To the editor:
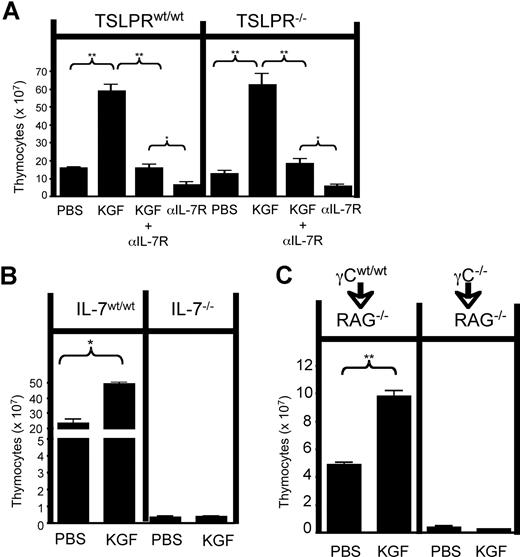
Thymic stromal lymphopoietin (TSLP) is a multifunctional cytokine, produced by epithelial tissues, that signals through TSLP receptors (TSLPRs) and interleukin (IL)-7Rα1 on B- and T-lymphocyte precursors, CD4+ T cells, and dendritic cells. TSLP plays an important role in allergic inflammation through dendritic cell and CD4+ T-cell–mediated activation,2,–4 can augment thymic function when administered directly to mice,5 and has recently been shown to be sufficient to mediate B and T lymphopoiesis in adult IL-7 deficient mice6 . Exogenous keratinocyte growth factor (KGF) potently augments thymopoiesis7 and protects from thymic damage8 by signaling via FGFR2IIIb expressed by thymic epithelium cells (TECs), but the exact mechanism by which KGF augments thymopoiesis remains unclear. Because KGF can expand TECs9 and has been reported to increase IL-7 production in treated mice7,8 and TSLP production in fetal thymic organ cultures (FTOC),10 we hypothesized that TSLP and/or IL-7 might mediate the thymopoietic effects of KGF. To investigate whether IL-7Rα signaling by thymocytes mediated KGF thymopoietic effects, we administered KGF to WT and Tslpr−/− mice in the presence or absence of anti-IL-7Rα mAb. The KGF effect was nearly completely dependent on IL-7Rα signaling, with only minimal KGF-induced thymic enlargement in mice treated with anti-IL-7Rα (Figure 1A). However, TSLPR signaling did not contribute substantially to the effect because the results were essentially identical in WT vs Tlspr−/− hosts. Thus, the thymopoietic effect of KGF is primarily mediated by IL-7 and does not require TSLP signaling, a result consistent with the known dominant role for IL-7 in thymopoiesis. However, a recent study published in Blood demonstrated that supraphysiologic TSLP levels generated by transgenesis “rescued” thympoiesis in Il7−/− mice.6 Previous work also demonstrated that exogenous TSLP augmented thymopoiesis in γc−/− mice5 and that KGF induced TSLP production in FTOC.10 We therefore hypothesized that TSLP might be sufficient to augment thymopoiesis in Il7−/− hosts after KGF therapy. To explore this, we first administered KGF to Il7−/− mice, but observed no significant increase in thymic size (Figure 1B), consistent with previous studies.8 Importantly however, Il7−/− mice have grossly disrupted thymic architecture, which could prevent the thymic stroma from supporting increased numbers of thymocytes. Therefore, we reconstituted lethally irradiated Rag1−/− mice with either γc−/− or WT hematopoieic stem cells (HSCs) and tested their responsiveness to KGF. Recipients receiving γc−/− marrow showed essentially no thymic reconstitution, demonstrating that endogenous TSLP was insufficient to support thymopoiesis after lethal irradiation. Moreover, KGF administration did not increase thymic cellularity in the Rag1−/− mice reconstituted with γc−/− HSC, whereas Rag1−/− mice reconstituted with WT HSC nearly doubled the size of their thymus after KGF therapy (Figure 1C). Thus, IL-7 is the primary mediator of KGF thymopoietic effects and these data rule out the possibility that TSLP plays any substantial role. Furthermore, despite the recent report demonstrating “rescue” of thymopoiesis with sustained, genetically induced levels of TSLP, endogenous TSLP production is not sufficient to support thymopoiesis after lethal irradiation. Moreover, even after KGF treatment that expands TECs and increased TSLP expression in FTOC,10 we saw no evidence of substantive thymopoiesis in the absence of IL-7.
TSLP is not necessary or sufficient to mediate the thymopoietic effects of KGF. All KGF-treated mice received 5 mg/kg KGF or PBS (intraperitoneally) on days 0, +2, and +4, and thymic cellularity +21 days after KGF therapy was initiated is shown. Values represent mean (±SEM). (A) Where indicated, 1 mg IL7Rα blocking antibody (A7R34) was given intraperitoneally on days −3, −1, +1, +3, and +5 (n=5-8 per group). (B) IL7−/− mice and littermate, age-matched controls (n=5 per group). (C) Rag1−/− mice were lethally irradiated (10 gy) and given 107 bone marrow cells intravenously from γc−/− or wild type mice (n = 3-5 per group). * indicates P < .01 and **, P < .001.
TSLP is not necessary or sufficient to mediate the thymopoietic effects of KGF. All KGF-treated mice received 5 mg/kg KGF or PBS (intraperitoneally) on days 0, +2, and +4, and thymic cellularity +21 days after KGF therapy was initiated is shown. Values represent mean (±SEM). (A) Where indicated, 1 mg IL7Rα blocking antibody (A7R34) was given intraperitoneally on days −3, −1, +1, +3, and +5 (n=5-8 per group). (B) IL7−/− mice and littermate, age-matched controls (n=5 per group). (C) Rag1−/− mice were lethally irradiated (10 gy) and given 107 bone marrow cells intravenously from γc−/− or wild type mice (n = 3-5 per group). * indicates P < .01 and **, P < .001.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: M.G. designed and performed experiments and wrote the manuscript; W.J.L. and B.R.B. supported the work, substantially contributed to experimental design, and critically reviewed the manuscript; R.S. and S.W.R. substantially contributed to experimental design and critically reviewed the manuscript; R.G.V. acquired data and critically reviewed the manuscript; G.H. supported the work and critically reviewed the manuscript; and C.L.M. supported the work, substantially contributed to experimental design, and wrote the manuscript.
C.L.M. and B.R.B. contributed equally to this manuscript.
Acknowledgments: This work was supported in part by the Intramural Program of the National Cancer Institute and National Heart, Lung, and Blood Institute as well as National Institutes of Health grants RO1 CA72669 (B.R.B.), HL55209 (B.R.B.), HL073794 (B.R.B.), RO1 A1057477 (G.A.H.), and a grant from the Swiss National Science Foundation (G.A.H.).
Correspondence: Crystal L. Mackall, MD, Acting Chief, Pediatric Oncology Branch, National Cancer Institute, Building 10-CRC, 1W-3940, 10 Center Dr, MSC 1104, Bethesda, MD 20892; e-mail: cm35c@nih.gov.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal