Abstract
Platelets, in addition to their function in hemostasis, play an important role in wound healing and tumor growth. Because platelets contain angiogenesis stimulators and inhibitors, the mechanisms by which platelets regulate angiogenesis remain unclear. As platelets adhere to activated endothelium, their action can enhance or inhibit local angiogenesis. We therefore suspected a higher organization of angiogenesis regulators in platelets. Using double immunofluorescence and immunoelectron microscopy, we show that pro- and antiangiogenic proteins are separated in distinct subpopulations of α-granules in platelets and megakaryocytes. Double immunofluorescence labeling of vascular endothelial growth factor (VEGF) (an angiogenesis stimulator) and endostatin (an angiogenesis inhibitor), or for thrombospondin-1 and basic fibroblast growth factor, confirms the segregation of stimulators and inhibitors into separate and distinct α-granules. These observations motivated the hypothesis that distinct populations of α-granules could undergo selective release. The treatment of human platelets with a selective PAR4 agonist (AYPGKF-NH2) resulted in release of endostatin-containing granules, but not VEGF-containing granules, whereas the selective PAR1 agonist (TFLLR-NH2) liberated VEGF, but not endostatin-containing granules. In conclusion, the separate packaging of angiogenesis regulators into pharmacologically and morphologically distinct populations of α-granules in megakaryocytes and platelets may provide a mechanism by which platelets can locally stimulate or inhibit angiogenesis.
Introduction
Angiogenesis, the process of new vessel development, plays an essential role in embryogenesis, but postnatal angiogenesis is limited to sites of abnormal vascular surface. An activated vascular endothelium can be induced by tissue injury or wound healing, by hormonal cycling such as in pregnancy and ovulation, or by tumor-induced vessel growth. In all of these circumstances, platelets act as the initial responder to vascular change and provide a flexible delivery system for angiogenesis-related molecules.1-4 The process of postnatal angiogenesis is regulated by a continuous interplay of stimulators and inhibitors of angiogenesis, and their imbalance contributes to numerous inflammatory, malignant, ischemic, and immune disorders.5 There is a revived interest in the overlap between angiogenesis and platelets6 because several clinical trials have now shown that anticoagulation can improve cancer survival7,8 beyond the benefit derived from the treatment of deep vein thrombosis alone
It is known that platelets stimulate endothelial cells in culture and can promote the assembly of capillary-like structures in vitro.9,10 Platelets may modulate angiogenesis by releasing promoters such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), platelet derived growth factor (PDGF), and matrix metalloproteinases (MMPs).1,6,11-18 The repertoire of angiogenesis inhibitors contained within platelets includes endostatin, platelet factor-4, thrombospondin-1, α2-macroglobulin, plasminogen activator inhibitor-1, and angiostatin.19,20 Although platelets contain 3 types of secretory granules (α-granules, dense granules, and lysosomes), most angiogenic regulatory proteins have been localized to α-granules. α-Granules are 200 to 500 nm in size and contain proteins that enhance the adhesive process, promote cell-cell interactions, and stimulate vascular repair. By adhering to the endothelium of injured organs and tissues and then secreting the contents of their α-granules, platelets may be capable of depositing high concentrations of angiogenesis regulatory proteins in a localized manner.
A body of experimental data and clinical investigations suggests that platelets are major regulators of angiogenesis.21 However, because platelets contain both pro- and antiangiogenic regulatory proteins and because it has been assumed that the contents of α-granules are homogeneous, it has been unclear how platelets could either stimulate or inhibit angiogenesis. We provide new details about the organization of angiogenesis regulatory proteins in the α-granules of platelets and address the mechanism of how the selective release of these granules leads to the regulation of angiogenesis. Here we report the novel finding that angiogenic and antiangiogenic proteins are segregated into different sets of α-granules in platelets. We provide a mechanism for the differential release of these α-granules and show that these distinct populations of α-granules may be regulated by differential G-protein–mediated signaling pathways.
Methods
Approval was obtained from the Partners Human Research Committee institutional review board, Boston, MA, for these studies. Informed consent was provided in accordance with the Declaration of Helsinki.
Preparation of resting platelets
Human blood from healthy volunteers, drawn into 0.1 volume of Aster-Jandl anticoagulant, was centrifuged at 110g for 10 minutes. None of the volunteers had ingested aspirin or other nonsteroidal anti-inflammatory drugs for at least 10 days before blood collection. The platelet-rich plasma was gel-filtered through a Sepharose 2B column equilibrated with a solution containing 145 mM NaCl, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 10 mM glucose, 0.5 mM Na2HPO4, 5 mM KCl, 2 mM MgCl2, and 0.3% bovine serum albumin (BSA), pH 7.4. The number of platelets was counted by fluorescence–activated cell sorting and adjusted to 2 × 108/mL. The isolated platelet suspension was incubated at 37°C for up to 1 hour. The resting state of the platelets was routinely confirmed by PAC1 antibody and antitubulin immunofluorescence staining.
Activation of platelets
Release of α-granules was examined in vitro in response to 10 μm AYPGK-NH2, a selective PAR4-activating peptide, or 8 μm TFLLR-NH2, a PAR1-activating peptide. Peptides were prepared by solid-phase synthesis at the Peptide Synthesis Facility of Synbiocsi (Livermore, CA). Isolated platelets were exposed to PAR activating peptide or vehicle for 10 minutes, fixed with 4% formaldehyde for 20 minutes, attached to polylysine-coated coverslips, and then processed for immunofluorescence microscopy.
Megakaryocyte cultures
Livers were recovered from mouse fetuses, and single-cell suspensions were generated using methods described previously.22 Between the fourth and sixth days of megakaryocyte culture, cells were placed on a 1.5% to 3% albumin step gradient and sedimented23 to obtain enriched populations of megakaryocytes.
Immunofluorescence microscopy
Rabbit anti-VEGF antibody (Ab-1) and mouse anti-VEGF (Ab-7) were obtained from Lab Visions (Fremont, CA). Rabbit antiendostatin antibody (Ab-1) was obtained from Lab Visions. Mouse antithrombospondin antibody (Ab-4, 6.1) was obtained from Lab Visions. Rabbit polyclonal antifibroblast growth factor basic was obtained from Abcam (Cambridge, MA). Mouse antifibrinogen was obtained from BD Biosciences (Franklin Lakes, NJ) and rabbit antifibrinogen was obtained from Santa Cruz Biotechnologies. Rabbit anti-von Willebrand factor was obtained from Chemicon (Billerica, MA) and Dako (Carpenteria, CA). Alexa 568 antimouse, Alexa 488 antirabbit, Alexa 568 antirabbit, and Alexa 488 antimouse secondary antibodies were purchased from Jackson Immuno-Research Laboratories (West Grove, PA). Actin filament integrity was assayed by fluorescence microscopy of fixed specimens stained with 1 mM phalloidin-Alexa 488 (Molecular Probes, Eugene, OR) for 30 minutes and washed 4 times with blocking buffer. Resting platelets were fixed for 20 minutes in suspension by the addition of 1 vol of 8% formaldehyde. Solutions of fixed platelets in suspension were placed in wells of a 24-well microliter plate, each containing a polylysine-coated coverslip, and the plate was centrifuged at 250g for 5 minutes to attach the cells to the coverslip. Megakaryocytes were fixed with 4% formaldehyde in Hanks' balanced salt solution (GIBCO BRL, Invitrogen, Carlsbad, CA) for 20 minutes, centrifuged at 500g for 4 minutes onto coverslips previously coated with poly-L-lysine, and permeabilized with 0.5% Triton X-100 in Hanks' balanced salt solution. Specimens were blocked overnight in phosphate-buffered saline (PBS) with 1% BSA, incubated in primary antibody for 2 to 3 hours, washed, and treated with appropriate secondary antibody for 1 hour, and then washed extensively. Primary antibodies were used at 1 μg/mL in PBS containing 1% BSA and secondary antibodies at 1:500 dilution in the same buffer. Controls were processed identically except for omission of the primary antibody. Controls consisted of either incubating cells with one or both primary antibodies without fluorescently labeled secondary antibodies, or cells incubated with one or both fluorescently labeled secondary antibodies in the absence of primary antibodies. Preparations were mounted in Aqua polymount from Polysciences (Warrington, PA) and analyzed at room temperature on a Nikon TE 2000 Eclipse microscope equipped with a Nikon 100×/1.4 NA objective and a 100-W mercury lamp. Images were acquired with a Hamamatsu (Bridgewater, NJ) Orca IIER CCD camera. Electronic shutters and image acquisition were under the control of Molecular Devices Metamorph software (Downington, PA). Images were acquired by fluorescence microscopy with an image capture time of 200 to 500 ms.
Immunogold-electron microscopy
For preparation of cryosections, isolated human platelets were fixed with 4% paraformaldehyde in 0.1 M Na phosphate buffer, pH 7.4. After 2 hours of fixation at room temperature, the cell pellets were washed with PBS containing 0.2 M glycine to quench free aldehyde groups from the fixative. Before freezing in liquid nitrogen, cell pellets were infiltrated with 2.3 M sucrose in PBS for 15 minutes. Frozen samples were sectioned at −120°C, and the sections were transferred to formvar-carbon coated copper grids and floated on PBS until the immunogold labeling was carried out. The gold labeling was carried out at room temperature on a piece of parafilm. All antibodies and protein A gold were diluted with 1% BSA. The diluted antibody solution was centrifuged for 1 minute at 5000g before labeling to avoid possible aggregates. All antibodies were used at a concentration of 1 μg/mL. Grids were floated on drops of 1% BSA for 10 minutes to block for nonspecific labeling, transferred to 5-μL drops of primary antibody, and incubated for 30 minutes. The grids were then washed in 4 drops of PBS for a total of 15 minutes, transferred to 5 μL drops of Protein-A gold for 20 minutes, and washed in 4 drops of PBS for 15 minutes and 6 drops of double distilled water. For double labeling, after the first Protein A gold incubation, grids were washed in 4 drops of PBS for a total of 15 minutes and then transferred to a drop of 1% glutaraldehyde in PBS for 5 minutes and washed in 4 drops of PBS/0.15 M glycine. The second primary antibody was then applied, followed by PBS washing and treatment with different size protein A gold as above. Contrasting/embedding of the labeled grids was carried out on ice in 0.3% uranyl acetate in 2% methyl cellulose for 10 minutes. Grids were picked up with metal loops, leaving a thin coat of methyl cellulose. The grids were examined in a Tecnai G2 Spirit BioTWIN transmission electron microscope (Hillsboro, OR) at 18 500× magnification at an accelerating voltage of 80 kV. Images were recorded with an AMT 2k CCD camera.
Preparation of photomicrographs
The digital images produced in Metamorph were assembled into composite images using Adobe Photoshop 8.0 (Adobe Systems, San Jose, CA).
Results
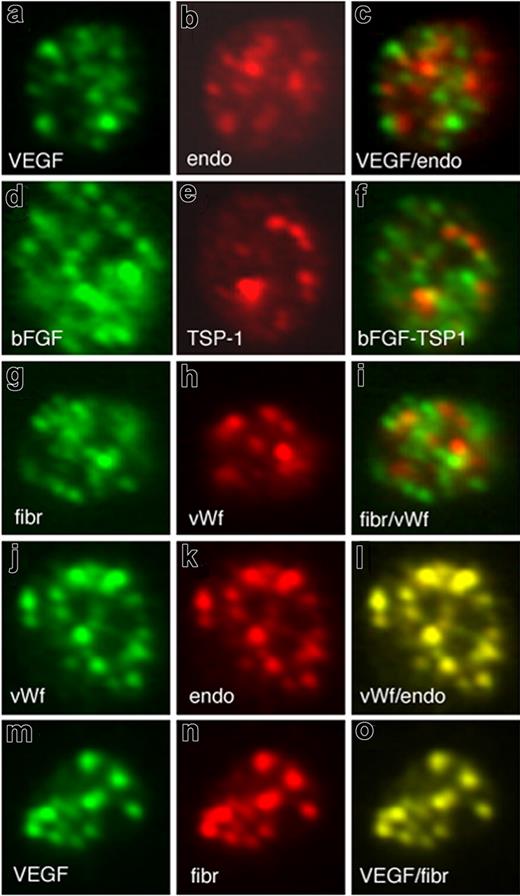
The localization of angiogenesis regulatory proteins within the platelet is important for understanding how platelets contribute to new blood vessel formation. The capacity of platelets to regulate angiogenesis could result from segregation of proangiogenic and antiangiogenic regulators into separate granules. To test this possibility, we compared the localization of the most well-characterized proangiogenic protein VEGF and the established antiangiogenic regulator endostatin, in resting platelets by immunofluorescence microscopy. The majority of α-granules stained for either VEGF (labeled green) or endostatin (labeled red), and little evidence of colocalization as would be indicated by yellow in the merged image was observed (Figure 1A-C). Similarly, double immunofluorescence microscopy comparing the localization of the endogenous angiogenesis inhibitor thrombospondin-1 and bFGF, another angiogenesis stimulator, also showed segregation of these proteins into separate, distinct granules (Figure 1D-F). To establish whether the segregation of proteins into distinct α-granules was specific to angiogenesis regulatory proteins, we examined the localization of von Willebrand factor (VWF) and fibrinogen. To evaluate the degree of overlap of proteins, we investigated the combination of fibrinogen and VWF. Surprisingly, fibrinogen and VWF also segregated into separate and distinct α-granules (Figure 1G-I). Immunofluorescence microscopy further revealed that VWF colocalized with endostatin (Figure 1J,K) and fibrinogen predominantly with the VEGF-containing α-granules (Figure 1M-O).
Pro- and antiangiogenic regulators organize into separate, distinct α-granules in resting platelets. Double immunofluorescence microscopy of resting platelets using antibodies against VEGF (A) and endostatin (B) and an overlay (C). Double immunofluorescence microscopy of resting platelets using antibodies against bFGF (D) and TSP-1 (E) and an overlay (F). Double immunofluorescence microscopy of resting platelets using antibodies against fibrinogen (G) and von Willebrand factor (H) and an overlay (I). Double immunofluorescence microscopy of resting platelets using antibodies against von Willebrand factor (J) and endostatin (K) and an overlay (L). Double immunofluorescence microscopy of resting platelets using antibodies against VEGF (M) and fibrinogen (N) and an overlay (O)..
Pro- and antiangiogenic regulators organize into separate, distinct α-granules in resting platelets. Double immunofluorescence microscopy of resting platelets using antibodies against VEGF (A) and endostatin (B) and an overlay (C). Double immunofluorescence microscopy of resting platelets using antibodies against bFGF (D) and TSP-1 (E) and an overlay (F). Double immunofluorescence microscopy of resting platelets using antibodies against fibrinogen (G) and von Willebrand factor (H) and an overlay (I). Double immunofluorescence microscopy of resting platelets using antibodies against von Willebrand factor (J) and endostatin (K) and an overlay (L). Double immunofluorescence microscopy of resting platelets using antibodies against VEGF (M) and fibrinogen (N) and an overlay (O)..
The organization of angiogenesis regulators into distinct αgranules is not exclusive to platelets. Megakaryocytes have been shown to generate platelets by remodeling their cytoplasm into long proplatelet extensions that transport individual α-granules on their microtubule tracks.24 To address whether inhibitors and stimulators of angiogenesis are packaged into distinct populations of α-granules in the precursor cells of platelets, we analyzed the distribution of angiogenic regulatory proteins in proplatelet-producing mouse megakaryocytes. As observed in platelets, VEGF and endostatin were localized to separate α-granules in the proplatelet extensions (Figure 2A-C). A similar segregated staining pattern was also observed for thrombospondin-1 and basic FGF (Figure 2D-F) as well as fibrinogen and VWF (Figure 2G-I). Most VWF colocalized with the endostatin-containing α-granules (Figure 2J-L). We confirmed the presence of distinct populations of α-granules in human platelets at the ultra-structural level using immuno-electron microscopy (Figure 3). As expected, anti-VEGF (Figure 3A) and antiendostatin antibodies (Figure 3B) label only a subpopulation of α-granules. Double immunogold microscopy confirmed that the majority of VEGF and endostatin are localized to separate and distinct granules in platelets (Figure 3C). Single immunogold studies revealed that antifibrinogen (Figure 3D) and anti-VWF antibodies (Figure 3E) label only a subpopulation of α-granules. Double immunogold microscopy confirmed that the majority of fibrinogen and VWF are localized to separate and distinct granules in resting platelets (Figure 3G). Anti–P-selectin antibodies specifically labeled almost all α-granules and the plasma membrane of resting platelets (Figure 3G). Quantitative analysis of gold labeling in serial sections revealed that antibodies to VEGF, endostatin, VWF, and fibrinogen each stain approximately 50% of the granule population (Figure 3H). In contrast, anti–P-selectin antibodies label the membrane of all α-granules as well as the surface of the resting platelet. Less than 10% of granules contained gold labeling for both endostatin and VEGF or VWF and fibrinogen together (Figure 3H).
Pro- and antiangiogenic regulatory proteins are segregated into separate, distinct α-granules in megakaryocyte proplatelets. Double immunofluorescence microscopy of proplatelets using antibodies against VEGF (A) and endostatin (B) and an overlay (C). Double immunofluorescence microscopy of proplatelets using antibodies against bFGF (D) and TSP-1 (E) and an overlay (F). Double immunofluorescence microscopy of proplatelets using antibodies against fibrinogen (G) and von Willebrand factor (H) and an overlay (I). Double immunofluorescence microscopy of proplatelets against VEGF (J) and fibrinogen (K) and an overlay (L).
Pro- and antiangiogenic regulatory proteins are segregated into separate, distinct α-granules in megakaryocyte proplatelets. Double immunofluorescence microscopy of proplatelets using antibodies against VEGF (A) and endostatin (B) and an overlay (C). Double immunofluorescence microscopy of proplatelets using antibodies against bFGF (D) and TSP-1 (E) and an overlay (F). Double immunofluorescence microscopy of proplatelets using antibodies against fibrinogen (G) and von Willebrand factor (H) and an overlay (I). Double immunofluorescence microscopy of proplatelets against VEGF (J) and fibrinogen (K) and an overlay (L).
Localization of proteins in resting, human platelets using immunoelectron microscopy of ultrathin cryosections. Single immunogold labeling on ultrathin platelet sections was performed with anti-VEGF (A) and antiendostatin (B) antibodies. Double immunogold labeling on platelet sections was performed with the use of anti-VEGF antibody and antiendostatin antibodies. Large gold particles representing anti-VEGF staining (15 nm, arrows) are evident on one population of α-granules and small gold particles (5 nm) representing endostatin staining are abundantly present on a different population of α-granules (arrowheads) (C). Single immunogold labeling on ultrathin platelet sections was performed with antifibrinogen (D) and anti-VWF (E) antibodies. Double immunogold labeling on platelet sections was performed with the use of antifibrinogen antibody, which was revealed with a 15-nm, gold conjugate (arrows) and then with an antibody to VWF, which was revealed with a 5-nm, gold conjugate (arrowheads) (F). Single immunogold labeling on ultrathin platelet sections was performed with anti–P-selectin antibody (G). Gold particles representing P-selectin staining are abundantly present on the α-granules as well as the cell-surface membrane. Bar represents 300 nm. (H) The bar graph shows the quantitation of the percentage of α-granules positive (via immunogold staining) for specific factors. The data represent 3 separate experiments; error bars represent SD. More than 100 granules were scored for each study.
Localization of proteins in resting, human platelets using immunoelectron microscopy of ultrathin cryosections. Single immunogold labeling on ultrathin platelet sections was performed with anti-VEGF (A) and antiendostatin (B) antibodies. Double immunogold labeling on platelet sections was performed with the use of anti-VEGF antibody and antiendostatin antibodies. Large gold particles representing anti-VEGF staining (15 nm, arrows) are evident on one population of α-granules and small gold particles (5 nm) representing endostatin staining are abundantly present on a different population of α-granules (arrowheads) (C). Single immunogold labeling on ultrathin platelet sections was performed with antifibrinogen (D) and anti-VWF (E) antibodies. Double immunogold labeling on platelet sections was performed with the use of antifibrinogen antibody, which was revealed with a 15-nm, gold conjugate (arrows) and then with an antibody to VWF, which was revealed with a 5-nm, gold conjugate (arrowheads) (F). Single immunogold labeling on ultrathin platelet sections was performed with anti–P-selectin antibody (G). Gold particles representing P-selectin staining are abundantly present on the α-granules as well as the cell-surface membrane. Bar represents 300 nm. (H) The bar graph shows the quantitation of the percentage of α-granules positive (via immunogold staining) for specific factors. The data represent 3 separate experiments; error bars represent SD. More than 100 granules were scored for each study.
The packaging of VEGF and endostatin into separate α-granules suggested that distinct granule populations may undergo selective release. We tested this hypothesis by stimulating platelets with either PAR4-activating peptide or PAR1-activating peptide. Selective granule release was assessed by immunofluorescence microscopy (Figure 4). Phalloidin staining demonstrated that exposure of platelets to either ligand resulted in aggregation and extension of lamellipodia and filopodia, leading to activation (Figure 4B,D,F,H). Immunofluorescence microscopy revealed that PAR4 treatment resulted in loss of the endostatin labeling, suggesting that most of the endostatin-containing granules were released from the platelets ligated with PAR4-activating peptide (Figure 4C). However, numerous VEGF-containing granules were retained in the cytoplasm of PAR4-treated platelets (Figure 4A). In contrast, immunofluorescence microscopy revealed that ligation of PAR1 resulted in the release of VEGF-containing (green) granules, suggesting that release of VEGF-containing granules was elicited by the PAR1 agonist (Figure 4E). However, a large number of endostatin-containing granules were still retained in the cytoplasm of PAR1-treated platelets. (Figure 4G). To confirm the phenomenon of differential granule release, we analyzed the agonist-mediated release of α-granules at higher resolution using immunoelectron microscopy. Stimulation of platelets with PAR4 agonist resulted in the release of almost all endostatin-containing granules; the majority (84%) of granules remaining in the activated plate-lets were positive for VEGF (Figure 4I). Treatment of platelets with PAR1 agonist induced the release of the majority of VEGF-containing granules; the majority (88%) of granules remaining in the PAR1-activated platelets were positive for endostatin (Figure 4J).
Activation of specific protease activated receptors stimulates the selective release of α-granules containing either endostatin or VEGF. Platelets were treated with platelet buffer in the presence of agonists for 10 minutes with PAR4-activating peptide (A-D), and PAR1-activating peptide (E-H) and then fixed and processed for immunofluorescence microscopy. Cells were stained with either anti-VEGF antibodies (Alexa 488 green labeling; A,E) or antiendostatin antibodies (Alexa 568 red labeling; C,G,) to assay for granule retention or release. All micrographs were taken at the same exposure time. Corresponding staining with Alexa-phalloidin (B,D,F,H) in the bottom panels highlights the morphology of the platelets. Negative controls consisting of incubation with both secondary fluorescently labeled antibodies only or incubation with only primary antibodies failed to show appreciable fluorescence (data not shown). Images are representative of at least 10 high-power fields for each experiment, and each experiment was performed 3 times. Representative images of immunoelectron microscopy of platelets treated with either PAR4-AP (I) or PAR1-AP (J). Double immunogold labeling on platelet sections was performed with the use of anti-VEGF antibody and antiendostatin antibodies. In the PAR4-treated samples (I), large gold particles representing antiendostatin staining (15 nm) are evident on one α-granule (arrow) and small gold particles (5 nm) representing VEGF staining are abundantly present on separate population of multiple α-granules. In the PAR1-treated samples (J), large gold particles representing anti-VEGF staining (15 nm, arrow) are evident on one α-granule (arrow) and small gold particles (5 nm) representing endostatin staining are abundantly present on separate population of multiple α-granules. (K) A model illustrating the mechanism of differential granule release from platelets. A simplified summary of the pathway is shown. Resting platelets contain both proangiogenic (green) and antiangiogenic (red) granules. Selective activation of the PAR1 receptor causes release of granules containing proangiogenic factors, whereas selective activation of the PAR4 receptor causes release of granules containing antiangiogenic factors.
Activation of specific protease activated receptors stimulates the selective release of α-granules containing either endostatin or VEGF. Platelets were treated with platelet buffer in the presence of agonists for 10 minutes with PAR4-activating peptide (A-D), and PAR1-activating peptide (E-H) and then fixed and processed for immunofluorescence microscopy. Cells were stained with either anti-VEGF antibodies (Alexa 488 green labeling; A,E) or antiendostatin antibodies (Alexa 568 red labeling; C,G,) to assay for granule retention or release. All micrographs were taken at the same exposure time. Corresponding staining with Alexa-phalloidin (B,D,F,H) in the bottom panels highlights the morphology of the platelets. Negative controls consisting of incubation with both secondary fluorescently labeled antibodies only or incubation with only primary antibodies failed to show appreciable fluorescence (data not shown). Images are representative of at least 10 high-power fields for each experiment, and each experiment was performed 3 times. Representative images of immunoelectron microscopy of platelets treated with either PAR4-AP (I) or PAR1-AP (J). Double immunogold labeling on platelet sections was performed with the use of anti-VEGF antibody and antiendostatin antibodies. In the PAR4-treated samples (I), large gold particles representing antiendostatin staining (15 nm) are evident on one α-granule (arrow) and small gold particles (5 nm) representing VEGF staining are abundantly present on separate population of multiple α-granules. In the PAR1-treated samples (J), large gold particles representing anti-VEGF staining (15 nm, arrow) are evident on one α-granule (arrow) and small gold particles (5 nm) representing endostatin staining are abundantly present on separate population of multiple α-granules. (K) A model illustrating the mechanism of differential granule release from platelets. A simplified summary of the pathway is shown. Resting platelets contain both proangiogenic (green) and antiangiogenic (red) granules. Selective activation of the PAR1 receptor causes release of granules containing proangiogenic factors, whereas selective activation of the PAR4 receptor causes release of granules containing antiangiogenic factors.
Discussion
Angiogenesis is a critical element of many physiologic processes such as wound healing, as well as pathologic processes such as tumor growth. In both situations, new blood vessel development is driven locally by the release of proangiogenic factors such as VEGF, bFGF, and PDGF. However, angiogenesis can also be inhibited by local release of antiangiogenic factors such as endostatin and thrombospondin. The proximity to and interaction with the endothelium allow platelets to strongly influence tumor development and wound healing. Platelets have been presumed to contribute to these angiogenesis-dependent processes by providing many pro- and antiangiogenic proteins, but their regulatory role is incompletely understood. In this study, we have shown that platelets contain distinct populations of α-granules that can undergo differential release in vitro. This study suggests that at least 2 populations of α-granules containing endogenous angiogenic regulatory proteins are present in platelets and raises the possibility that platelets contain multiple types of α-granules. Platelets contain a large number of angiogenic regulatory proteins, whose localization will need to be thoroughly established to understand the complexity of α-granule organization within resting platelets. Yet, it can be inferred that this subcellular organization has a physiologic purpose in facilitating the differential release of these proteins in response to tissue stimuli. Our findings of differential granule release also support and provide a mechanistic explanation for earlier studies examining the secretion reaction of platelets. Two independent groups have documented the differential release of α-granule proteins from platelets.25,26 In addition, morphometric evaluation of the platelet release reaction during thrombogenesis has demonstrated that platelets do not release all of their granules when they are incorporated into a thrombus.27 The above results showing distinct granules also raise the question of whether other cell types containing secretory granules segregate angiogenic regulatory proteins to regulate differential release. For example, Weibel Palade bodies, the specific secretory organelles of endothelial cells, contain several angiogenesis regulators and have been recently shown to differentially package and release P-selectin and VWF through protease-activated receptors.28
What molecular mechanisms regulate the differential packaging of specific proteins into α-granules? α-granules contain a mixture of proteins synthesized by the megakaryocyte as well as proteins endocytosed from the circulation by both megakaryocytes and the platelets. Although the formation of α-granules is poorly understood,29,30 it appears that α-granules develop from budding vesicles in the Golgi complex within megakaryocytes, where they transform into multivesicular bodies, which also fuse with endocytic vesicles. Coated pits and vesicles have been observed in platelets and function to take up proteins, such as fibrinogen, by receptor-mediated endocytosis. These endocytic vesicles fuse with the multivesicular bodies. Multivesicular bodies, which are prevalent in early megakaryocytes, are thought to be a common precursor of both α- and dense granules. However, the mechanisms by which α- and dense granules develop into distinct entities are unknown. It is tempting to speculate that a similar process may be used to segregate proteins into distinct subsets of α-granules and that genetic defects that affect the α-granule segregation or differential release may provide an explanation of the wide range of angiogenic responses manifested by different patients.31 Several angiogenesis-dependent processes may be explained by the sequential release of angiogenesis regulators. For example, in early endothelial injury, an unstable platelet clot is formed and the high-affinity thrombin receptor (PAR1) signals to release by majority pro-angiogenic proteins such as VEGF. In the late stage of tissue reconstruction, a high thrombin state occurs when the majority of the clot is crosslinked by factor XIII, and the low affinity protease-activated receptor (PAR4) is engaged and mainly inhibitors of angiogenesis are released.
Recognizing that activated platelets release growth factors, investigators have begun to enhance tissue regeneration by applying platelets and their derivatives into sites of injuries or surgical intervention. The concept of platelet and tissue interaction and the resulting release of angiogenesis regulators have already been used in the empirical application of platelet preparations to chronic diabetic ulcers,32,33 chronic cutaneous ulcers,34 dehiscent wounds,35-37 and tissue regeneration.38 Although the majority of evidence indicates that platelets and their derivatives (gels, releasates, and lysates) are promising therapeutic agents for regenerative medicine, little is known about the specific mechanisms underlying platelet-accelerated tissue repair.39 The ability to generate selective platelet releasates by manipulating protease-activated receptors may provide new opportunities for research and applications of tissue engineering and may aid in therapeutic strategies to promote or inhibit angiogenesis.
Our findings of distinct populations of α-granules that can be differentially released suggest implications and potential for a substantial role in antiangiogenic therapy. It is now well accepted that the growth of a tumor beyond approximately 1 mm is dependent on the development of a neovasculature. One possibility is that tumors also hijack the angiogenic properties of platelets to promote new blood vessel growth by manipulating the protease activated receptors on platelets and triggering the selective release of predominantly proangiogenic factors. The protease-activated receptors on platelets and endothelial cells are likely to play an important role in the sequential and highly selective contribution of angiogenesis regulators to tissues. If confirmed, then it may be possible to develop drugs that instruct platelets, which interact with tumors to release predominantly antiangiogenic proteins.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maria Ericsson for assistance with electron microscopy, Dr John Hartwig and Eva Alden for critical reading of the manuscript, and Jenny Grillo for formatting the manuscript.
The work was supported by the National Institutes of Health (grant HL068130, J.E.I.), the Breast Cancer Research Foundation (J.F.), the Department of Defense (grant W81XWH-04-1-0316, J.F.), and NASA (grant NNH04ZUU002N).
Authorship
Contribution: J.E.I. designed and performed experiments and data analysis, interpreted results, provided guidance for the group, and drafted the manuscript; J.L.R., S.P.H., E.B., A.Z., and S.S. performed experiments and data analysis and interpreted results; S.R. and J.F. designed experiments, interpreted results, formulated discussions, and assisted in manuscript preparation and editing; G.L.K. designed experiments, provided guidance for the group, interpreted results, formulated discussions, and assisted in manuscript preparation and editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph E. Italiano Jr, Translational Medicine Division, Brigham and Women's Hospital, One Blackfan Circle, Boston, MA 02115; e-mail: jitaliano@rics.bwh.harvard.edu; or Giannoula Klement, Children's Hospital Boston, Karp Family Research Laboratories, One Blackfan Circle, Boston, MA 02115; e-mail: giannoula.klement@childrens.harvard.edu.