Abstract
Heterozygous mutations in the telomerase components TERT, the reverse transcriptase, and TERC, the RNA template, cause autosomal dominant dyskeratosis congenita due to telomere shortening. Anticipation, whereby the disease severity increases in succeeding generations due to inheritance of shorter telomeres, is a feature of this condition. Here we describe 2 families in which 2 TERT mutations are segregating. Both families contain compound heterozygotes. In one case the proband is homozygous for a novel mutation causing a P704S substitution, while his father's second allele encodes an H412Y mutation. The proband in the second family has mutant alleles Y846C and H876Q. Transfection studies show codominant expression of the mutated alleles with no evidence of a dominant negative effect or of intragenic complementation. Thus in these families the expression of both TERT alleles and the inherited telomere length contribute to the clinical phenotype.
Introduction
Mutations in genes encoding components of the telomerase ribonucleoprotein complex resulting in very short telomeres have been identified in patients with dyskeratosis congenita (DC), a rare inherited bone marrow failure syndrome.1-6 X-linked DC is caused by mutations in the DKC1 gene, encoding a protein necessary for the stabilization of the TERC RNA. Individuals with autosomal dominant DC (AD DC) are heterozygous for mutations in the telomerase RNA TERC or the gene encoding the catalytic subunit TERT.2-5 In contrast to patients with X-linked DC, who usually develop severe disease with a high penetrance, disease penetrance and expressivity in AD DC are highly variable and, in addition to the gene mutation, the inheritance of short telomeres is required for the manifestation of the disease.7,8 Here we demonstrate that the inheritance of AD DC may be complex. We report a DC patient homozygous for a TERT mutation and compound heterozygotes in 2 separate families with apparent codominance of the 2 mutations
Methods
Clinical and genetic information was obtained through our ongoing study on the molecular mechanisms of bone marrow failure (http://bmf.im.wustl.edu). The study is approved by the Washington University School of Medicine Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki. DNA for mutation analysis was extracted from peripheral blood cells (Qiagen, Valencia, CA). Telomere length measurements in peripheral blood mononuclear (PBMC) by flow-FISH and direct DNA sequencing were previously described.8 Primers used are shown in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
The mutations identified were introduced in the p3.1+ TERT plasmid9 using the QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). Wild-type (WT) or mutant TERT plasmid (4 μg) were transfected into WI-38 VA-13 cells at 80% confluence in the presence of an equal amount of pUC TERC using lipofectamine 2000 (Invitrogen, Carlsbad, CA).10 In cotransfection experiments, 2 μg of each mutant TERT plasmid were used. Thirty-six hours after transfection, telomerase activities were determined in cell lysates at protein concentrations of 40, 10, 2.5, and 0.625 ng using a quantitative polymerase chain reaction (Q-PCR)–based TRAP assay as previously described.11
Results and discussion
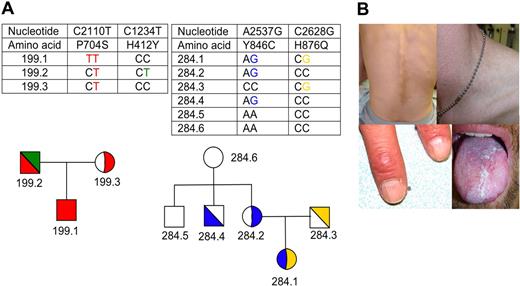
Figure 1A shows the pedigrees of families 199 and 284. Patient 199.1 is a 31-year-old man of Scottish descent. His clinical manifestations include short stature; elfin appearance; esophageal stricture; leukoplakia of the buccal mucosa, anus, and penis; abnormal pigmentation of his neck, trunk, and back; hyperkeratosis of his palms; ridged fingernails; avascular necrosis of both hips; tooth loss; chronic diarrhea; learning difficulties; pulmonary infiltrates; and progressive bone marrow failure (Figure 1B). His 61-year-old father was diagnosed with osteoporosis at the age of 60. His 60-year-old mother is healthy. Both parents have normal peripheral blood cell counts. The paternal grandmother (age, 84 years) has a history of anemia, osteoporosis, and pulmonary fibrosis. The maternal grandmother was reported to have died at the age of 60 years because of pulmonary fibrosis.
Pedigrees and clinical manifestations in TERT mutations (A) Pedigrees and identified TERT gene mutations in families 199 and 284. Circles, females; squares, males; white, wild type; color, mutant as indicated in the chart. Half-filled symbols indicate heterozygosity. The TERT gene haplotype is shown. (B) Clinical manifestations in patient 199.1. are characteristic of DC, including hyperpigmentation of the back and reticular hyperpigmentation of neck, ridged and brittle fingernails, and leukoplakia of the tongue (Canon EOS 300D).
Pedigrees and clinical manifestations in TERT mutations (A) Pedigrees and identified TERT gene mutations in families 199 and 284. Circles, females; squares, males; white, wild type; color, mutant as indicated in the chart. Half-filled symbols indicate heterozygosity. The TERT gene haplotype is shown. (B) Clinical manifestations in patient 199.1. are characteristic of DC, including hyperpigmentation of the back and reticular hyperpigmentation of neck, ridged and brittle fingernails, and leukoplakia of the tongue (Canon EOS 300D).
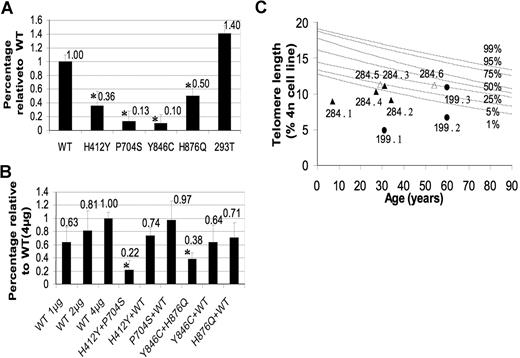
Mutation analysis revealed that patient 199.1 is homozygous for a C to T transition in exon 5 of the TERT gene (cDNA nt C2110T) causing a proline to serine substitution at amino acid 704 (P704S). Functional analysis in WI-38 VA-13 cells demonstrated that the TERT P704S mutation severely reduces telomerase activity to 13% of normal (P < .001; Figure 2A). Both parents are heterozygous for the TERT P704S mutation (Figure 1A). Interestingly, however, the father carries a second TERT mutation in exon 2. This C1234T mutation (H412Y), has been previously described in an unrelated family.3 This mutation reduced telomerase activity to 36% of normal in our transfection experiments (P < .001; Figure 2A). Coexpression of WT TERT with either the P704S or H412Y variants did not show evidence of a dominant negative effect. The coexpression of the 2 TERT mutations resulted in an intermediate telomerase activity of 22% (P < .001; Figure 2B), suggesting a synergic effect on telomerase activity and no intragenic complementation. Careful analysis of the family tree revealed that the parents are fourth cousins, explaining the presence of the TERT P704S mutation in both parents.
Telomerase activity and telomere lengths of wild-type and mutant individuals. (A) In vitro telomerase activity of the mutant TERT proteins in WI-38 VA-13 cells. WI-38 VA-13 cells were transfected with a plasmid expressing the mutant TERT cDNA sequences and a plasmid expressing the WT TERC RNA. Telomerase activity was determined using a Q-PCR–based TRAP assay. Activity is shown in comparison to the activity obtained after transfection with WT TERT cDNA (=1.0). Telomerase activity in 293T cells served as a positive control. (B) In vitro telomerase activity of 2 mutants or 1 mutant and WT TERT proteins in WI-38 VA-13 cells. WI-38 VA-13 cells were cotransfected with 2 plasmids expressing 2 different mutants, or 1 mutant and the WT TERT cDNA sequences. Equal amounts of plasmid expressing the TERC RNA were cotransfected in all experiments. Activity is shown in comparison to the activity obtained after the transfection of equal amounts of the WT TERT (=1.0). Experiments were performed 4 times and cotransfection experiments twice. The comparison of telomerase activity between the variants was performed by ANOVA analysis followed by post hoc test. Statistically significant reduction of telomerase activity (P < .05) is indicated with  . Error bars represent SD. (C) Telomere lengths measured in PBMN cells. Lines represent percentiles (1%-99%) of telomere length measured in 234 healthy individuals between the ages of 0.3 to 94 years old. The telomere lengths of family 199 are represented as ● and those of 284 as △ and ▲. Filled symbols indicate those with mutations (see text and Figure 1), empty symbols indicate family members carrying 2 wild-type alleles.
. Error bars represent SD. (C) Telomere lengths measured in PBMN cells. Lines represent percentiles (1%-99%) of telomere length measured in 234 healthy individuals between the ages of 0.3 to 94 years old. The telomere lengths of family 199 are represented as ● and those of 284 as △ and ▲. Filled symbols indicate those with mutations (see text and Figure 1), empty symbols indicate family members carrying 2 wild-type alleles.
Telomerase activity and telomere lengths of wild-type and mutant individuals. (A) In vitro telomerase activity of the mutant TERT proteins in WI-38 VA-13 cells. WI-38 VA-13 cells were transfected with a plasmid expressing the mutant TERT cDNA sequences and a plasmid expressing the WT TERC RNA. Telomerase activity was determined using a Q-PCR–based TRAP assay. Activity is shown in comparison to the activity obtained after transfection with WT TERT cDNA (=1.0). Telomerase activity in 293T cells served as a positive control. (B) In vitro telomerase activity of 2 mutants or 1 mutant and WT TERT proteins in WI-38 VA-13 cells. WI-38 VA-13 cells were cotransfected with 2 plasmids expressing 2 different mutants, or 1 mutant and the WT TERT cDNA sequences. Equal amounts of plasmid expressing the TERC RNA were cotransfected in all experiments. Activity is shown in comparison to the activity obtained after the transfection of equal amounts of the WT TERT (=1.0). Experiments were performed 4 times and cotransfection experiments twice. The comparison of telomerase activity between the variants was performed by ANOVA analysis followed by post hoc test. Statistically significant reduction of telomerase activity (P < .05) is indicated with  . Error bars represent SD. (C) Telomere lengths measured in PBMN cells. Lines represent percentiles (1%-99%) of telomere length measured in 234 healthy individuals between the ages of 0.3 to 94 years old. The telomere lengths of family 199 are represented as ● and those of 284 as △ and ▲. Filled symbols indicate those with mutations (see text and Figure 1), empty symbols indicate family members carrying 2 wild-type alleles.
. Error bars represent SD. (C) Telomere lengths measured in PBMN cells. Lines represent percentiles (1%-99%) of telomere length measured in 234 healthy individuals between the ages of 0.3 to 94 years old. The telomere lengths of family 199 are represented as ● and those of 284 as △ and ▲. Filled symbols indicate those with mutations (see text and Figure 1), empty symbols indicate family members carrying 2 wild-type alleles.
Telomere length measurement in family 199 revealed that patient 199.1 has very short telomeres (below the 1st percentile of the normal telomere length distribution; Figure 2C). Interestingly, the father (199.2), who is compound heterozygous for the TERT P704S and H412Y mutations, has also very short telomeres, whereas the mother (199.3), who is heterozygous for TERT P704S mutation, has a normal telomere length.
Patient 284.1 is an 8-year-old girl of European descent, originally diagnosed with moderate but progressive aplastic anemia. Both of her parents are healthy with no abnormalities in the peripheral blood. Family history was negative for blood diseases, pulmonary fibrosis, or cancer.
Mutation analysis revealed 2 different TERT gene sequence alterations. The A2537G in exon 9 (Y846C) and C2628G mutation in exon 10 (H876Q). Further analysis showed that the TERT Y846C mutation was inherited from the mother, whereas the TERT H876Q mutation was inherited from her father, indicating that patient 284.1 is a compound heterozygote for the 2 TERT gene mutations (Figure 1A). Both TERT gene mutations result in a significantly reduced telomerase activity after transfection into WI-38 VA-13 cells to 10% (P < .001) and 50% (P < .001) of normal (Figure 2A), whereas the cotransfection of the 2 mutants results in a telomerase activity of 38% (P = .004; Figure 2B).
Telomere length in peripheral blood cells from patient 284.1 was very short, below the 1st percentile of normal and so were those measured in her mother (284.2) and in one of her uncles (284.4), both of whom carry the TERT Y846C mutation. Telomere length in her father (284.3) heterozygous for the TERT H876Q mutation was between the 1st and 5th percentile of normal (Figure 2C).
In conclusion, we have identified 3 novel and 1 recurrent TERT gene mutation in 2 families who were thought to have sporadic DC and idiopathic aplastic anemia. All 4 mutations are hypomorphic mutations, impairing, but not eliminating telomerase activity. Homozygous hypomorphic TERT mutations have recently been found to cause disease in 2 consanguineous families.12 Here we demonstrate that in a nonconsanguineous family compound heterozygosity for TERT can cause disease and that the involvement of TERT in the pathogenesis of DC is probably more complex than initially anticipated. Our data indicate that in compound heterozygosity or homozygosity for hypomorphic TERT mutations the mutant alleles are codominant and suggest that severity of telomerase dysfunction and the inheritance of short telomeres determine the clinical phenotype and onset of disease. Codominant inheritance has also been found in one family with 2 hypomorphic TERC gene mutations,13 whereas compound heterozygosity or homozygosity for TERC or TERT null mutations have never been reported, suggesting that in humans, in contrast to mice, biallelic TERC or TERT null mutations are probably not compatible with life. The consideration that both sides of the family may be affected even in nonconsanguineous families might have important implications for the patient in the selection of a potential sibling donor as well as for the prognosis and management of other family members carrying one or 2 of the identified gene mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank both families for study participation, Jennifer Ivanovich from the Hereditary Cancer Core, and the Procurement Core of the Alvin J. Siteman Cancer Center for assistance (National Cancer Institute Cancer Center Support Grant P30 CA91842).
This work was supported by National Institutes of Health grants CA105312 (M.B and D.B.W) and CA106995 (P.J.M).
Authorship
Contribution: H.Y.D. designed and performed the experiments, analyzed the data, and drafted the manuscript; M.B. and P.J.M. designed the experiments and drafted the manuscript. E.P. carried out some of the experiments; D.B.W., P.M., J.J.F., and S.J.B. aided in the collection of the families' clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monica Bessler, Department of Internal Medicine, Washington University School of Medicine, 660 S Euclid Ave, St Louis, MO 63110; e-mail: mbessler@im.wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal