Regulatory T cells (Tregs) prevent graft-versus-host disease (GvHD) by inhibiting the proliferation and function of conventional T cells (Tcons). However, the impact of Tregs on T-cell development and immunity following hematopoietic cell transplantation (HCT) is unknown. Using a murine GvHD model induced by Tcons, we demonstrate that adoptive transfer of Tregs leads to (1) abrogration of GvHD, (2) preservation of thymic and peripheral lymph node architecture, and (3) an accelerated donor lymphoid reconstitution of a diverse TCR-Vβ repertoire. The resultant enhanced lymphoid reconstitution in Treg recipients protects them from lethal cytomegalovirus (MCMV) infection. By contrast, mice that receive Tcons alone have disrupted lymphoid organs from GvHD and remain lymphopenic with a restricted TCR-Vβ repertoire and rapid death on MCMV challenge. Lymphocytes from previously infected Treg recipients generate secondary response specific to MCMV, indicating long-term protective immunity with transferred Tregs. Thymectomy significantly reduces survival after MCMV challenge in Treg recipients compared with euthymic controls. Our results indicate that Tregs enhance immune reconstitution by preventing GvHD-induced damage of the thymic and secondary lymphoid microenvironment. These findings provide new insights into the role of Tregs in affording protection to lymphoid stromal elements important for T-cell immunity.
Introduction
Naturally arising CD4+CD25+Foxp3+ regulatory T cells (Tregs) are a major regulator of adaptive immunity.1 Depletion of Tregs enhances microbial and tumor immunity,2,3 while their adoptive transfer protects animals from autoimmune diseases,1,4,5 induces tolerance following organ transplantation,5 and prevents graft-versus-host disease (GvHD) following hematopoietic cell transplantation (HCT).6,–8 Given these observations, immunotherapy with Tregs is being explored for clinical applications.9 However, the impact of Treg transfer on short- and long-term host immunity remains not well understood.
In HCT, GvHD damages the stroma of the thymus and secondary lymphoid organs, thereby decreasing thymic output, impairing peripheral expansion of T cells,10,,,,–15 and consequently leading to a prolonged immunodeficiency state.11,16 Given the pathophysiologic link between GvHD and immune reconstitution, protection from GvHD by Tregs would potentially promote timely and complete immune recovery. However, quantitative immune reconstitution does not necessarily translate into recovery of cellular function,17 and the long-term persistence of transferred Tregs in vivo18 may diminish or abrogate functional effector responses.
Tregs suppress the priming, expansion, and/or function of conventional T cells (Tcons).19,–21 However, it is unclear whether this regulation is antigen specific. Following HCT, specific suppression of alloreactive T cells is beneficial, as donor T cells have dual and opposing roles of mounting antitumor and antimicrobial responses and inducing GvHD. Observations from autoimmune disease models that Tregs can induce bystander suppression5,22,23 and infectious tolerance24,25 suggest nonspecific inhibition following their activation. Given the multiorgan involvement of GvHD, we anticipate broader nonspecific suppression by polyclonal donor Tregs. Thus, we hypothesized that the adoptive transfer of Tregs would attenuate GvHD via nonspecific suppression of effector responses, which could in turn compromise viral and tumor immunity following HCT.
The aims of our studies were to determine if Tregs affect the generation and expansion of thymic-derived naive donor T cells and mature CD4+ and CD8+ T cells transplanted with the allograft, and if effective immune responses can be generated in the presence of Tregs. Our results demonstrate that Tregs prevent damage of the thymus and peripheral lymphoid tissues associated with GvHD. Protection of both central and peripheral lymphoid compartments by Tregs in turn facilitates the development and expansion of an enhanced T-cell repertoire that results in improved reconstitution of functional immune responses following HCT.
Methods
Mice
FVB/N (H-2q), C57Bl/6 (H-2b), and Balb/c (H-2d) mice were purchased from Charles River Laboratory (Wilmington, MA) and Jackson Laboratory (Bar Spring Harbor, ME) at age 8 to 14 weeks. Thymectomies of Balb/c mice were performed at 4 weeks of age. Animal protocols were approved by the Institutional Animal Care and Use Committee at Stanford University.
Cell-sorting and flow cytometric analysis
Antibodies were purchased from BD-Pharmingen and eBiosciences (San Diego, CA): CD4(RM4-5), CD8α(53-6.7), CD25(PC61), CD45/B220(RA3-6B2), CD62L(MEL14), H-2Kq(KH114), H-2Dq(KH117), H-2Dd(34-2-12), and NK1.1(PK136). Analysis of Vβ TCR included monoclonal antibodies to Vβ 2, 3, 4, 5.1 and 5.2, 6, 7, 8.1 and 8.2, 8.3, 9, 10b, 11, 12, 13, 14, and 17a T-cell receptors. Antibody-conjugated microbeads (CD4, CD8, and PE) were obtained from Miltenyi (Auburn, CA).
T-cell subsets were isolated from spleen and lymph nodes of donor mice. To enrich for CD25+ cells, single-cell suspension was stained with anti–CD25-PE and anti–PE-conjugated magnetic beads and separated using the AutoMACS (Miltenyi). Positively selected cells were then stained with anti–CD4-APC and sorted on MoFlo (Cytomation, Fort Collins, CO) to a purity of 98% to 99% for CD4+CD25hi cells (Tregs), with greater than 96% expressing Foxp3 (unpublished data, August 2005). CD4+ and CD8+ cells at a physiologic ratio (Tcons) were isolated to a purity of 95% to 98% as previously described.18 Bone marrow was harvested and prepared into single-cell suspension as previously described.8 T cells were depleted by magnetic separation with anti-CD4 and anti-CD8 microbeads with more than 99% purity.
Transplantation and MCMV infection
Wild-type or thymectomized Balb/c recipients were lethally irradiated with 8 Gy TB, given as 2 separate doses of 4 Gy 4 hours apart. Within 24 hours of irradiation, recipient mice were injected via tail vein with FVB donor cells and included one of the following:(1) TCD-BM (5 × 106) alone; (2) TCD-BM (5 × 106) and Tcons (5.0 × 105); (3) TCD-BM (5 × 106), Tcons (5.0 × 105), and Tregs (5.0 × 105). Tcon cell dose was determined via titration studies to establish a subacute GvHD course, which permits long-term immune reconstitution studies (unpublished data, March 2005). Animals were monitored and clinical evidence of GvHD was evaluated and scored as previously described.52
Groups of mice were challenged intraperitoneally with 5 × 105 plaque-forming units (pfu) of lacZ-tagged MCMV RM427+ administered via intraperitoneal injection53 at days 14, 30, or 63 after transplantation. In a series of titration experiments using Tcon recipients, this dose and administration route resulted in a median time to death of 13 days (range, 12-16) following infection 14 days after HCT (unpublished data, March 2005).
Viral load determination
Liver, kidney, lung, and salivary glands from killed mice were weighed and stored at −80°C in complete Dulbecco modified Eagle medium and triple-sterilized dry milk. Organs were sonicated and titered by plaque assay on 3T3 cell (American Type Culture Collection, Manassas, VA) monolayers as described previously.54
Histopathology
Sections of spleen, lymph nodes, and thymus were collected and fixed in 10% formalin and placed in paraffin blocks. Tissue sections of 5 μm were H&E stained, coded (D.D.), and analyzed (N.K.).
Analysis of peripheral blood
Peripheral blood was obtained by heart puncture following sacrifice of the animal, collected into a heparin-coated tube, and measured using the Celldyn Analyzer (Abbott Diagnostics, Mountain View, CA) by the Department of Comparative Medicine at Stanford University.
IFN-γ ELISPOT assay
Polyvinylidene fluoride 96-well plates (Millipore, Billerica, MA) were coated with anti–IFN-γ mAb (Mabtech, Sweden) at 0.5 μg/well. MCMV 1E1 peptide (YPHFMPTNL-COOH, synthesized at Stanford PAN facility) was added at 0.11 μg/100 μL/well. Sorted donor (H2Kq) lymphocytes (2 × 105) were added to their respective wells. Lymphocytes from uninfected animals were negative controls. As a positive control, lymphocytes from uninfected animals were stimulated with anti-CD3/anti-CD28 antibodies (1 μg/mL in media). Secondary anti–IFN-γ-biotinylated antibody (1 μg/mL) was added following overnight incubation and kept at RT for 2 hours. After a phosphate buffered saline (PBS) wash, streptavidin-alkaline-phosphatase was added, and plates were incubated at RT for 1 hour, followed by incubation with BCIP/NBT substrate dye (Moss, Pasadena, MD) until color development. Plates were analyzed (Zellnet, Fort Lee, NJ) for the number of spots/well, with each spot representing individual IFN-γ–secreting cells.
TCR Vβ spectratyping
RNA was extracted from splenic single-cell suspensions using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse-transcribed into cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Polymerase chain reaction (PCR) was performed with 21 different 5′ primers that specifically amplify all functional TCR-Vβ genes from C57Bl/6 animals. These variable primers were used in combination with a common 3′ primer based in the β-chain constant region, (5′-GACCTCCTTGCCATTCACCCACCAG-3′). PCR of the run-off products was performed using a FAM-labeled common 3′ primer based in the β-chain constant region, (5′-CAAACAAGGAGACCTTGGGTGGAGTCAC-3′). 6-carboxy-fluorescein (FAM)-labeled PCR fragments were then run on an ABI Prism 3700 DNA-Sequencer (Applied Biosystems, Foster City, CA) and analyzed using GeneScan software (Applied Biosystems).
Statistical analysis
The log-rank test was used to compare groups in Kaplan-Meier survival analysis. For comparison of absolute cell counts between experimental groups, the rank sum or Mann-Whitney test was used, with their nominal P values reported. Fisher exact test was used to compare proportions (or percent) survival without virus between groups.
Results
Tregs attenuate GvHD and do not interfere with engraftment and donor chimerism
We established a model of subacute GvHD following complete MHC-mismatched HCT to assess the effect of Tregs on GvHD, engraftment, and immune reconstitution. In this model, lethally irradiated Balb/c H2d recipients received 5 × 106 T cell–depleted bone marrow cells (BM) from H2k mice. Co-transfer of donor 5 × 105 CD4+ and CD8+ T cells (Tcons) at a physiologic dose ratio of 3:1 resulted in lethal GvHD with a median survival of 85 to 90 days. Infusion of donor CD4+CD25hi regulatory T cells (Tregs) at a 1:1 dose ratio to Tcons protected animals from GvHD-related mortality.
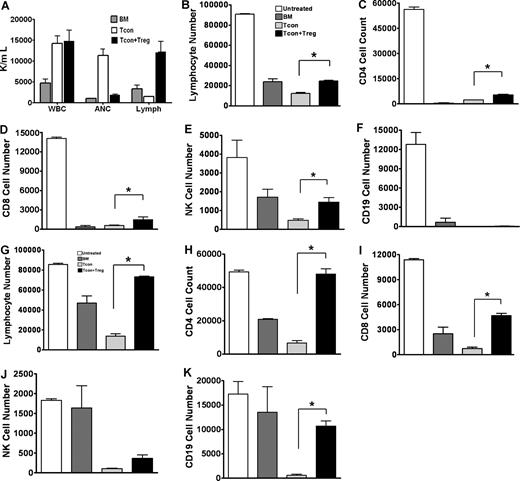
By day 30 after HCT, peripheral blood analysis showed complete donor chimerism in animals that received Tcons alone (Tcon) or both Tcons and Tregs (Tcon + Treg) and remained stable on days 60 and 100 after HCT (unpublished data, December 2005). Peripheral blood counts on day 60 showed engraftment of white blood cells (WBCs), red blood cells, and platelets, with no obvious disparity between animals that received Tcons or Tcon + Treg (unpublished data, December 2005). However, in the analysis of WBC subsets, recipients of Tcons had a significantly elevated number of neutrophils and were lymphopenic. In contrast, animals that received Tcon + Treg had normal levels of neutrophils (P = .004) and increased numbers of the lymphocyte subsets (P = .014), suggesting improved lymphoid engraftment (Figure 1A).
Impact of regulatory T cells on engraftment and quantitative immune reconstitution. (A) Peripheral blood with normal total white blood cells (WBCs) in animals on day 40 after transplantation. Analysis of subpopulations of WBCs showed abnormally low numbers of lymphocytes (Lymph) and high numbers of neutrophils (ANC) in recipients of Tcons alone, the latter accounting for the increased total WBC. Animals that received Tregs with Tcons had normal levels of ANC and enhanced numbers of lymphocytes. Peripheral blood reconstitution of total lymphocytes and lymphoid subsets on days 14 (B-F) and 40 (G-K) after transplantation. Statistical differences (*P < .05) were noted between Tcons versus Tcon + Treg recipients in total lymphocytes, CD4+, CD8+, and NK+ cells on day 14. Differences were more significant on day 40 and included CD19+ cells. Results represent the mean plus or minus SE (3 to 4 mice per group, one of 3 experiments).
Impact of regulatory T cells on engraftment and quantitative immune reconstitution. (A) Peripheral blood with normal total white blood cells (WBCs) in animals on day 40 after transplantation. Analysis of subpopulations of WBCs showed abnormally low numbers of lymphocytes (Lymph) and high numbers of neutrophils (ANC) in recipients of Tcons alone, the latter accounting for the increased total WBC. Animals that received Tregs with Tcons had normal levels of ANC and enhanced numbers of lymphocytes. Peripheral blood reconstitution of total lymphocytes and lymphoid subsets on days 14 (B-F) and 40 (G-K) after transplantation. Statistical differences (*P < .05) were noted between Tcons versus Tcon + Treg recipients in total lymphocytes, CD4+, CD8+, and NK+ cells on day 14. Differences were more significant on day 40 and included CD19+ cells. Results represent the mean plus or minus SE (3 to 4 mice per group, one of 3 experiments).
To evaluate the differences in lymphoid reconstitution, we measured early and late quantitative recovery of donor lymphocyte subsets on days 14 and 40 following HCT. These time points were selected based on preliminary assessments defining the temporal sequence of hematopoietic chimerism. On day 14, recipients of Tcon + Treg had increased numbers of total lymphocytes as well as subsets of CD4+, CD8+, and NK cells when compared with Tcon recipients (P < .05) (Figure 1B-F). By day 40, lymphoid recovery, including CD19+ B cells, in Tcon + Treg recipients was even more robust and comparable to age-matched untreated animals or animals that received only BM transplants and were without GvHD (P < .05) (Figure 1G-K).
Polyclonal Vβ usage and diverse TCR repertoire in recipients of Tregs
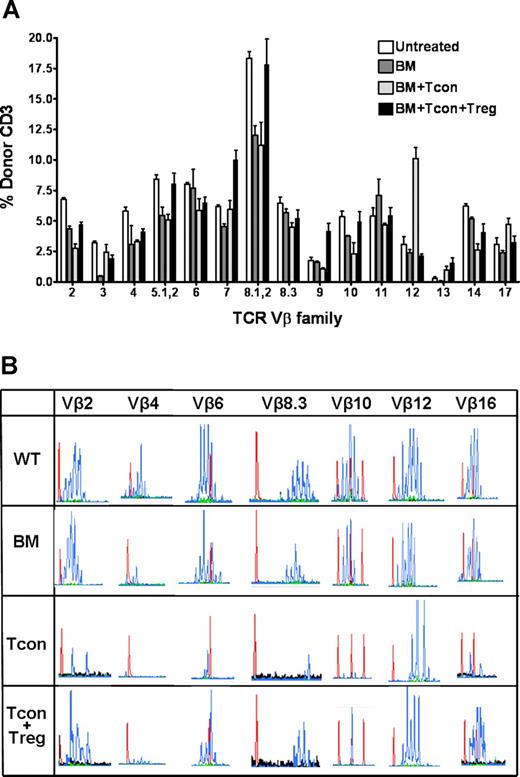
We next determined the impact of Tregs on the developing T-cell repertoire after HCT. Specifically, we evaluated the TCR-Vβ expression of donor-derived T cells isolated from transplant recipients on day 30 (Figure 2A). TCR-Vβ usage was broad among animals within each experimental group and comparable to age-matched untreated controls. However, Tcon recipients generally had lower levels of donor T cells at all Vβ elements except at Vβ12, where a higher percentage of T cells was noted. These findings suggest that Tregs do not compromise the generation and maintenance of a broad TCR-Vβ repertoire and appear to attenuate the loss of Vβ diversity that results from Tcon infusion.
Influence of regulatory T cells on the TCR Vβ repertoire following hematopoietic cell transplantation. (A) TCR Vβ profile of donor FVB (unpublished data) or C57Bl6 (shown) CD3+ T cells from Balb/c recipients on day 30 following transplantation. Flow cytometry shows a broad overall Vβ usage in all groups, with a higher percentage of donor CD3+ T cells at the majority of Vβ regions in animals that received Tregs in addition to Tcons. TCR Vβ profiles of untreated age-matched C57Bl/6 animals (Untreated) and of Balb/c recipients which received only C57Bl/6 T cell–depleted bone marrow cells (BM) served as controls. Data presented as the mean plus or minus SE (3 to 4 mice per group). (B) CDR3 size spectratyping analysis was performed using C57Bl/6-specific Vβ primers to generate PCR products from purified splenic cells from untreated C57Bl/6 wild-type controls (WT) or transplant recipients which received TCD-BM alone, with Tcons (Tcon) or with Tcons and Tregs (Tcon + Treg), on day 30 following transplantation. Shown are representative spectratypes of Vβ2, 4, 6, 8.3, 10, 12, and 16 in the histogram format. Histograms (blue peaks) depict CDR3 sequence length (abscissa) vs frequency of occurrence (ordinate). Red peaks within each histogram represent nucleic acid reference sequence of defined length. Data are representative of at least 3 animals per group, from one of 2 experiments.
Influence of regulatory T cells on the TCR Vβ repertoire following hematopoietic cell transplantation. (A) TCR Vβ profile of donor FVB (unpublished data) or C57Bl6 (shown) CD3+ T cells from Balb/c recipients on day 30 following transplantation. Flow cytometry shows a broad overall Vβ usage in all groups, with a higher percentage of donor CD3+ T cells at the majority of Vβ regions in animals that received Tregs in addition to Tcons. TCR Vβ profiles of untreated age-matched C57Bl/6 animals (Untreated) and of Balb/c recipients which received only C57Bl/6 T cell–depleted bone marrow cells (BM) served as controls. Data presented as the mean plus or minus SE (3 to 4 mice per group). (B) CDR3 size spectratyping analysis was performed using C57Bl/6-specific Vβ primers to generate PCR products from purified splenic cells from untreated C57Bl/6 wild-type controls (WT) or transplant recipients which received TCD-BM alone, with Tcons (Tcon) or with Tcons and Tregs (Tcon + Treg), on day 30 following transplantation. Shown are representative spectratypes of Vβ2, 4, 6, 8.3, 10, 12, and 16 in the histogram format. Histograms (blue peaks) depict CDR3 sequence length (abscissa) vs frequency of occurrence (ordinate). Red peaks within each histogram represent nucleic acid reference sequence of defined length. Data are representative of at least 3 animals per group, from one of 2 experiments.
To more precisely assess the diversity and clonality of the T-cell repertoire, we performed spectratyping DNA sequence analyses of the CDR3 region within each Vβ region.26,27 Age-matched untreated donor animals had a diverse TCR-Vβ, evident by the Gaussian distribution of peaks that represent a range and frequency of CDR3 sequence lengths within each Vβ locus (Figure 2B). Tcon recipients exhibited a decreased number and shifting of the peaks, resulting in an oligoclonal (Vβ2,10,12,16) or monoclonal (Vβ4,6,8.3) TCR-Vβ profile, indicating a contracted and skewed TCR-Vβ repertoire. At the Vβ12 locus however, there was a more diverse pool of CDR3 sequences, with a higher percentage of donor CD3+ T cells. In contrast, BM recipients that received Tcon + Treg had a more diverse and polyclonal distribution of TCR at most Vβ regions, comparable to control animals that received BM only.
Tregs enhanced functional lymphoid reconstitution, leading to protection from MCMV challenge
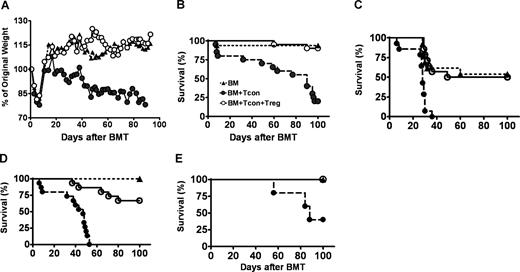
To determine if improved lymphoid recovery in Tcon + Treg recipients is associated with enhanced immunity against foreign antigens, animals were challenged with murine cytomegalovirus (MCMV) following HCT. Uninfected recipients in each respective experimental group served as controls to delineate the contribution of MCMV infection and GvHD on survival. Recipients of Tcon + Treg had minimal or no evidence of GvHD, while animals that received Tcons had significant GvHD, with a median survival of 85 days (Figure 3A,B). At day 100, 20% of uninfected Tcon recipients were alive, compared with 90% of animals co-transplanted with Tcon + Treg (P < .001), permitting longitudinal studies of immune reconstitution and responses to MCMV infection in these animals.
Viral immunity is preserved in the presence of Tregs. (A,B) Lethally irradiated Balb/c received FVB T cell–depleted BM alone (BM, ▴), with Tcons (●), or with Tcon + Treg (O). Shown are the weight change (A), a surrogate measure of graft-versus-host disease, and survival (B) following bone marrow transplantation (BMT) (Tcons vs Tcon + Treg, n = 20, P < .001). (C-E) Balb/c recipient mice transplanted with BM alone (▴), with Tcons (●) or Tcon + Treg (O) as described above are challenged with murine CMV on days 14 (C), 30 (D), or 63 (E). P value for Tcons vsersus Tcon + Treg: < .001, < .001, and = .08, respectively. P is NS for BM versus Tcon + Treg. Data combined from 3 separate experiments.
Viral immunity is preserved in the presence of Tregs. (A,B) Lethally irradiated Balb/c received FVB T cell–depleted BM alone (BM, ▴), with Tcons (●), or with Tcon + Treg (O). Shown are the weight change (A), a surrogate measure of graft-versus-host disease, and survival (B) following bone marrow transplantation (BMT) (Tcons vs Tcon + Treg, n = 20, P < .001). (C-E) Balb/c recipient mice transplanted with BM alone (▴), with Tcons (●) or Tcon + Treg (O) as described above are challenged with murine CMV on days 14 (C), 30 (D), or 63 (E). P value for Tcons vsersus Tcon + Treg: < .001, < .001, and = .08, respectively. P is NS for BM versus Tcon + Treg. Data combined from 3 separate experiments.
Animals were challenged with MCMV on days 14, 30, or 63 following HCT (Figure 3C-E). These time points represent incomplete, nearly complete, and complete lymphoid recovery in control animals that received TCD-BM only (unpublished data, December 2005). When animals were inoculated with MCMV on days 14 or 30 after transplantation, 100% of Tcon recipients died acutely from infection with a median time to death of 14 and 18 days after initial challenge, respectively. By contrast, animals that received Tcon + Treg and were infected on days 14 or 30 had a survival of 50% and 67% at day 100 after transplantation, respectively (Tcon vs Tcon + Treg, P < .001). When challenged with MCMV on day 63, 40% of Tcon recipients and 100% of Tcon + Treg recipients survived at day 100 after transplantation (P = .08), consistent with improved functional lymphoid reconstitution in both groups at this time. However, Tcon recipients that survived the acute infection also were significantly ill from severe GvHD. Survival of Tcon + Treg recipients was not statistically different from animals that received BM only.
Preserved thymus structure in Treg recipients correlates with improved lymphoid recovery
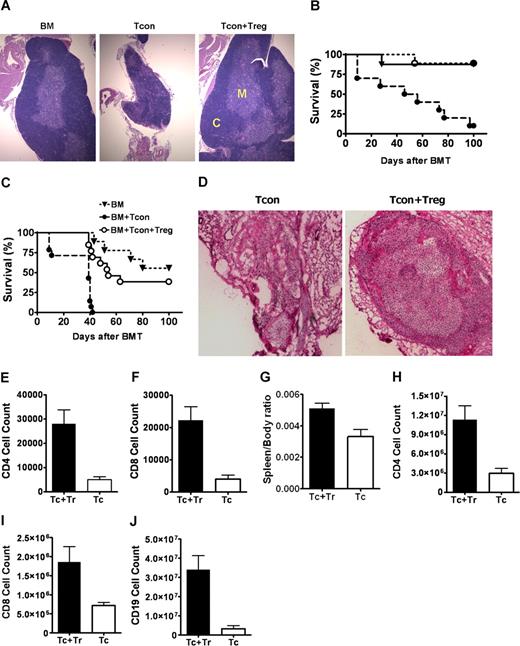
Thus far, our findings indicate that animals receiving Tcon + Treg develop a timely, diverse, and functional T-cell repertoire following HCT. In contrast, lymphoid recovery in Tcon recipients is delayed and incomplete, leading to poor MCMV immunity. We hypothesized that Tregs may protect thymic epithelial cells and stromal elements from GvHD-related damage, thus attenuating GvHD-induced thymic dysplasia, which arrest or block T-cell differentiation,12 resulting in decreased thymic output.11 Gross and histologic examination of thymi on days 30 and 60 after HCT confirmed the preservation of thymic integrity and architecture in BM controls and Tcon + Treg recipients compared with smaller involuted and degenerated thymi of Tcon recipients (Figure 4A).
Protection of thymic stroma and secondary lymphoid organs by regulatory T cells improves T-cell immunity. (A) H&E staining of thymus extracted from Balb/c recipients which received FVB TCD-BM alone (BM), with Tcons (Tcon), or with Tcons and Tregs (Tcon + Treg) (200 × magnification). Results from day 60 show small and involuted thymus with disorganized medullary, M, and cortical, C, regions in Tcon recipients compared with animals that received Tcons and Tregs. (B,C) Survival of adult thymectomized Balb/c recipients co-transplanted with BM alone (▾), with Tcons (●) or with Tcons and Tregs (O), uninfected (B). ●, n = 10 vs O, n = 9, P = .001), or challenged with MCMV on day 30 after transplantation (C): ●, n = 14 vs O, n = 13, P < .001). P is NS for BM versus Tcon + Treg. Data combined from 2 separate experiments. (D) H&E staining of peripheral lymph node sections from Balb/c recipients, which had received donor FVB TCD-BM with Tcons alone (Tcon) or with Tcons and Tregs (Tcon + Treg) on day 60 after transplantation (200 × magnification). In Tcon recipients, peripheral lymph nodes are collapsed with hyalinalization and hypoplasia. (E,F) Number of CD4+ and CD8+ T cells in peripheral lymph node of animals with Tcons alone (Tc, ▭) or with Tcons and Tregs (Tc + Tr, —) (CD4+, P = .002; CD8+, P = .002). (G-J). Spleen to body weight ratio (P = .037) and number of CD4+ (P = .011), CD8+ (P = .032), and CD19+ cells (.006) in spleens of animals that received BM and Tcons alone (Tc, ▭) or with BM with Tcons and Tregs (Tc + Tr, —). Data shown for day 60, representing at least 4 animals, with mean values (± SE).
Protection of thymic stroma and secondary lymphoid organs by regulatory T cells improves T-cell immunity. (A) H&E staining of thymus extracted from Balb/c recipients which received FVB TCD-BM alone (BM), with Tcons (Tcon), or with Tcons and Tregs (Tcon + Treg) (200 × magnification). Results from day 60 show small and involuted thymus with disorganized medullary, M, and cortical, C, regions in Tcon recipients compared with animals that received Tcons and Tregs. (B,C) Survival of adult thymectomized Balb/c recipients co-transplanted with BM alone (▾), with Tcons (●) or with Tcons and Tregs (O), uninfected (B). ●, n = 10 vs O, n = 9, P = .001), or challenged with MCMV on day 30 after transplantation (C): ●, n = 14 vs O, n = 13, P < .001). P is NS for BM versus Tcon + Treg. Data combined from 2 separate experiments. (D) H&E staining of peripheral lymph node sections from Balb/c recipients, which had received donor FVB TCD-BM with Tcons alone (Tcon) or with Tcons and Tregs (Tcon + Treg) on day 60 after transplantation (200 × magnification). In Tcon recipients, peripheral lymph nodes are collapsed with hyalinalization and hypoplasia. (E,F) Number of CD4+ and CD8+ T cells in peripheral lymph node of animals with Tcons alone (Tc, ▭) or with Tcons and Tregs (Tc + Tr, —) (CD4+, P = .002; CD8+, P = .002). (G-J). Spleen to body weight ratio (P = .037) and number of CD4+ (P = .011), CD8+ (P = .032), and CD19+ cells (.006) in spleens of animals that received BM and Tcons alone (Tc, ▭) or with BM with Tcons and Tregs (Tc + Tr, —). Data shown for day 60, representing at least 4 animals, with mean values (± SE).
To assess if the thymus is required for protection from adoptively transferred Tregs, adult thymectomized Balb/c animals were lethally irradiated and transplanted BM, with Tcons, and with Tcon + Treg, as previously described. Thymectomized uninfected HCT controls have a similar survival pattern as their euthymic counterparts (Figure 4B). Recipients of Tcons that were challenged with MCMV on day 30 after transplantation had a 100% mortality, with a median time to death of 10 days following initial infection. However, at day 100, 38% of Treg recipients survived without clinical infection or GvHD (P < .001) (Figure 4C). These data suggest that either extrathymic generation of new T cells or a secondary site of protection by Tregs account for the partial survival.
Secondary protection of peripheral nodal niches by Tregs promotes lymphoid recovery
Improved survival (Figure 4C) and reduced viral burden (Figure 5) in infected thymectomized Tcon + Treg recipients compared with thymectomized Tcon animals suggested that Tregs may exert their protection not only via the thymus but elsewhere. We evaluated the impact of Tregs on secondary lymphoid organs following HCT. Spleens and lymph nodes were isolated on days 30 and 60 after transplantation. Animals receiving Tcons had significant lymph node atrophy and hypoplasia resulting from GvHD (Figure 4D), with a reduction in CD4+ (P = .002) and CD8+ T cells (P = .002) compared with animals transplanted with Tcon + Treg (Figure 4E,F). Splenic fibrosis from GvHD in Tcon recipients was associated with significant reduction in numbers of CD4+ (P = .011), CD8+ (P = .032), and CD19+ cells (.006) (Figure 4G-J). These results indicate that the peripheral lymphoid niche is important for T-cell homeostasis following HCT11 and suggest that Tregs enhance immune reconstitution by preventing GvHD-induced damage to both primary and secondary lymphoid structures.
Improved survival in Treg recipients correlate with lower viral load. Viral load (A) and percent viral-free survival (B) of BM only or BM + Treg recipients on day 100 following transplantation. (A) Euthymic and athymic animals that received BM alone or with Tcons and Tregs are killed and organs (kidney, lungs, and liver) are extracted for viral load assessment. Shown is the kidney viral load (log plaque forming units [pfu] per gram tissue) from euthymic mice that received BM only (open symbol) or with Tcon + Treg (filled symbol) on day 0 and infected on day 14, day 30, or day 63, and athymic mice that received BM only (open symbol) or with Tcon + Treg (filled symbol) on day 0 and infected on day 30. Solid lines indicate mean viral load. Dotted line indicates the detection limit of the plaque assay. P = NS for viral load in recipients of BM only vs BM + Tcon + Treg; P = NS and .02, for ● versus ▴, and ▴ versus ■, respectively; P = .04 and .003, for O versus ▵, and ▵ versus □, respectively). (B) Euthymic or athymic animals that received BM alone (open bar) or with Tcon + Treg (filled bar) and were infected on days 14 or 30 or 63 were assessed on day 100 for the presence of virus in the salivary glands, kidneys, liver, and lungs. Uninfected animals in the respective groups served as controls. Data are shown for virus detectable in the kidneys. All thymectomized recipients that survived had detectable viral load. P = NS for survival without virus in BM vs BM + Tcon + Treg animals. In BM group, euthymic animals infected on day 14 versus 30 (P = .06); euthymic versus thymectomized animals infected on day 30 (P = .04). In BM + Tcon + Treg group, euthymic animals infected on day 14 versus 30 (P = 1.0); euthymic versus thymectomized animals infected on day 30 (P = .08).
Improved survival in Treg recipients correlate with lower viral load. Viral load (A) and percent viral-free survival (B) of BM only or BM + Treg recipients on day 100 following transplantation. (A) Euthymic and athymic animals that received BM alone or with Tcons and Tregs are killed and organs (kidney, lungs, and liver) are extracted for viral load assessment. Shown is the kidney viral load (log plaque forming units [pfu] per gram tissue) from euthymic mice that received BM only (open symbol) or with Tcon + Treg (filled symbol) on day 0 and infected on day 14, day 30, or day 63, and athymic mice that received BM only (open symbol) or with Tcon + Treg (filled symbol) on day 0 and infected on day 30. Solid lines indicate mean viral load. Dotted line indicates the detection limit of the plaque assay. P = NS for viral load in recipients of BM only vs BM + Tcon + Treg; P = NS and .02, for ● versus ▴, and ▴ versus ■, respectively; P = .04 and .003, for O versus ▵, and ▵ versus □, respectively). (B) Euthymic or athymic animals that received BM alone (open bar) or with Tcon + Treg (filled bar) and were infected on days 14 or 30 or 63 were assessed on day 100 for the presence of virus in the salivary glands, kidneys, liver, and lungs. Uninfected animals in the respective groups served as controls. Data are shown for virus detectable in the kidneys. All thymectomized recipients that survived had detectable viral load. P = NS for survival without virus in BM vs BM + Tcon + Treg animals. In BM group, euthymic animals infected on day 14 versus 30 (P = .06); euthymic versus thymectomized animals infected on day 30 (P = .04). In BM + Tcon + Treg group, euthymic animals infected on day 14 versus 30 (P = 1.0); euthymic versus thymectomized animals infected on day 30 (P = .08).
Improved survival from MCMV infection in Treg recipients correlates with lower viral load
To determine whether improved survival from MCMV is the result of viral clearance, we measured the MCMV load of the kidneys, liver, and lungs of Tcon + Treg recipients on day 100 after HCT. In euthymic recipients, MCMV infection at day 30 and beyond post-HCT resulted in higher overall and virus-free survival and lower viral load (Figure 5). No virus was detected in the tissues of animals challenged with MCMV on day 63. Infection on day 14 after HCT led to 50% overall survival in which 50% of animals still had detectable virus in the kidneys or liver, although there were no clinical signs of infection. These findings indicate that ultimate viral control is determined by the status of functional immunity at the time of viral exposure. In contrast, all thymectomized animals that survived MCMV infection had detectable virus titers that were several log fold higher than their surviving euthymic controls (P = .02). Importantly, when compared with animals that received only BM, the virus-free survival and viral load (Figure 5) of surviving Tcon + Treg recipients were not significantly different. In both groups, despite undetectable levels in the kidneys, liver, and lungs in most animals, and no clinical evidence of infection, virus was found at comparable titer in the salivary glands of animals at day 100 after HCT (unpublished data), an observation that is consistent with known MCMV tropism. These results suggest that the enhanced immune reconstitution due to Tregs leads to increased viral clearance and improved survival without impacting the subclinical persistence of MCMV.
MCMV-specific response due to T cells derived from donor stem cells
To determine the relative contribution of thymic-derived T cells and mature T cells (Tcons) transferred with the allograft toward MCMV immunity, we evaluated immune reconstitution in lethally irradiated animals that received BM only (BM) and do not have GvHD. In these animals, quantitative lymphoid reconstitution (Figure 1) and TCR-Vβ repertoire (Figure 2) was not statistically different from recipients of Tcon + Treg, but was significantly enhanced compared with BM recipients that had been transplanted with Tcons. MCMV challenge of euthymic BM recipients on days 14, 30, or 63 after transplantation showed increasing overall survival with later infection relative to HCT (Figure 3C-E) despite the absence of transferred Tcons. The percentage of animals that successfully cleared MCMV also improved with later infection, with rates of 43%, 90%, and 100% for infection on days 14, 30, and 63 (Figure 5). These findings are consistent with increasing lymphoid recovery from donor stem cells over time. In addition, BM recipients that had undergone pretransplant thymectomy had a significantly decreased survival and detectable viral loads compared with euthymic controls (Figure 5). These results indicate that newly generated T cells from the thymus constitute the major population involved in the observed responses to MCMV.
Generation of antiviral T-cell response with adoptive transfer of Tregs
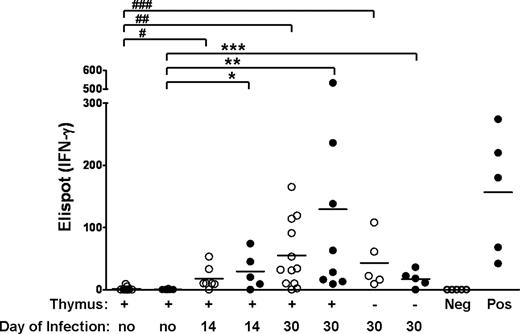
Given that animals receiving Tcon + Treg are able to generate an effective primary immune response to a novel antigen such as MCMV, we next evaluated the recipients' ability to generate MCMV-specific memory T cells in the presence of Tregs. In the absence of tetramers available for the FVB background, we used the ELISPOT assay to measure interferon-γ (IFN-γ) release from equivalent number of donor T cells isolated on day 100 from previously infected animals and incubated with MCMV 1E1 peptide (Figure 6). T cells from uninfected Tcon + Treg recipients did not release interferon; however, T cells from previously infected animals that received Tcon + Treg or BM only secreted a similar level of IFN-γ upon exposure to CMV 1E1, with increasing IFN-γ values measured for animals infected with MCMV at later time points after HCT (P = .01 for day 14 infection and .001 for day 30 infection, Tcon + Treg compared with uninfected controls). In addition, T cells from thymectomized Tcon + Treg recipients had a lower secondary response (P = .003), consistent with the survival data and is not unexpected since lymphoid reconstitution in Tcon + Treg recipients is more complete with an intact thymus. These data provides evidence for the ability of previously infected Tcon + Treg recipients to mount a secondary response to MCMV, particularly when immune reconstitution is more complete, and is consistent with our findings that MCMV-specific immunity is due mainly to T cells generated from the donor stem cells. We were unable to evaluate secondary responses in animals that had received Tcons, as they rapidly died from primary MCMV infection within approximately 2 weeks of initial challenge.
Tregs permit the generation of secondary responses to CMV antigen. Euthymic or thymectomized Balb/c animals that were co-transplanted with FVB TCD-BM, Tcons, and Tregs (●) or with TCD-BM alone (○) on day 0 and infected with CMV on day 14 or 30 were killed on day 100. Donor splenic lymphocytes were sorted and CMV-specific responses to E1 antigen were measured by Elispot assay for Interferon-γ release. Each symbol represents the Elispot count from a different mouse. Solid line indicates mean number of elipsots. P values: * = .01, ** = .006, *** = .01, # = .002, ## = .001, ### = .001. P values are not significant between • versus ○, and not significant between euthymic versus thymectomized animals infected on day 30 for both groups. Positive (pos) and negative (neg) controls are as described in “IFN-γ ELISPOT assay.”
Tregs permit the generation of secondary responses to CMV antigen. Euthymic or thymectomized Balb/c animals that were co-transplanted with FVB TCD-BM, Tcons, and Tregs (●) or with TCD-BM alone (○) on day 0 and infected with CMV on day 14 or 30 were killed on day 100. Donor splenic lymphocytes were sorted and CMV-specific responses to E1 antigen were measured by Elispot assay for Interferon-γ release. Each symbol represents the Elispot count from a different mouse. Solid line indicates mean number of elipsots. P values: * = .01, ** = .006, *** = .01, # = .002, ## = .001, ### = .001. P values are not significant between • versus ○, and not significant between euthymic versus thymectomized animals infected on day 30 for both groups. Positive (pos) and negative (neg) controls are as described in “IFN-γ ELISPOT assay.”
Discussion
Regulatory T cells have potential as an adoptive cellular therapy to prevent GvHD.6,7,9,21 However, the absence of known GvHD epitopes required for expansion of antigen-specific Tregs necessitates the transfer of polyclonal Treg populations that some had hypothesized may lead to inadvertent and bystander inhibition of general immunity and may compromise responses to microbes and tumor following HCT. Our studies demonstrate that Tregs in fact do not adversely impact long-term engraftment or stable donor chimerism. We showed that adoptively transferred Tregs promote lymphoid reconstitution in addition to providing protection from GvHD. Enhanced T-cell number and diversity in Tcon + Treg recipients led to improved immunity to MCMV, with significant reduction in viral load and increased survival from an otherwise lethal CMV infection in animals transplanted with Tcons alone. Importantly, the transfer of Tregs did not compromise long-term immunity following HCT, which is required for effective vaccination28 and adequate responses to recall antigens, such as CMV reactivation and tumor relapse.17,29 Our studies were performed to assess the impact of Tregs on total T cells without further distinction of CD4+ and CD8+ T cells since both subpopulations are required for sustained antiviral protection.30,,–33 Further studies will evaluate the potential differential regulation of Tregs on the repertoire and function of T-cell subsets, including naive versus memory CD4+ T cells. In addition, we will assess the newly generated Treg repertoire as its scope may influence the level of specific and nonspecific suppression in our model of GvHD.
Our data raise the possibility that adoptive transfer of Tregs contributes to the viral persistence which otherwise may be cleared by the co-transfer of Tcons. This delayed or incomplete pathogen clearance thus may enable immunological memory to develop.34 Previous studies showed that endogenous Tregs can be both beneficial and detrimental to the infected host, limiting aggressive immune responses against pathogens that may cause collateral damage of host tissues35,36 and the development of autoimmunity,37 while facilitating pathogen persistence by suppressing rapid effector responses.34,38,,–41 In our BMT model, animals that received Tcon + Treg generated primary and secondary immune responses to the same extent as BM recipients, indicating that Tregs do not adversely affect host MCMV immunity after HCT and that viral persistence at a subclinical level is part of the natural history of CMV infection. Our data showed that the protective role of Tregs may be their ability to reduce the immunopathology induced by the co-transferred Tcons which compromises immune reconstitution. Indeed, we demonstrated that Tcon + Treg recipients had preserved thymic and peripheral lymphoid architecture and size similarly found in BM control animals, with significantly increased blood and nodal output of newly generated lymphocytes following HCT. The differences in outcomes of the contradictory effects of Tregs in different experimental models, including ours, may be explained by the balance between Tregs and effector T cells3,18,42 under different disease settings, and is further demonstrated by the ability of the immune system in normal immunocompetent individuals to prevent the development of autoimmune diseases while able to mount effective responses to a range of pathogenic agents.
In our model of HCT, lymphoid reconstitution and immunity results mainly from thymic-dependent recapitulation of lymphoid ontogeny with donor stem cells and thymic-independent expansion of mature T cells (Tcons) from the allograft.43 Our studies showed that Tcons cause GvHD18,21 while thymic-derived T cells are the major effectors of MCMV and long-term immunity. Our previous reports on the in vivo kinetics of adoptively transferred Tregs18,21 indicate that the apparent selective inhibition by Tregs of GvHD but not MCMV immunity is due to early and robust suppression of Tcons in the first week after HCT, which prevents GvHD, followed by homeostatic immune responses of Tregs during the non-inflammatory phase of HCT which allow and accelerate recapitulation of lymphoid ontogeny of the thymic-derived donor T-cell repertoire. We showed that low levels of Tregs during this latter phase of HCT do not adversely impact viral immunity; Tcon + Treg recipients had comparable control of MCMV to animals that received BM only and immunity improved with later infection consistent with increased lymphoid recovery. We expect that MCMV challenge at the time of Treg transfer would lead to significant reduction in viral control due to the significant Treg-mediated reduction of Tcons which mediate immunity early after HCT. The protective role of Tregs for both GvHD and MCMV immunity therefore occurs early, with direct inhibition of Tcons and prevention of immunopathology of thymic and nodal niches that afford enhanced lymphoid reconstitution. Studies using CMV-specific tetramers and congenic markers will further define the contributions of Tcons versus thymic-derived T cells toward MCMV immunity in presence of Tregs.
Resolution of CMV infection is primarily mediated by T cells.30,,–33 However, NK cells,44,45 B cells,46,47 and dendritic cells48,49 contribute to a variable degree to the immune control of CMV and may also account for partial survival in thymectomized Tcon + Treg recipients challenged with MCMV. Tcon + Treg recipients have increased reconstitution of donor NK cells and B cells at early and later time points after HCT, respectively, which likely contributes to CMV immunity following HCT. Further studies are needed to determine the relative contribution of B and NK cells as well as the potential contribution of residual host T and NK cells in the control of CMV infection in our model.50 Extrathymic de novo generation of donor T cells observed in our studies may also provide some protection from MCMV infection,50 despite their lower number and poorer function compared with thymic-derived T cells.11,51
In summary, our findings offer new insights into the contribution of Tregs in maintaining an optimal microenvironment for the reconstitution of functional immunity. Protection of the thymic and nodal niches in which T-cell generation and homeostasis occur is a major mechanism by which Tregs mediate their protective effects, and may help explain the intrinsic link between the suppression of GvHD and an enhancement of immune reconstitution following adoptive transfer of Tregs. The ability of Tregs recipients to generate a diverse TCR repertoire and to mount effective functional immune responses supports further consideration of Tregs immunotherapy for clinical allogeneic HCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ruby Wong for statistical support and Dan daSilva for technical assistance.
This work was supported by the National Institutes of Health grant nos. K08 AI060888 (V.H.N.), R01 CA0800065, and P01 HL075462 (R.S.N. and J.M.B.).
National Institutes of Health
Authorship
Contribution: V.H.N. designed and conceptualized the research, performed the experiments, analyzed the data, and wrote the paper; S.S. did the ELISA and viral load assessment and helped analyze data; D.C., L.H., and M.B. helped with the spectratyping studies; N.K. analyzed the histology; R.S.N. and J.M.B. provided overall research advice and guidance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Science Research, 269 W Campus Dr, Rm 2205, Division of Bone Marrow Transplantation, Stanford University School of Medicine, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
Author notes
S.S. and D.S.C. contributed equally to the manuscript.
J.M.B. and R.S.N. are co-senior authors.

![Figure 5. Improved survival in Treg recipients correlate with lower viral load. Viral load (A) and percent viral-free survival (B) of BM only or BM + Treg recipients on day 100 following transplantation. (A) Euthymic and athymic animals that received BM alone or with Tcons and Tregs are killed and organs (kidney, lungs, and liver) are extracted for viral load assessment. Shown is the kidney viral load (log plaque forming units [pfu] per gram tissue) from euthymic mice that received BM only (open symbol) or with Tcon + Treg (filled symbol) on day 0 and infected on day 14, day 30, or day 63, and athymic mice that received BM only (open symbol) or with Tcon + Treg (filled symbol) on day 0 and infected on day 30. Solid lines indicate mean viral load. Dotted line indicates the detection limit of the plaque assay. P = NS for viral load in recipients of BM only vs BM + Tcon + Treg; P = NS and .02, for ● versus ▴, and ▴ versus ■, respectively; P = .04 and .003, for O versus ▵, and ▵ versus □, respectively). (B) Euthymic or athymic animals that received BM alone (open bar) or with Tcon + Treg (filled bar) and were infected on days 14 or 30 or 63 were assessed on day 100 for the presence of virus in the salivary glands, kidneys, liver, and lungs. Uninfected animals in the respective groups served as controls. Data are shown for virus detectable in the kidneys. All thymectomized recipients that survived had detectable viral load. P = NS for survival without virus in BM vs BM + Tcon + Treg animals. In BM group, euthymic animals infected on day 14 versus 30 (P = .06); euthymic versus thymectomized animals infected on day 30 (P = .04). In BM + Tcon + Treg group, euthymic animals infected on day 14 versus 30 (P = 1.0); euthymic versus thymectomized animals infected on day 30 (P = .08).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/2/10.1182_blood-2007-07-103895/4/m_zh80030812110005.jpeg?Expires=1769159899&Signature=ncvd8GoPY05lOlwWaN~pbVcw18FYpt695mrRRTL-UUC7Zs-PhXE750OEzR3kGWZs9GEmVctpsjtHg1NTDqaTH7dOIM9pWe6baaZ-amMvDrx0CoxX95EyZDEJxI5KqSuLoa5Sa4gWcHTqbu-BUV-zzH~gs6ANP6R4JL-IhkFtt2etFVngSZ1MKth2x3UqdCmD2nI~UK2DMGL9fls7KM6dwrzM3TbU5v3kya7~WKqW23wApzlPyqXUJxZZ8OvHy7GJfu98l1-OnX8iUV8qOypCZVrvT6jrnz8eaVReVSrVMQ6eL5Pdbtvi5lhX8Ne8GFwroAMWRzJZsHk~IQe61FIdTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal