Previous studies using intravital microscopy in a sickle cell disease (SCD) mouse model suggest that adherent white blood cells (WBCs) play a key role in vaso-occlusion by capturing circulating red blood cells (RBCs) in venules. Commercial intravenous immunoglobulin (IVIG) given before the inflammatory stimuli increased microcirculatory blood flow and survival. To mimic the clinical situation in which SCD patients seek medical attention after the onset of symptoms, we developed an in vivo model in which the therapeutic intervention (eg, IVIG) was administered after in the inflammatory challenge. In this setting, IVIG rapidly (< 10 minutes) reduced adherent leukocyte numbers and dramatically inhibited interactions between RBCs and WBCs, resulting in improved microcirculatory blood flow and survival of sickle cell “Berkeley” mice. Longer survival correlated positively with blood flow (P = .001) and negatively with the number of adherent leukocytes (P = .001) and RBC-WBC interactions (P = .002). Using multichannel digital fluorescence videomicroscopy, we found that IVIG affected specifically the recruitment of neutrophils. Moreover, further analyses of leukocyte behavior revealed that IVIG significantly increased rolling velocities, indicating that it alters adhesion pathways involved in slow rolling. These data suggest that the potential therapeutic benefits of IVIG in SCD crises should be evaluated in a clinical trial.
Introduction
Sickle cell disease (SCD) is one of the most common inherited hematologic diseases in the world. It arises from a single missense mutation in the β-chain of hemoglobin, resulting in the substitution of valine for glutamic acid (β6Glu→Val), which renders the hemoglobin molecule less soluble upon deoxygenation.1,–3 This may lead to the polymerization of hemoglobin, resulting in alterations in the red blood cell (RBC) physiologic discoid shape. Hemoglobin polymerization also induces marked changes on the cell surface, resulting in an increased propensity of RBCs to adhere and providing the basis for understanding the pathophysiology of vascular occlusion, the hallmark of sickle cell disease.4 Although the propensity of sickle RBCs to stick to one another was recognized many years before cell adhesion was conceptualized at the molecular level,5 the increased adherence to endothelial cells was characterized in a series of seminal studies in the 1980s.6,–8 Many adhesion pathways have been suggested to participate in sickle cell adhesion to the endothelium, but their pathophysiologic functions are unclear because very few studies have evaluated the mechanisms mediating sickle cell adhesion in vivo, when plasma and all blood cell elements are present. In vivo studies are critical to identify valuable targets because vaso-occlusion is a complex phenomenon; sickle RBCs can indeed adhere to other blood cells, including leukocytes9 and platelets.10
Our previous studies revealed that the adhesion of sickle RBCs to leukocytes (WBCs) plays a key role in the pathophysiology of vaso-occlusion induced by the cytokine tumor necrosis factor-α (TNF-α).11 We originally developed this model using TNF-α because the response in the microcirculation had been extensively studied and shown to increase the expression of key adhesion molecules on the endothelium.12,–14 In addition, TNF-α levels are chronically elevated in the plasma of steady-state sickle cell patients compared with healthy controls.15,–17 Further, a proinflammatory mutation in the TNF gene promoter (TNF(-308)G/A promoter polymorphism) was shown to be associated with large vessel stroke, suggesting that it may contribute to the pathophysiology of SCD.18 Our previous intravital microscopy observations of sickle cell mice, challenged by the surgical trauma and TNF-α, have revealed that adherent leukocytes in small venules can capture circulating RBCs, producing a progressive reduction in microcirculatory blood flow and eventually a complete vascular occlusion. Although the molecular mechanisms mediating these interactions are still unclear, the infusion of normal immunoglobulins was shown to reduce significantly the number of interactions between RBCs and WBCs and to improve hemodynamics in the cremasteric microcirculation.19 Because intravenous immunoglobulin (IVIG) administration is an approved drug for hypogammaglobulinemia and several autoimmune diseases, it may provide a potentially novel therapeutic approach for the treatment of sickle cell crises.
Acute vaso-occlusive crises represent the most common complication in SCD, but there is currently no specific treatment for this condition. A significant proportion of patients admitted with a sickle cell crisis will subsequently develop an acute chest syndrome, a life-threatening complication.20 However, treatment of acutely ill patients represents a special challenge because the tested therapy may conceivably aggravate the acute problem. This concern is relevant for IVIG therapy because the administration of high doses of IVIG to patients without SCD is associated with a low but meaningful incidence of stroke,21 a common complication in sickle cell patients.22
To our knowledge, all previously published in vivo preclinical studies in this disease have evaluated the impact of a gene or drug before a challenge. These types of studies are less relevant for therapies aimed at acute complications because patients generally seek medical attention after a crisis is firmly established. Here, we have developed a model in which IVIG is administered after the onset of a crisis experimentally induced by surgical trauma and the cytokine TNF-α. We show that IVIG can dramatically alter the progress of vaso-occlusion, leading to marked improvement in microcirculatory blood flow and survival of “Berkeley” sickle cell mice. In addition, detailed intravital microscopy analyses provide new insight into the mechanisms of action of IVIG in SCD. These data provide the proof-of-principle that acute vaso-occlusive crises are therapeutically reversible. Thus, these results underscore the encouraging possibility that therapeutic interventions initiated when patients have an established crisis may improve the outcome of acute episodes, and may prevent their progression into life-threatening complications.
Methods
Animals
Sickle cell mice [Tg(Hu-miniLCRα1GγAγδβS) Hba−/−Hbb−/−],23 a kind gift from Dr Narla Mohandas (New York Blood Center, New York, NY), were housed and bred at Mount Sinai School of Medicine. Genetically identical cohorts of male sickle cell mice were generated by bone marrow transplantation (BMT) as described previously except that the preparative regimen was 1500 cGy in 2 split doses.11 We have found that this dose of radiation yields full chimerism at high frequency (> 90%) as assessed using acid–urea hemoglobin electrophoresis. Mice carrying more than 97% donor chimerism at 3 to 5 months after transplantation were used for intravital microscopy studies. Other intravital studies were carried out on parental sickle mice without transplantation or wild-type C57BL/6 mice at about 3 months of age. All experimental procedures in this study were approved by the Animal Care and Use Committee of Mount Sinai School of Medicine.
Immunoglobulins
Human γ-globulin (IVIG; 10% caprylate/chromatography purified Gammunex) was a generous gift from Talecris Biotherapeutics (Clayton, NC).
Intravital experimental protocol
Sickle cell mice were anesthetized by intraperitoneal (ip) injection of a mixture of 2% chloralose (Sigma-Aldrich, St Louis, MO) and 10% α-urethane (Sigma-Aldrich) in phosphate-buffered saline (PBS; 6 mL/kg). A polyethylene tube was inserted into the trachea to facilitate spontaneous respiration, and the right carotid artery was cannulated for injection of IVIG or PBS. The cremaster muscle was gently exteriorized and then continuously superfused throughout the experiment with warmed (37°C) bicarbonate-buffered (pH 7.4) saline aerated with a mixture of 95% N2 and 5% CO2. Seventy minutes after administration of TNF-α (0.5 μg ip), IVIG (800 mg/kg) or an equivalent volume of control PBS or albumin (800 mg/kg) was administered by a programmable syringe pump (PHD 4400, Harvard Apparatus, Holliston, MA) at the rate of 667 μL/kg/min. Twenty minutes after IVIG, PBS, or albumin exposure, 8 to 12 venules were videotaped over a period of 60 minutes, with each venule recorded continuously for at least 3 minutes. Venules were visualized with a custom-designed Nikon MM-40 intravital microscope (Nikon, Tokyo, Japan), using a 60× water immersion objective lens (Nikon). Images were recorded using a charge-coupled device video camera (Hamamatsu, Bridgewater, NJ) and a Sony SVHS SVO-9500 video recorder (Sony, Tokyo, Japan). Venular diameter was measured with a video caliper. Centerline red cell velocity (VRBC) was measured for each venule in real time using an optical Doppler velocitometer (Texas A&M University, College Station, TX). Wall shear rate (γ) was calculated based on Poiseuille's law for a Newtonian fluid, γ = 2.12 (8Vmean)/Dv, where Dv is the venule diameter, Vmean is estimated as VRBC/1.6, and 2.12 is a median empirical correction factor obtained from actual velocity profiles measured in microvessels in vivo.24 Blood flow rate (Q) was calculated as Q = Vmean πd2/4, where d is venule diameter and is expressed as nL/sec. Survival time, defined as the time interval from TNF-α administration to the death of mouse, was recorded. Blood samples taken immediately after death, through a cardiac puncture, were used to determine complete blood counts using the ADVIA 120 Hematology Analyzer System (Bayer, Holliston, MA)
Image analyses for brightfield intravital microscopy
All analyses were made using playback assessment of videotapes as described previously.11 Briefly, rolling WBCs were defined as those moving at a velocity less than that of free-flowing erythrocytes in a given vessel and counted over 3 1-minute intervals per venule. Adherent WBCs were defined as those remaining stationary for at least 30 seconds over a 100-μm venular segment. RBCs were identified by their size and shape (discoid and sickle-shaped cells). An interaction between RBCs and adherent WBCs was defined as the arrest of an RBC on an adherent WBC for more than 2 video frames (> 0.07 second). This time interval corresponds to a readily discernable adhesion event when the videotape is played in real time. Leukocyte rolling velocity (VWBC) was calculated by dividing the traveled distance by the tracking time or as the average translation over 2 seconds of 10 WBCs per venule and expressed as μm/sec. Leukocyte transit time was calculated as 100 μm/VWBC. The flux fraction (F) of rolling leukocytes, corresponding to the percentage of leukocytes that are rolling per minutes, was calculated by F = WBCr /(0.25πd2 Vmean 60 [WBC]), where WBCr is the number of leukocytes rolling past a fixed reference point in the venule per minute, d is venule diameter, VRBC is centerline velocity, and [WBC] is the total systemic leukocyte count.25
Multichannel digital fluorescence intravital microscopy
We used multichannel fluorescence videomicroscopy to identify leukocyte subsets recruited in venules. Our digital intravital microscopy system was described recently.26 Briefly, the system is built on an Olympus BX61WI work station mounted on a motorized X,Y stage (Applied Scientific Instrumentation, Eugene, OR), allowing precise computer-controlled lateral movement between XY positions and a Z focusing drive to allow the focal plane of a LUMPlanFI 60× numeric aperture 0.90 ∞ water-immersion objective (Olympus, Melville, NY) to be rapidly changed. The microscope is equipped with a Lambda DG-4 high-speed wavelength switcher (Sutter Instrument, Novato, CA) from a 175-watt xenon light source with excitation filters (360 nm, 480 nm, and 590 nm) matched to a triple-band filter for 4,6 diamidino-2-phenylindole (DAPI), fluorescein isothiocyanate (FITC), and cyanin 3 (Cy3), and individual filter sets for DAPI, FITC, Cy3, Texas Red, and Cy5 (Chroma, Brattleboro, VT) in the body of the microscope. Transmitted brightfield light was delivered through a shutter (Uniblitz VS25; Vincent Associates, Rochester, NY), allowing the acquisition of both brightfield and fluorescence images without interruption through computer control. Images were collected with a SensiCam camera (9.9 μm2 pixel, 640 × 480 pixel format; Cooke, Auburn Hills, MI) mounted on a Videoscope image intensifier (Sterling, VA). Coordinated image acquisition and offline data analyses were carried out using SlideBook software (Intelligent Imaging Innovations, Denver, CO) and run on a Dell Precision 650 computer system (Dell, Round Rock, TX) with dual Xeon 3.06 GHz microprocessors and 3.0 GB of RAM.
Antibodies were purchased from BD PharMingen (San Diego, CA), with the exception of rat anti-F4/80, which was purified from hybridoma supernatants (American Type Culture Collection, Manassas, VA), and labeled with AlexaFluor dyes per manufacturer instruction (Invitrogen, Carlsbad, CA). To assess the subsets of leukocytes recruited in cremasteric venules, we injected AlexaFluor 555–labeled rat anti-mouse CD45 (0.12 mg/kg), AlexaFluor 488–labeled Gr-1 (Ly-6C and Ly-6G; 0.12 mg/kg), and AlexaFluor 660–labeled anti-F4/80 (0.06 mg/kg) through the right carotid artery. We have shown previously that this combination of antibodies can discriminate among neutrophils (CD45 + Gr-1 + F4/80−), monocytes (CD45 + F4/80 +), and lymphocytes (CD45 + Gr-1−F4/80−).26 For offline analyses of differential counts, leukocytes were scored as positive when the fluorescence intensity per pixel of a cell surface marker was 10% greater than of the neighboring acellular plasma. Positive events were also confirmed from comparative analyses of multiple frame series by evaluating the shape and movement of the fluorescence signal.
Flow cytometry
Blood samples were sequentially collected into sterile tubes containing ethylenediaminetetraacetic acid (EDTA) from a mouse tail. The time points sampled include the baseline (T0, pre–TNF-α), 70 minutes after TNF-α administration but before IVIG injection (T70), and 120 minutes (T120) and 150 minutes (T150) after TNF-α administration (both after IVIG exposure). After RBC lysis, leukocytes were stained with allophycocyanin C (APC)–labeled anti–Gr-1, FITC-labeled anti-CD11a, and R-phycoerythrin-labeled anti–P-selectin glycoprotein ligand-1 (PSGL-1; Biosciences). Polymorphonuclear neutrophils (PMNs) were gated on the basis of forward- and side-scatter characteristics and high Gr-1 expression. To detect the binding of E-selectin to PMNs, cells were incubated with APC-labeled anti–Gr-1 and E-selectin/immunoglobulin M (IgM) chimera followed by incubation with FITC-conjugated anti-human IgM antibody (1:50 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) as described previously. All incubations were performed at 6°C for 15 minutes. Samples were analyzed using a FACSCalibur flow cytometer and CellQuest version pro software (BD Biosciences, San Jose, CA).
Statistical analyses
All data are displayed as mean (±SEM). Statistical analyses were done with Statview version 4.5 software (Abacus Concepts, Berkeley, CA). Parametric data were analyzed using analysis of variance. Statistical significance for nonparametric distributions (RBC-WBC interactions) was assessed using the Mann-Whitney test. Univariate regression analyses were carried out to assess the dependency of survival on various hematologic and intravital microscopy parameters. Independent variables that showed significant association were tested in multiple regression analyses. Correlation between RBC-WBC interactions and survival was tested with the Spearman rank correlation. Statistical significance for Kaplan-Meier plots was assessed using the log-rank test. A value of P less than .05 was deemed significant.
Results
Intravital microscopy model of sickle vasoocclusion
Sickle cell patients generally seek medical attention once a sickle “crisis” is already established. To evaluate the effect of IVIG in an animal model that resembles a clinical situation, we modified our previous protocol by infusing IVIG into sickle mice during a crisis, using the occurrence of sickle RBC interactions with adherent WBCs as a surrogate marker of vaso-occlusive activity. Our previous studies revealed that the interactions between RBCs and WBCs are initiated approximately 40 minutes after cremasteric exposure and are further enhanced by the administration of TNF-α.11,27 We inserted a catheter through the carotid artery of tracheostomized sickle mice to inject IVIG by continuous infusion. Immediately after the cremasteric surgery, we injected TNF-α i.p. to provide an additional inflammatory trigger producing a systemic sickle cell crisis. Mice were infused 70 minutes after TNF-α administration with either IVIG (800 mg/kg) or PBS control over 10 minutes. The corresponding IVIG infusion rate was approximately 10 times faster than the maximal recommended rate of infusion in humans. Several venules were then recorded during the interval between 90 and 150 minutes, and sickle mice were observed for up to 11 hours to determine the time of death. Thus, this approach allowed us to evaluate the effect of IVIG in the microcirculation during active inflammation and to determine whether IVIG can alter the progression of an established crisis.
IVIG rapidly alters the recruitment of adherent leukocytes and their ability to capture circulating sickle RBCs
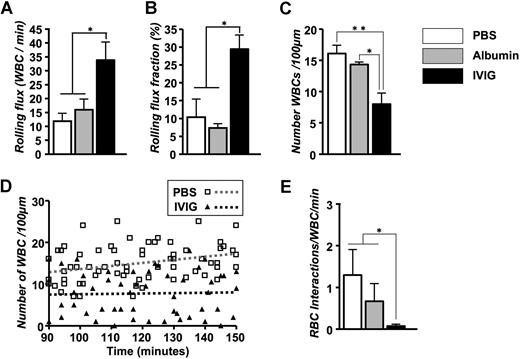
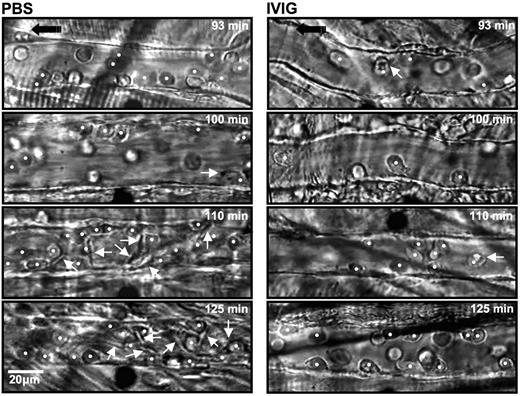
The numbers of circulating leukocytes, RBCs, reticulocytes, and platelets were not significantly different between the PBS- and IVIG-treated groups (Table 1). Careful analyses of recording revealed a 3-fold increase in the number of rolling leukocytes in IVIG-treated mice (Figure 1A). Because of the slight difference in the number of circulating WBCs and animal-to-animal variability, we also expressed these data as a rolling flux fraction. In PBS-injected mice, approximately 10% of the circulating leukocytes were rolling, whereas the rolling flux fraction was approximately 30% in IVIG-treated animals (Figure 1B; P < .05). In contrast, the average number of WBCs adherent to endothelium was reduced by approximately 50% in the IVIG-treated animals compared with PBS-injected sickle mice (Figure 1C; P < .01). The effect of IVIG on leukocyte adhesion was rapid because it was already detectable (P < .05) in the first recorded venules approximately 10 minutes after the end of infusion (Figure 1D). The number of adherent leukocytes increased progressively over time in PBS control mice, whereas it remained lower throughout the recording period in IVIG-treated mice. To assess the effect of IVIG on sickle cell adhesion, we quantified the interactions between circulating RBCs and adherent WBCs in venules recorded between 90 and 150 minutes. IVIG treatment dramatically reduced the number of RBCs interacting per adherent WBC (19-fold reduction; Figure 1E; P < .05), indicating that IVIG can affect the capture of RBCs even when adherent leukocytes are already recruited. This effect did not result from the possible colloid expansion by the infusion of immunoglobulins because leukocyte behavior after the administration of albumin (800 mg/kg) was not significantly different from the PBS group (Figure 1). Representative sequences of images to illustrate these observations are depicted in Figure 2.
Effect of IVIG on peripheral blood counts in transplanted sickle cell mice
| Treatment . | Mice, no. . | WBC, 103 cells/μL . | RBC, 106 cells/μL . | Reticulocytes, 109 cells/L . | Platelets, 103 cells/μL . |
|---|---|---|---|---|---|
| PBS | 14 | 10.7 ± 2.2 | 3.8 ± 0.3 | 1284 ± 132 | 285 ± 35 |
| IVIG | 11 | 9.5 ± 1.3 | 3.8 ± 0.2 | 1543 ± 100 | 307 ± 27 |
| Treatment . | Mice, no. . | WBC, 103 cells/μL . | RBC, 106 cells/μL . | Reticulocytes, 109 cells/L . | Platelets, 103 cells/μL . |
|---|---|---|---|---|---|
| PBS | 14 | 10.7 ± 2.2 | 3.8 ± 0.3 | 1284 ± 132 | 285 ± 35 |
| IVIG | 11 | 9.5 ± 1.3 | 3.8 ± 0.2 | 1543 ± 100 | 307 ± 27 |
Data are presented as mean (±SEM). There was no statistical difference between the PBS and IVIG groups.
Effect of IVIG on the leukocyte behavior and RBC capture in TNF-α treated sickle mice. IVIG increases the number of rolling leukocytes. (A) Leukocyte rolling flux represents the number of rolling leukocytes per minute. *P < .05. (B) Leukocyte rolling flux fraction. *P < .05. (C) Number of adherent leukocytes per 100 μm. *P < .05; **P < .01. (D) Scatter plots of the number of leukocytes during the experimental period. Each symbol represents data from a single venule. The increase in leukocyte adhesion over time after TNF-α exposure was abrogated in IVIG-treated compared with PBS-treated control mice. The gray and black dashed regression lines represent PBS-treated and IVIG-treated mice, respectively. (E) Number of interactions between RBCs and adherent WBCs per minute. *P < .05.
Effect of IVIG on the leukocyte behavior and RBC capture in TNF-α treated sickle mice. IVIG increases the number of rolling leukocytes. (A) Leukocyte rolling flux represents the number of rolling leukocytes per minute. *P < .05. (B) Leukocyte rolling flux fraction. *P < .05. (C) Number of adherent leukocytes per 100 μm. *P < .05; **P < .01. (D) Scatter plots of the number of leukocytes during the experimental period. Each symbol represents data from a single venule. The increase in leukocyte adhesion over time after TNF-α exposure was abrogated in IVIG-treated compared with PBS-treated control mice. The gray and black dashed regression lines represent PBS-treated and IVIG-treated mice, respectively. (E) Number of interactions between RBCs and adherent WBCs per minute. *P < .05.
Sequential representative images of venules from sickle mice treated with PBS and IVIG. Each still frame was taken at 93-, 100-, 110-, and 125-minute time points after TNF-α injection. IVIG significantly reduced the number of adherent leukocytes (white circles) and RBCs interacting with adherent leukocytes (white arrows).  indicates the direction of flow.
indicates the direction of flow.
Sequential representative images of venules from sickle mice treated with PBS and IVIG. Each still frame was taken at 93-, 100-, 110-, and 125-minute time points after TNF-α injection. IVIG significantly reduced the number of adherent leukocytes (white circles) and RBCs interacting with adherent leukocytes (white arrows).  indicates the direction of flow.
indicates the direction of flow.
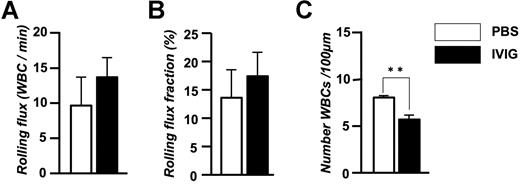
To exclude the possibility that irradiation and graft-versus-host disease were confounding factors, we also evaluated leukocyte behavior and RBC-WBC interactions in parental sickle Berkeley mice and found similar results (rolling flux fraction: PBS, 5.1 ± 0.3%; IVIG, 9.0 ± 3.2%; adherent WBCs/100 μM: PBS, 14.0 ± 0.8, IVIG, 10.4 ± 0.4; RBC interactions/WBC/minutes: PBS, 1.53 ± 0.5, IVIG, 0.04 ± 0.02; data are mean of n = 2 mice per group). We also evaluated leukocyte behavior after IVIG administration in C57BL/6 mice to determine whether IVIG had similar effects in nonsickle mice. We found similar trends with IVIG treatment on leukocyte rolling (Figure 3A,B) and a significant reduction in the recruitment of adherent leukocytes (Figure 3C). Thus, IVIG infusion of sickle cell mice that have ongoing disease activity can rapidly alter the behavior of leukocytes, reducing leukocyte recruitment and inhibiting the ability of RBCs and WBCs to interact with each other.
Effect of IVIG on the leukocyte behavior in TNF-α–treated C57BL/6 wild-type mice. (A) Leukocyte rolling flux. (B) Leukocyte rolling flux fraction. (C) Number of adherent leukocytes per 100 μm. n = 5-6 mice per group in which 51-52 venules were analyzed. **P = .002.
Effect of IVIG on the leukocyte behavior in TNF-α–treated C57BL/6 wild-type mice. (A) Leukocyte rolling flux. (B) Leukocyte rolling flux fraction. (C) Number of adherent leukocytes per 100 μm. n = 5-6 mice per group in which 51-52 venules were analyzed. **P = .002.
IVIG treatment during ongoing crisis improves microcirculatory blood flow
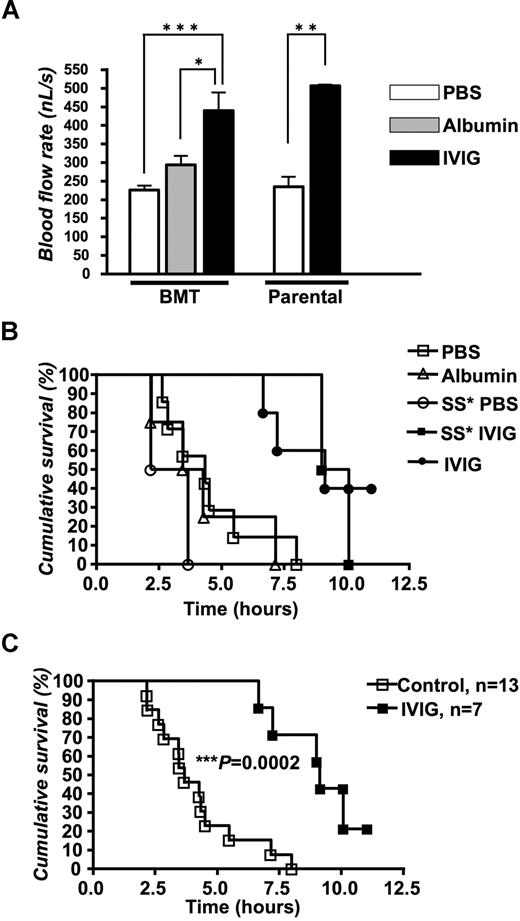
Centerline velocity (VRBC) was measured using an optical velocimeter. The mean VRBC in IVIG-treated sickle cell mice was almost 2-fold higher than in PBS- or albumin-treated controls (P < .001 and P < .01, respectively; Table 2). This difference was not attributable to venular size because the average venular diameter was nearly identical among the 3 groups (Table 2). A similar response to IVIG on the VRBC was observed in parental Berkeley mice (Table 2). The calculated mean blood flow rate, which can be regarded as a surrogate measure for vaso-occlusion, was almost 2-fold higher in IVIG-treated mice than control animals (Figure 4A; P < .001).
Hemodynamic parameters in TNF-α–primed sickle mice treated with IVIG
| Sickle mouse generation . | Treatment . | Mice, no. . | Venules, no. . | Venular diameter, μM . | Centerline velocity, μM/sec . | Shear rate, s−1 . |
|---|---|---|---|---|---|---|
| BMT | PBS | 7 | 68 | 20.0 ± 0.2 | 1117 ± 53*** | 595 ± 31*** |
| BMT | Albumin | 4 | 45 | 19.8 ± 0.2 | 1488 ± 118 | 797 ± 63 |
| BMT | IVIG | 5 | 55 | 19.9 ± 0.3 | 2162 ± 221* | 1147 ± 116* |
| Parental | PBS | 2 | 20 | 19.9 ± 0.3 | 1202 ± 68** | 648 ± 17** |
| Parental | IVIG | 2 | 31 | 20.5 ± 0.5 | 2294 ± 39 | 1179 ± 30 |
| Sickle mouse generation . | Treatment . | Mice, no. . | Venules, no. . | Venular diameter, μM . | Centerline velocity, μM/sec . | Shear rate, s−1 . |
|---|---|---|---|---|---|---|
| BMT | PBS | 7 | 68 | 20.0 ± 0.2 | 1117 ± 53*** | 595 ± 31*** |
| BMT | Albumin | 4 | 45 | 19.8 ± 0.2 | 1488 ± 118 | 797 ± 63 |
| BMT | IVIG | 5 | 55 | 19.9 ± 0.3 | 2162 ± 221* | 1147 ± 116* |
| Parental | PBS | 2 | 20 | 19.9 ± 0.3 | 1202 ± 68** | 648 ± 17** |
| Parental | IVIG | 2 | 31 | 20.5 ± 0.5 | 2294 ± 39 | 1179 ± 30 |
Data are presented as mean (± SEM).
P < .05 compared with albumin.
P < .01 compared with PBS control.
P < .001 compared with PBS control.
IVIG sustains blood flow rates and prolongs survival in TNF-á–exposed sickle mice. (A) Blood flow rates (nL/sec). *P < .05, **P < .01, and ***P < .001. (B) The Kaplan-Meier survival curves for individual IVIG-treated or control sickle mouse subsets. SS indicates parental sickle mice without transplantation. P = .008, IVIG versus PBS; P = .01, IVIG versus albumin. (C) Survival probabilities when control (PBS and albumin) and IVIG (transplanted and parental) groups are combined. Survival was significantly improved in IVIG-treated mice; log-rank (Mantel-Cox) test; ***P = .0002.
IVIG sustains blood flow rates and prolongs survival in TNF-á–exposed sickle mice. (A) Blood flow rates (nL/sec). *P < .05, **P < .01, and ***P < .001. (B) The Kaplan-Meier survival curves for individual IVIG-treated or control sickle mouse subsets. SS indicates parental sickle mice without transplantation. P = .008, IVIG versus PBS; P = .01, IVIG versus albumin. (C) Survival probabilities when control (PBS and albumin) and IVIG (transplanted and parental) groups are combined. Survival was significantly improved in IVIG-treated mice; log-rank (Mantel-Cox) test; ***P = .0002.
Increased survival of sickle cell mice treated with IVIG during acute crisis
After the end of the recording period, mice were monitored for up to 11 hours after the injection of TNF-α to assess the effect of the IVIG therapy on mortality. Kaplan-Meier survival curves revealed a dramatic improvement in survival of IVIG-treated compared with either PBS- or albumin-treated control sickle cell mice (Figure 4B; P = .008 for IVIG vs PBS and P = .01 for IVIG vs albumin; log-rank test). A similar protection was observed in parental sickle Berkeley mice without transplantation. Figure 4C shows the Kaplan-Meier curves when the 2 control and 2 sickle groups are grouped, illustrating a major effect of IVIG on survival. In the control group, approximately one half of sickle cell mice survived 4 hours after TNF-α exposure, but none survived more than 8 hours. By contrast, 40% of sickle cell mice treated with IVIG survived more than 10 hours. Taken together, these results strongly suggest that IVIG therapy may be beneficial in SCD during acute crisis.
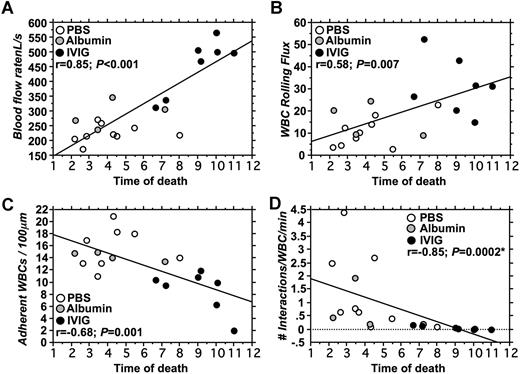
To get further insight into which microvascular or hematologic parameters are associated with improved survival, we evaluated correlations between key variables in the 2 groups of mice. Univariate analyses revealed no significant correlation between survival and counts of circulating RBCs (r = −0.1; P = .76), reticulocytes (r = 0.01; P = .97), platelets (r = 0.13; P = .69), or WBCs (r = 0.03; P = .92; data not shown). However, as shown in Figure 5, parameters measured in the cremasteric microcirculation correlated significantly with survival. There was a strong positive correlation between blood flow rates and survival (r = 0.85; P < .001; Figure 5A). Survival also correlated positively with the number of rolling leukocytes (r = 0.58; P = .007; Figure 5B) and negatively with the number of adherent leukocytes (r = −0.68; P = .001; Figure 5C). Moreover, the number of interactions between RBCs and WBCs correlated negatively with survival (P = < .001; r = −0.85; Spearman nonparametric correlation; Figure 5D). Multiple regression analyses to identify variables related to survival revealed a significant association only with blood flow rates (P = .03) when adherent and rolling leukocytes and RBC–WBC interactions were also included in the model. Blood flow rates correlated negatively with the number of adherent leukocytes (r = −0.77; P = .003) but not with any other intravital microscopy or hematologic variables (data not shown). Thus, these results indicate that poor flow rates in cremasteric venules are a good predictor of short survival in this model of vaso-occlusion, and that these flow rates are significantly influenced by the number of adherent leukocytes.
Correlations between survival and intravital microscopy parameters. (A) Blood flow rates correlated positively with survival. Most mice treated with IVIG had higher blood flow rates and survived longer than control mice (PBS; albumin). (B) Leukocyte rolling flux correlated positively with survival. (C) A negative correlation between the number of adherent leukocytes and survival was observed. (D) The number of interactions between RBCs and adherent WBCs correlated negatively with survival. *Spearman correlation for nonparametric values.
Correlations between survival and intravital microscopy parameters. (A) Blood flow rates correlated positively with survival. Most mice treated with IVIG had higher blood flow rates and survived longer than control mice (PBS; albumin). (B) Leukocyte rolling flux correlated positively with survival. (C) A negative correlation between the number of adherent leukocytes and survival was observed. (D) The number of interactions between RBCs and adherent WBCs correlated negatively with survival. *Spearman correlation for nonparametric values.
IVIG dramatically increases leukocyte rolling velocities
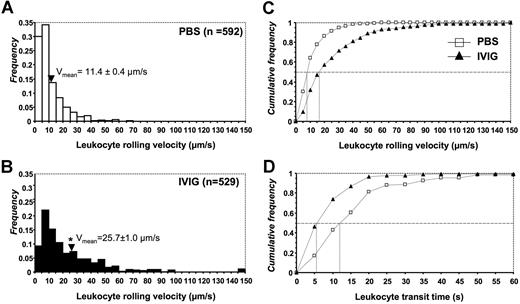
To begin to understand the mechanisms involved in the reduced WBC recruitment by IVIG, we analyzed in more detail leukocyte behavior by intravital microscopy. To this end, the velocity of leukocyte rolling was evaluated from 592 leukocytes in 68 venules of 7 PBS-treated sickle cell mice and 529 leukocytes in 55 venules of 5 IVIG-treated mice. Leukocytes in sickle cell mouse venules treated with PBS rolled at an average velocity of 11.4 plus or minus 0.4 μm/sec, ranging from 0.5 to 70 μm/sec (Figure 6A). In contrast, leukocyte rolling velocities in IVIG-treated sickle mouse venules were significantly greater than PBS-treated controls, with an average rolling velocity of 25.7 plus or minus 1.0 μm/sec, ranging from 1 to 200 μm/sec (Figure 6B). Furthermore, a cumulative frequency histogram for these 2 groups demonstrated that IVIG shifted leukocyte rolling from slower to faster with approximately 2-fold higher median rolling velocities (Figure 6C). Consistent with the velocity histograms, the transit times of leukocytes were much shorter in IVIG-treated mice. The median time of rolling leukocytes to travel a 100-μm venular segment was approximately 5 seconds in IVIG-treated mice, whereas the transit time was approximately 13 seconds for leukocytes of control mice treated with PBS (Figure 6D).
Leukocyte rolling velocity histograms. Leukocyte rolling velocities in (A) control PBS and (B) IVIG-treated sickle cell mice. Arrowheads indicate the means. The mean is significantly higher in the IVIG group than in PBS controls. *P < .05. Cumulative frequency histograms of (C) rolling velocities and (D) leukocyte transit times. Median values are indicated by vertical lines.
Leukocyte rolling velocity histograms. Leukocyte rolling velocities in (A) control PBS and (B) IVIG-treated sickle cell mice. Arrowheads indicate the means. The mean is significantly higher in the IVIG group than in PBS controls. *P < .05. Cumulative frequency histograms of (C) rolling velocities and (D) leukocyte transit times. Median values are indicated by vertical lines.
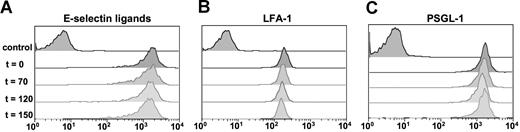
Because previous studies have revealed that slow rolling is mediated by E-selectin and the β2 integrin leukocyte function-associated antigen-1 (LFA-1),28 we evaluated whether IVIG might alter leukocyte rolling velocities by reducing LFA-1 or E-selectin ligand (ESL) expression. To test this possibility, we harvested peripheral blood at baseline (T0), after TNF-α injection but before IVIG infusion (T70), and at 2 other time points after the injection of IVIG or PBS control (T120 and T150). As shown in Figure 7, the expression of LFA-1 and PSGL-1 and the binding of an E-selectin–IgM chimera were not affected by IVIG infusion.
Effects of TNF-α and IVIG on adhesion molecule expression on Gr-1 + leukocytes by flow cytometric analyses. Blood from sickle cell mice was harvested at baseline, 70 minutes after TNF-α (IVIG not yet administered); 120 minutes after TNF-α (30 minutes after IVIG infusion); and 150 minutes after TNF-α (60 minutes after IVIG infusion). (A) E-selectin binding. ESLs were detected using a chimeric E-selectin–IgM recombinant protein. Control sample was incubated in the presence of 10 mM EDTA. (B) LFA-1 and (C) PSGL-1 expression. Controls were stained with isotype-matched IgG.
Effects of TNF-α and IVIG on adhesion molecule expression on Gr-1 + leukocytes by flow cytometric analyses. Blood from sickle cell mice was harvested at baseline, 70 minutes after TNF-α (IVIG not yet administered); 120 minutes after TNF-α (30 minutes after IVIG infusion); and 150 minutes after TNF-α (60 minutes after IVIG infusion). (A) E-selectin binding. ESLs were detected using a chimeric E-selectin–IgM recombinant protein. Control sample was incubated in the presence of 10 mM EDTA. (B) LFA-1 and (C) PSGL-1 expression. Controls were stained with isotype-matched IgG.
IVIG specifically alters neutrophil recruitment
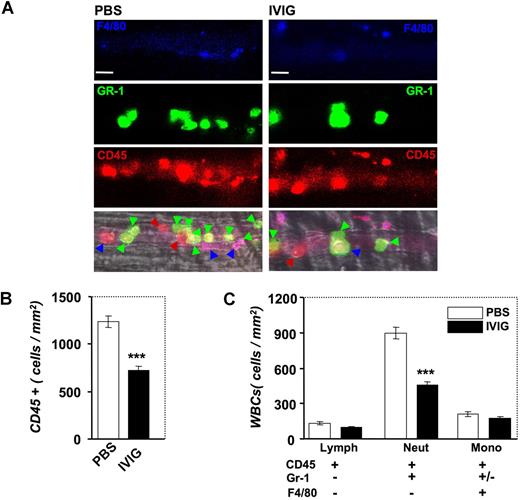
To acquire additional insight into the mechanism of action of IVIG, we took advantage of a novel method that we developed recently to identify in live mice the subsets of adherent leukocytes using high-speed multichannel videomicroscopy.26 In these experiments, low doses of fluorescently conjugated antibodies against CD45, Gr-1, and F4/80 antigens were injected via the carotid artery catheter after IVIG or PBS infusion and image sequences were captured between T90 and T150 in several venules per mouse (Figure 8A). Labeling with the pan-leukocyte marker CD45 revealed a significant reduction after IVIG treatment in the number of adherent CD45 + leukocytes per surface area in inflamed venules, confirming our previous studies using brightfield microscopy (compare Figure 1C and Figure 8B). To determine which leukocyte subset was affected by IVIG administration, we analyzed the differential expression of Gr-1 and F4/80 among CD45 + adherent leukocytes. Strikingly, IVIG specifically reduced the number of recruited PMNs (CD45 + Gr-1 + F4/80−; Figure 8C; P < .001) but did not significantly affect the recruitment of monocytes (CD45 + F4/80 +) or lymphocytes (CD45 + Gr-1−F4/80−).
Effect of IVIG on the recruitment of leukocyte subsets by multichannel fluorescence intravital microscopy. (A) Leukocyte subsets were identified by injection of low doses of antibodies against CD45 (red), Gr-1 (green), and F4/80 (blue). Colored arrowheads indicate adherent leukocytes in representative venules according to their subset identification with this method. Bar, 20 μm. (B) IVIG reduces the number of CD45 + cells. ***P < .001. (C) IVIG significantly reduced the recruitment of neutrophils (CD45 + Gr-1 + F4/80−) but did not affect the recruitment of monocytes (CD45 + F4/80 +) and lymphocytes (CD45 + Gr-1−F4/80−). ***P < .001.
Effect of IVIG on the recruitment of leukocyte subsets by multichannel fluorescence intravital microscopy. (A) Leukocyte subsets were identified by injection of low doses of antibodies against CD45 (red), Gr-1 (green), and F4/80 (blue). Colored arrowheads indicate adherent leukocytes in representative venules according to their subset identification with this method. Bar, 20 μm. (B) IVIG reduces the number of CD45 + cells. ***P < .001. (C) IVIG significantly reduced the recruitment of neutrophils (CD45 + Gr-1 + F4/80−) but did not affect the recruitment of monocytes (CD45 + F4/80 +) and lymphocytes (CD45 + Gr-1−F4/80−). ***P < .001.
Discussion
Here we demonstrate that the administration of high doses of immunoglobulins, even when administered after the onset of TNF-α–triggered sickle cell crisis, can profoundly alter the course of acute vaso-occlusive episodes in sickle cell mice. We show that IVIG rapidly reduces the number of adherent leukocytes and the interactions between RBCs and WBCs, resulting in improved blood flow and reversal of an otherwise lethal crisis. Our results indicate that IVIG appears to affect specifically the behavior of neutrophils in inflamed venules, resulting in higher rolling velocities and specific reductions in PMN adhesion. Because this model reflects clinical vaso-occlusive painful crises, these data suggest that IVIG may be beneficial in the context of acute sickle cell crises.
We have previously shown that IVIG given approximately 100 minutes before TNF-α–induced vaso-occlusion dramatically inhibits RBC-WBC interactions, improves blood flow, and increases the survival of sickle cell mice.19 However, clinically the time onset of a vaso-occlusive crisis is unpredictable. In the present studies, we developed the first model of vaso-occlusion reflecting the clinical situation in which increased disease activity precedes medical intervention. In this model, IVIG was infused after sickle cell mice were challenged with TNF-α for 70 minutes, a time period during which interactions between RBCs and adherent WBCs can be observed. We administered a higher dose of IVIG (800 mg/kg) than in our previous study19 to assess whether standard clinical dosing (up to 1000 mg/kg) would incur any toxicity. We found that high doses of IVIG, even in the setting of an acute inflammatory challenge, rapidly reversed the course of sickle cell crisis and improved microcirculatory blood flow. The apparent benefit of IVIG during an active lethal disease in an animal model is reassuring given the reports of thrombotic events and acute renal failure associated with IVIG in the nonsickle cell population.29 Because these complications have been associated with rapid infusion rates, the high infusion rate of this study (≈10× faster than the maximal recommended rate in humans) reduces concerns about overt toxicity in the setting of active disease. The fact that the same beneficial effect on vaso-occlusion was observed in parental sickle mice rules out an influence of the transplantation model used to generate genetically identical sickle mice.11 In addition, the absence of a specific effect of albumin administration eliminates the possibility that the observed benefits are attributable to a putative increase in the oncotic pressure after the administration of IVIG. Our results indeed suggest that IVIG may ameliorate sickle cell vasoocclusion through its anti-inflammatory activities.
Although IVIG has been used for decades to modulate the immune response, the exact operative mechanisms are still unclear. Several nonmutually exclusive mechanisms have been suggested, including the blockade of Fc receptors or induction of inhibitory FcγRIIB, and modulation of the immune response.30 Autoantibody-dependent models of idiopathic thrombocytopenic purpura (ITP) and acute arthritis have revealed that FcγRIIB was a key mediator of the suppressive effects of IVIG in macrophages.31,32 Further studies have suggested that the anti-inflammatory activity of IVIG is contained in the sialylated IgG fraction.33 Other recent studies have suggested that small soluble immune complexes might play important roles through interactions with the “activating” FcγRIII on CD11c + dendritic cells.34 Further, other works have suggested that IVIG may exert inhibitory functions in human macrophages mediated by FcγRIII through the down-regulation of interferon-γ receptor 2 and unresponsiveness to interferon-γ.35 Although IVIG has clearly been shown to induce the secretion of numerous cytokines, whether these cytokines play a direct role in the mechanisms of IVIG action is still unresolved.36 The various anti-inflammatory effects in multiple models underscores the multifaceted actions of IVIG, suggesting the possibility of multiple mechanisms.
Our previous studies established that IVIG reduces leukocyte recruitment in sickle cell mice in a dose-dependent manner.19 Similar observations have been noted by intravital microscopy in a feline ischemia–reperfusion model.37 We have shown in the present studies that IVIG acts rapidly because the effects on leukocyte adhesion are seen within 10 minutes after its administration. This rapid action probably results from the high turnover and the dynamism of leukocyte recruitment in inflamed venules. Indeed, time-lapse studies show that leukocytes are recruited continuously to and dismissed from the venular wall and moreover that adherent leukocytes are highly mobile on the inflamed endothelium, exhibiting a polarized expression of PSGL-1 at the trailing edge.26
Detailed analyses of leukocyte subsets revealed that IVIG affects specifically the recruitment of neutrophils and that it significantly increased the rolling velocity of these leukocytes, suggesting that this effect may result from altered function of adhesion molecules involved in slow leukocyte rolling. Slow leukocyte rolling has been largely attributed to ESLs and also to β2 integrins and their endothelial counter-receptors. Indeed, deficiency in the integrin αLβ2 (LFA-1) or E-selectin is associated with a significant increase in leukocyte rolling velocities.28,38 Our recent analyses of all ESLs on leukocytes have revealed that PSGL-1, ESL-1, and CD44 comprised all ESL activity on neutrophils.25,39 Among these, CD44 and ESL-1, but not PSGL-1, mediate the slow rolling on TNF-α–stimulated venules.25,39 However, we have not found any alteration in the expression of PSGL-1 or LFA-1, or in the binding of leukocytes to E-selectin after the administration of TNF-α or IVIG. These results appear to differ from other studies in which a reduced binding to P- and E-selectins and to β2 integrin was noted after the incubation of blood with IVIG in vitro.37 Although the exact mechanisms remain to be clarified, these studies indicate that IVIG can clearly inhibit leukocyte adhesion in systemic venules of normal cats and sickle cell mice.19,37 The beneficial effect of IVIG in our model of vaso-occlusion further supports a major role for leukocyte adhesion in occlusive disease and is consistent with other studies that have shown benefits of antibodies against intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin in other models.40,41
In summary, our studies in a novel model of sickle cell vaso-occlusion in which the therapeutic intervention is administered after the inflammatory challenge clearly shows that IVIG can dramatically improve microcirculatory blood flow and survival during vaso-occlusive crisis in sickle Berkeley mice. Given these favorable observations, we initiated a randomized, double-blind, placebo-controlled dose-escalation study of the safety and efficacy of a single dose of IVIG in patients with SCD presenting with a simple vaso-occlusive crisis under a Food and Drug Administration–approved Investigational New Drug application.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Barry Coller for providing access to the ADVIA automated cell counter and Dr Andres Hidalgo for help with FACS analyses. We are also grateful to Anna Peired for excellent technical assistance with sickle mouse genotyping and husbandry.
This work was supported by grants R01 HL69438 (P.S.F.) and T32 HL07824 (J.C.) from the National Institutes of Health and a Glorney-Raisbeck fellowship from the New York Academy of Medicine (E.Y.C.).
National Institutes of Health
Authorship
Contribution: J.C. and P.A.S. performed research, analyzed the data, and participated in writing the manuscript. E.Y.C. contributed to a critical analytical tool and participated in writing the manuscript. P.S.F. designed the research, analyzed the data, and participated in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul S. Frenette, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1079, New York, NY 10029; e-mail: paul.frenette@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal