgE pretreated BMMCs were incubated with or without OVA (50 μg/mL) or DNP-OVA (0.5 μg/mL), respectively, for the indicated time periods. Data are represented as mean plus or minus SEM of 3 independent experiments (*P < .05 between the 2 groups by paired t test). (C) Anti-OVA IgE pretreated MigR1- (vector) or BCL-XL–transduced BMMCs were incubated with OVA (50 μg/mL) with or without IL-3 (10 ng/mL) for the indicated time periods. BMMC death was measured by examining the proportion of annexin V–positive cells by flow cytometry. (D) Anti-OVA IgE pretreated MigR1- (left) or BCL-XL–transduced (right) MHC class II−/− BMMCs were incubated for 48 hours with OVA (50 μg/mL), washed, and cocultured with unsorted splenocytes from OT-II mice. (E) Anti-OVA IgE pretreated MHC class II−/− BMMCs were incubated for 48 hours with OVA (50 μg/mL), washed, and cocultured with unsorted splenocytes from OT-II mice in the presence (right) or absence (left) of IL-3 (10 ng/mL). CD69 was detected 48 hours later on CD4+ T cells by flow cytometry. The number in the upper right corner indicates the percentage of CD69+CD4+ T cells.
This FcϵRI-activated cell death could be prevented with addition of the mast cell survival-promoting cytokine IL-3 or by retroviral transduction with the antiapoptotic protein BCL-XL (Figure 6C). To test whether BMMC apoptosis was related to antigen-specific activation of T cells, OT-II splenocytes were cocultured with OVA-incorporated BMMCs that were protected from cell death with IL-3 or BCL-XL. Compared with BMMCs retrovirally transduced with MigR1 vector alone, BMMCs expressing BCL-XL induced less CD69 expression on OT-II T cells (Figure 6D). A similar effect was observed when BMMC/OT-II splenocyte cocultures were given IL-3 (Figure 6E). These results suggest that apoptosis of BMMCs is required for efficient presentation of OVA-incorporated BMMCs by DCs.
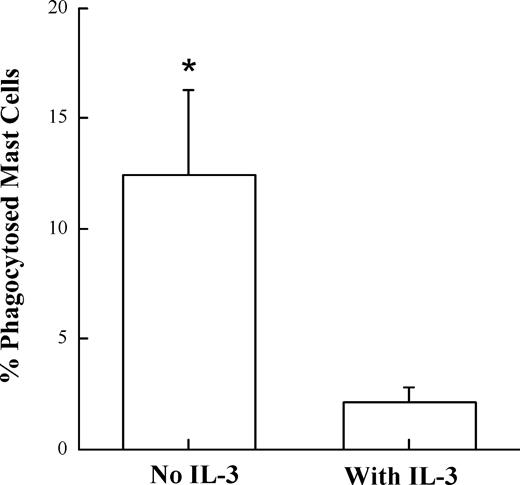
To document the encounter of BMDCs with apoptotic BMMCs, we used live cell fluorescent imaging. Anti-OVA IgE pretreated BMMCs were allowed to incorporate fluorescent OVA and were cocultured with BMDCs fluorescently labeled with PKH26 dye. The interaction of DCs and mast cells were then monitored overnight via fluorescence microscopy. During this overnight coculture, we obtained real-time movies of BMDCs phagocytosing apoptotic BMMCs (Video S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). A significant decrease in the number of phagocytosed mast cells was observed when mast cells were protected against cell death with IL-3 (Figure 7). Together, these data suggest that phagocytosis of antigen-incorporated apoptotic BMMCs represents a mechanism by which antigens can be transferred from mast cells to DCs for the antigen-specific activation of T cells.
Protection of mast-cell death by IL-3 results in significantly decreased phagocytosis by DCs. Fluorescently labeled BMDCs were cocultured with BMMCs that previously incorporated fluorescent OVA through anti-OVA IgE in the presence or absence of IL-3. Images of the cocultures were captured by a fluorescent microscope every 7 minutes for 10 hours. The number of phagocytosed mast cells was counted and divided by the total number of mast cells observed per 400× field. The mean plus or minus SEM of 5 independent fields for each condition is represented in the bar graph (*P < .03).
Protection of mast-cell death by IL-3 results in significantly decreased phagocytosis by DCs. Fluorescently labeled BMDCs were cocultured with BMMCs that previously incorporated fluorescent OVA through anti-OVA IgE in the presence or absence of IL-3. Images of the cocultures were captured by a fluorescent microscope every 7 minutes for 10 hours. The number of phagocytosed mast cells was counted and divided by the total number of mast cells observed per 400× field. The mean plus or minus SEM of 5 independent fields for each condition is represented in the bar graph (*P < .03).
Discussion
We investigated the fate of antigens that bind to FcϵRI on mast cells and found that uptake of specific antigens can be greatly enhanced by antigen-specific IgE molecules. Coculture of OVA-incorporated mast cells with OVA-specific CD4+ T cells resulted in T-cell activation in an antigen-specific manner. Activation of T cells did not require MHC class II expression by mast cells, suggesting that mast cells were not directly involved in antigen presentation. Rather, the antigen-captured mast cells were presented by other APCs. To investigate how presentation of OVA-incorporated mast cells by DCs may occur, we investigated the fate of mast cells stimulated by antigen and found that FcϵRI crosslinking enhanced mast-cell apoptosis. This cell death resulted in ingestion of mast cells by DCs and was required for efficient presentation because protection against mast-cell death significantly decreased T-cell activation. These results suggest that antigen-captured mast cells may be involved in antigen presentation by acting as an antigen reservoir for propagation of ongoing T-cell responses.
Our results demonstrating antigen uptake by mast cells are in agreement with a recent report showing that large particulate antigens can be phagocytosed by mast cells through an IgE-dependent mechanism.35 This report also demonstrated that mast cells could incorporate these particulate antigens in vivo. Using a similar mechanism involving FcϵRI and IgE, circulating basophils were also shown to be efficient antigen-capturing cells in vivo.36 Thus, IgE molecules appear to be an efficient vehicle for FcϵRI-bearing cells to incorporate specific antigens. B cells use a similar mechanism of antigen uptake through their B-cell receptor. The IgE-bound FcϵRI, however, offers several advantages over the B-cell receptor. First, the antigens that are incorporated by IgE/FcϵRI must have been rendered immunogenic in a previous immune response because IgE molecules are produced by antigen-activated B cells and are not mast cell intrinsic. Second, IgE of various specificities can be present on any given mast cell, whereas B cells express a cell-surface antibody of a single fixed specificity. Finally, empty FcϵRI molecules are constantly created at the cell surface and are stabilized on binding of new IgE molecules,37 and thus the mast cells can adapt rapidly to the continuously changing specificities of host-produced IgE. Taken together, these attributes offer mast cells a flexible approach to specifically incorporate antigens of minute quantities.
Once specific antigens were incorporated, mast cells were capable of activating CD4+ T cells in an antigen-specific manner. This activation appeared to occur independently of MHC class II expression by mast cells because resting or FcϵRI-activated mast cells did not express MHC class II. Moreover, MHC class II−/− mast cells were equally effective at inducing T-cell activation as wild-type mast cells. Thus, rather than directly activating T cells, the mast cells served as a source of antigen for other APCs such as DCs to present to T cells. This finding differs from several previous reports demonstrating that mast cells constitutively express MHC class II and can directly activate CD4+ T-cell hybridomas in an antigen-specific manner.34 Initially, we thought that the discrepancy was a result of differences in the type of mast cell used. In contrast to our culture conditions that use IL-3 and SCF to generate BMMCs, the former studies used protocols that involve WEHI-conditioned medium or IL-3 alone. In addition, cytokines such as IL-4, granulocyte-macrophage colony-stimulating factor,38 and interferonγ39 were used to boost the antigen-presenting capacity of mast cells. However, using a similar approach, we were still unable to detect MHC class II expression by mast cells on the cell surface or intracellularly (data not shown).
Activation of FcϵRI resulted in enhancement of cell death by mast cells. This finding is consistent with another report demonstrating that FcϵRI crosslinking enhances IL-4/IL-10–induced apoptosis of mast cells.40 This report showed that cytokines such as IL-4 and IL-10 decrease BCL-2 and BCL-XL expression by mast cells, rendering them susceptible to apoptosis. Thus, under Th2 inflammatory conditions, FcϵRI crosslinking may result in increased apoptosis of mast cells. In our present model, efficient activation of T cells appeared to require cell death by mast cells because protection of mast-cell death with IL-3 or BCL-XL attenuated the induction of CD69 by T cells. In previous studies, apoptotic bodies have been shown to be taken up by DCs and macrophages and presented to T cells.41 Under noninflammatory circumstances, apoptotic bodies are thought to induce a tolerogenic response to self-antigens,41 which is partly due to the absence of an inflammatory milieu with naturally occurring apoptotic bodies. However, in the case of mast cells, crosslinking of FcϵRI by specific antigens will result not only in antigen incorporation but also in proinflammatory mediator release. Furthermore, in contrast to naturally occurring apoptotic cells, FcϵRI-activated apoptotic mast cells have incorporated previously encountered immunogenic antigens. Thus, the context in which apoptotic mast cells are presented may yield a stimulatory rather than tolerogenic response. However, in light of a recent report demonstrating that mast cells are required for skin allograft tolerance induction,42 it is also possible that antigen-incorporated mast cells could induce tolerance under certain conditions.
There may be other mechanisms by which antigen-incorporated mast cells transfer antigen to APCs. Exosomes derived from MHC class II haplotype-mismatched mast cells that contain specific antigens have been shown to induce maturation of DCs and be presented to antigen-specific T cells.43 Another report demonstrated that antigens incorporated by mast cells remain undegraded, colocalize with secretory compartments, and are released on reactivation of mast cells.35 It is unlikely that either mechanism plays a major role in our system because T-cell activation by mast cells in our coculture system was largely dependent on mast-cell apoptosis. Furthermore, using fluorescent live cell imaging, we have demonstrated that apoptotic mast cells are phagocytosed by DCs in our coculture system. However, it remains likely that all 3 proposed mechanisms could play a role in vivo for mast cells to influence antigen-specific T-cell responses.
Mast cells not only play a role in immediate hypersensitivity but also are involved in the activation of T cells in a variety of disease models. Although the mechanism by which this occurs is largely thought to occur through inflammatory mediator release by mast cells, we propose that mast cells that have incorporated antigen through FcϵRI can also augment T-cell responses by participating indirectly in the antigen presentation process. One may envisage that mast cells can accumulate specific antigens present at low concentrations and serve as an ongoing source of antigen as they undergo apoptosis. Apoptosis may occur in situ at the antigen-encountered site or could possibly occur in lymph nodes because activated mast cells have been found to migrate to lymph nodes.44 In addition, the mast cells may gradually release their antigens through mechanisms that have been proposed by others.35,43 Nevertheless, we propose that antigens that are incorporated by mast cells are meaningful for modulation of an ongoing T cell–mediated immune response. Further studies are required to determine precisely how mast cells contribute to T-cell activation under a variety of stimulating conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Laurence Turka for providing valuable reagents, Drs Terri Laufer, Gregory Wu and members of the Koretzky laboratory for helpful discussions, Justina Stadanlick, Drs. Martha Jordan, and Jennifer Smith for careful reading of our manuscript, and Mariko Okumura, Emily Chen, and Mercy Gohil for excellent technical support.
This work was supported by grants from the Sandler Program for Asthma Research and from the National Institutes of Health.
Authorship
Contribution: T.K. wrote the paper, designed and performed research, and analyzed data; J.D.B., R.G.B., T.Z., E.J.A., and J.E.S. performed research; P.L.J. contributed vital analytical tools; G.A.K. designed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gary A. Koretzky, Abramson Family Cancer Research Institute, University of Pennsylvania, BRB II/III Room 415, 421 Curie Boulevard, Philadelphia, PA 19104-6160; e-mail: koretzky@mail.med.upenn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal