The present study shows that tetanus toxoid (tet) booster releases to the human circulation 2 subsets of specific plasma cells (PCs), as defined by phenotype and morphology, which clearly differed in the staining capacity of their cytoplasmic antibodies (Abs) with fluorescein isothiocyanate (FITC)–labeled tet–fragment C (tetC). These cells, called tetCHIGH and tetCINT PCs according to their either high or intermediate FITC-tetC staining capacity, exhibit similar rapid temporary kinetics in the blood (5-8 days after boost), contain many cycling cells, express equivalent amounts of BLIMP-1 mRNA, and produce similar quantities of IgG. However, Abs synthesized by tetCHIGH PCs show a tetC affinity more than 10 times higher than that exhibited by tetCINT PC Abs, and indicated by IGVH sequence analysis. Chemotaxis to CXCL12, a requisite for bone marrow (BM) PC homing, is similar for both cell types. Circulating nonspecific and tetCINT PCs, but not tetCHIGH PCs, tend to undergo spontaneous apoptosis, as demonstrated by APO2.7 and activated caspase-3 expression, and cell recovery. These results indicate that tet booster generates 2 discrete subsets of specific PCs exhibiting different ranges of Ab affinity for the immunogen, and that only those synthesizing high-affinity Abs show enhanced survival. This inherent property may be essential for determining the BM fate of PCs secreting high-affinity Ab.
Introduction
Plasma cells (PCs) represent the final and effector phase of the B lymphocyte differentiation process. There is accumulating evidence to support the view that in mammals, the PC compartment comprises a biologically complex variety of cell stages. In systemic humoral responses, specific PCs initially appear in antigen (Ag)–induced extrafollicular foci of secondary lymphoid tissues. In the presence of sufficient Ag, PCs are subsequently generated in the follicular germinal centers.1,,,–5 Most of the early-generated PCs die within a few days by apoptosis.5,–7 Later, some of the recently generated PCs migrate through the circulation and home into specialized survival niches present mainly in the bone marrow (BM)5,8,,,,,,–15 and, less commonly, in the spleen and probably in other lymphoid organs,16,–18 as well as in inflamed tissues.19 In these locations, PCs are thought to produce antibodies (Abs) for prolonged periods of time. Thus, it is well established that the BM PC pool is responsible for the generation of long-lasting serum Ab levels.5,9,,–12,20
The mechanisms involved in the arrival and retention of PCs into the BM niches are beginning to be known. In this respect, the interaction between the chemokine receptor CXCR4 expressed on PCs and its ligand CXCL12 is required for the establishment of the BM PC compartment in mice,21 and probably in humans.22,–24 Although the auxiliary cellular components of the BM PC niches still remain poorly defined,13,–15,25 they are believed to convey the necessary signals to support the prolonged survival of immigrant PCs. Some of the molecular mechanisms that participate in this process have been identified, including IL-6, APRIL, and certain ligands of PC adhesion molecules.13,–15,25,26 In spite of these findings, it remains uncertain whether occupancy of BM PC niches is merely a random phenomenon, or whether intrinsic factors of PCs are involved in the generation of the BM PC compartment.
It is now clear that, a few days after booster immunization, Ag-specific PCs are transiently observed in the blood, and this circulating PC subset appears to contain the precursors of BM PCs.27,28 In humans, a conventional booster immunization with tetanus-toxoid (tet) induces the temporary appearance in the blood of tet-specific PCs.23,24,29,–31 These tet-specific circulating PCs exhibit features of intermediate maturity between early PCs and PCs present in deposit organs such as the BM,23,28,31 and the occurrence of these PCs is temporarily and functionally associated with the high-rate phase of serum anti-tet Ab formation and, accordingly, with the establishment of the BM pool of anti-tet Ab-producing PCs.29,,,–33 Therefore, blood tet-specific PCs may be an appropriate model for exploring the factors involved in the selection of the BM PC compartment. In this regard, it is well established that long-lived BM PCs predominantly synthesize Ab with high-affinity for the Ag.9,,–12,28,34 This observation suggests that Ab affinity might play a role in this selection process.
The present study shows that tet-booster immunization induces the release to the human blood of 2 subsets of tet-specific PCs, which differ in the staining capacity of their cytoplasmic Ab with FITC-labeled tet–fragment C (tetC), a major and immunogenic fragment of the tet molecule.35,36 Further results indicate that the distinctive staining is due to differences in Ab affinity for tetC. Moreover, the PC subset synthesizing high-affinity anti-tetC Ab also showed increased resistance to apoptosis, suggesting that the formation of the BM PC compartment is not a merely stochastic process, and that Ab affinity–associated signals contribute to the selection of this pool by conferring improved survival capacity to high-affinity Ab-secreting PCs.
Methods
Materials
RPMI 1640 culture medium, l-glutamine, fetal calf serum (FCS), penicillin, and streptomycin were purchased from Gibco BRL Life Technologies (Paisley, United Kingdom). Fragment C tetanus toxin bound to fluorescein isothiocyanate (FITC-tetC), bovine serum albumin (BSA), o-phenylenediamine dihydrochloride (OPD), and Triton X-100 were purchased from Sigma (St Louis, MO). Purified tet and FITC-tetC were also provided by Calbiochem (Darmstadt, Germany). Phycoerythrin (PE)–labeled mAb against CD19, CD27, CD138, and HLA-DR; Igλ and Igκ light chains; activated caspase-3; peridinin chlorophyll protein (PerCP)–Cy5.5–labeled mAb against CD38; Alexa Fluor 647–labeled mAb against CD19; allophycocyanin (APC)–labeled mAb against CD19; and BrdU, as well as the corresponding negative isotypic controls and BrdU flow kit, were provided by Becton Dickinson (San Jose, CA). Cy5-conjugated mouse anti–human Igγ was obtained from Jackson ImmunoResearch (West Grove, PA). PE-labeled mAbs against APO2.7 were provided by Coulter (Fullerton, CA). Recombinant human CXCL12 was purchased from PeproTech (London, United Kingdom), anti–SDF-1α and the anti-CXCL12 kit were provided by R&D Systems (Minneapolis, MN). Intrastain kit and FITC-labeled rabbit anti–human IgG polyclonal Ab were from Dako (Glostrup, Denmark). For enzyme-linked immunosorbent assay (ELISA), unconjugated and peroxidase-conjugated goat anti–human IgG antibodies were provided by Biosource (Camarillo, CA).
Preparation of blood mononuclear cells and flow cytometric analysis
Heparinized peripheral blood was obtained from healthy volunteers (18 to 51 years old) at various dates (generally 6 days) after a conventional tet-booster immunization (40 IU), and peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll/Hypaque density-gradient centrifugation. Approval for these experiments was obtained from the institutional review board (Comisión Ética de Investigación), and informed consent was obtained. Cell staining and fluorescence-activated cell sorter (FACS) analysis was performed as previously described.31 PCs identified by specific external labeling were subsequently explored for the additional detection of internal markers with FITC-tetC (2 μg/mL, unless otherwise indicated) and with mAb for γ Ig-heavy chain, and Igλ and Igκ Ig light chains, APO2.7, and activated caspase-3 by using an Intrastain kit. FACS analysis was performed on a FACScalibur cytometer (Becton Dickinson). CD19+ CD38HIGH cells were gated on a CD19/CD38 dot plot of PBMCs using the FL2-FL3 or FL3-FL4 plots depending on the mAb combination used. The free channels were used to explore the expression of additional markers on these PCs. Data from 5000 CD19+ CD38HIGH cells/sample were collected, and the percentage as well as the mean fluorescence intensity (MFI) of the CD19+ CD38HIGH cells positive for each analyzed molecule were monitored. FACS analysis was also used for examining affinity between anti-tet Ab synthesized by human blood PCs and FITC-tetC, since it had been demonstrated that the Scatchard analysis was feasible via this technique.37,38 In brief, PBMCs were incubated with increasing concentrations of FITC-tetC (from 100 ng/mL to 20 μg/mL), and the FITC-tetC binding to cytoplasmic anti-tet Ab of the different PC subsets was determined. The peak of the fluorescence intensity histogram (≈ MFI in a narrow Gaussian curve) can be considered an estimation of the mean amount of fluorochrome-labeled molecules bound per cell.39 Accordingly, the MFI of FITC-tetC–labeled PCs was used as a reference of the quantity of the FITC-tetC bound. These binding data were used to construct the Scatchard plots, and each point represented the mean value for triplicate determinations. Data were analyzed using GraphPad Prism software (San Diego, CA).
Cell culture
PBMC cultures (106 cells/mL) were set up in a medium consisting of RPMI 1640 supplemented with 10% FCS, 10 mM l-glutamine, and 100 U/mL penicillin and 100 μg/mL streptomycin, in 96-well flat-bottomed plates, in a final volume of 250 μL per well.31 To study spontaneous apoptosis, intrastaining with APO2.7 mAb and with anti–activated caspase-3 mAb was performed in freshly obtained and in 24-hour–cultured PBMCs. Analysis of proliferating cells was performed by detecting BrdU incorporation to the cells during an 18-hour culture period using the BrdU Flow kit. Cell recovery of the different blood PC subsets under study was determined in PBMCs freshly obtained and cultured for 1 and 2 days by FACS analysis.
Chemotaxis of blood PC subsets to CXCL12
Duplicate tests were carried out in 24-well plates with transwell inserts (5-μm pore size; Costar Corning, Corning, NY), and RPMI 1640 medium was used as assay medium. PBMCs were diluted in medium at a concentration of 2 × 106 cells/mL. The lower transwell chamber was filled with 600 μL of medium either alone (control) or containing 0.8 μg CXCL12, and then 200 μL of the cell suspension was added to the upper chamber. Cells were allowed to migrate overnight. Finally, the cells were collected from the lower chamber and were stained and analyzed by FACS as above. In blocking experiments, PBMCs were cultured for 2 hours at 37°C, and then were incubated for 30 minutes at 4°C with the anti-CXCR4 mAb (10 μg/mL), washed, and tested for migration; in additional assays, anti-CXCL12 mAb (10 μg/mL) and CXCL12 (0.8 μg/mL) were also added to the lower transwell chamber, and cells were tested for migration. The total number of migrated CD19+ CD38HIGH cells, and the percentages of every PC subset in the migrated PCs, were evaluated.
Isolation of blood PC subsets by FAC sorting and PC identification
The PCs present in PBMCs were externally stained for CD19 and CD38, and internally labeled with FITC-tetC. Blood CD19+ CD38HIGH PCs negative and positive for FITC-tetC staining were identified by flow cytometry. Different PC subsets were purified by sterile FAC sorting on a FACSAria instrument (Becton Dickinson). Following sorting, isolated cells were collected and frozen for further studies, or transferred onto glass microscope slides by cytocentrifugation, stained with May/Grunwald/Giemsa, and PCs were identified by morphology under a light microscope. Purity was more than 96%. In addition, PCs were also stained for the intracytoplasmic IgG by an immunofluorescent technique.23 For detection of FITC-tetC, freshly sorted cell subsets were transferred onto glass microscope slides and directly analyzed. Cells were visualized using an Olympus BX40 epifluorescence microscope (Olympus, Hamburg, Germany) with a 40×/0.75 NA objective, an Olympus DP71 camera, and Cell° software version 2.6 (Olympus). Immunofluorescence localization was performed using a laser confocal imaging system (TCS-SL; Leica Microsystems, Heidelberg, Germany). Cytoplasmic IgG content was assessed in samples of sorted blood PC subsets by ELISA.40
Quantification of BLIMP-1 and IGγ1 transcripts
Total RNA was isolated from sorted PC cells as reported.31 cDNA synthesis was carried out using random hexamer primers and the components from the Affinity Script QPCR cDNA Synthesis Kit (Stratagene, La Jolla, CA). IGγ1, BLIMP-1, and GAPDH transcripts were quantified by real-time quantitative polymerase chain reaction (PCR) with a Light Cycler Instrument (Roche, Basel, Switzerland) using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA); assay references were as follows: Hs00153357 m1 for BLIMP-1 and Hs00378340 m1 for IGγ1. PCR conditions were as follows: 1 cycle of 10 minutes at 95°C, followed by 50 cycles of 1 second at 95°C, and 30 seconds at 60°C. Relative gene expression levels were calculated using the 2−ΔΔCT method. The data are presented as the fold change in each gene expression normalized to the GAPDH gene and relative to the samples indicated in the figure legends. A total of 2 independent analyses were performed for each sample and for each gene.
IGVH3 gene sequences analysis
The IGVH3 gene family was amplified from cDNA of isolated PC subsets by PCR, followed by subsequent cloning and sequencing, as previously reported.31 Nucleotide sequences were analyzed and compared with the IMGT IgVH database.41 A total of 86 sequences from 4 individuals were obtained. GenBank accession numbers for these sequences are EF633748 to EF633828.42
Statistical analysis
Data are expressed as means plus or minus SEM. Differences between paired experimental points were established by using the Wilcoxon test. Differences were accepted as significant when P values are less than .05.
Results
A total of 2 subsets of intracytoplasmic FITC-tetC–labeled cells were detectable in the circulation 6 days after human tet vaccination
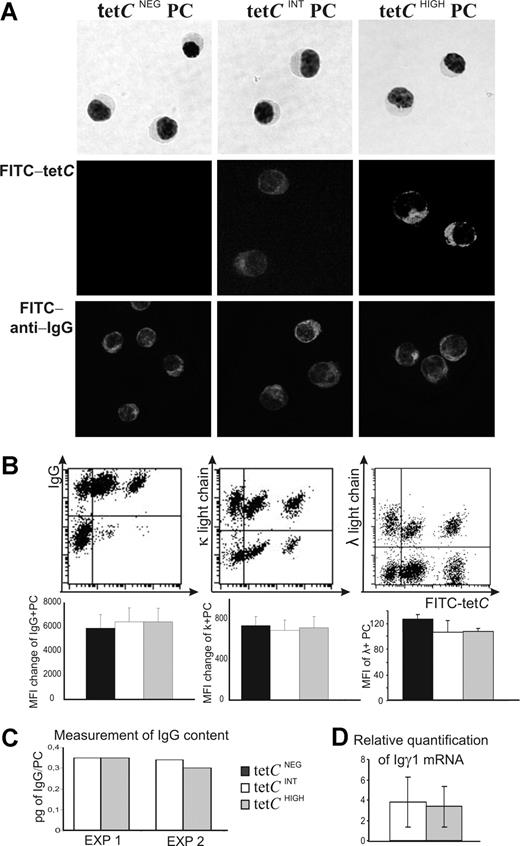
Booster immunization with tet induces the transient release to the blood of tet-specific PCs,23,24,30 which can be detected by staining of intracytoplasmic anti-tet IgG with FITC-labeled tetC and FACS analysis of CD19+ CD38HIGH cells.24,31 These circulating tetC-specific PCs are detected 5 to 7 days after tet booster and, when the labeled Ag was used at a concentration of 500 ng/mL, a unique tetC+ cell subset was observed31 (Figure 1A). However, the use of increasing concentrations of FITC-tetC in this technique revealed the existence of 2 discrete subsets of cells differing in their capacity of labeling, which stained with high and intermediate intensity, respectively (Figure 1A; tetCHIGH and tetCINT). Similar results were obtained with 2 different sources of FITC-tetC (data not shown). A concentration of 2 μg/mL was sufficient to discriminate clearly these 2 FITC-tetC–stained cell subsets (Figure 1A) in every donor tested (n = 51). Additional FITC-tetC+ subsets were not observed even using a concentration of 100 μg/mL of labeled Ag (data not shown). Further, the major CD19+ CD38HIGH cell subset in these samples consisted of cells whose FITC-tetC staining was similar to the background staining control (Figure 1A; tetCNEG). Detection of tetCHIGH and tetCINT cell subsets was specific, since the staining could be blocked by incubating the cells with unlabeled tet, but not with 5% BSA (Figure 1B; right panel) or 10% FCS (data not shown). In fact, optimal staining of tetCHIGH and tetCINT PC obtained with FITC-tetC (2 μg/mL) was blocked by coincubation with increasing quantities of unlabeled tet. As can be seen in Figure 1B, a concentration of 1 μg/mL of unlabeled tet was required to inhibit tetCINT PC detection, whereas 20 times more unlabeled tet was needed to prevent the detection of the tetCHIGH PC (Figure 1B; left panel, third and fourth histograms, respectively). FITC-tetC staining in these cell samples depended on the previous permeabilization of the cells, as there was no staining in the absence of this step (Figure 1B; right panel). The temporary kinetics of appearance of these cell subsets after tet vaccination was also explored. As can be seen in Figure 1C, tetCHIGH and tetCINT cell subsets showed a similar curve of presence in the blood between 4 and 8 days after tet booster, and the peak of the response for both subsets was commonly observed on the sixth day after the challenge.
Detection of tetC− and 2 different subsets of tetC+ PCs in the peripheral blood of volunteers, 6 days after tet immunization. (A) PCs were identified as CD19+ CD38HIGH cells in a CD19/CD38 dot plot obtained by FACS (left panel). The presence of tetCINT and tetCHIGH cell subsets was established by labeling permeabilized CD19 CD38HIGH cells with increasing quantities of FITC-tetC. Right panel shows a representative experiment. FITC-tetC concentrations of 500 ng/mL or less only distinguished 2 cell subsets (tetC− and tetC+; top left), while the use of higher concentrations revealed the presence of 3 different cell subsets (tetCNEG, tetCINT, and tetCHIGH; bottom left). (B) Representative control experiments showing the expression histograms of FITC-tetC staining (2 μg/mL) when the cells were coincubated with increasing quantities of unlabeled tet (left panels) or with 5% BSA (middle right), and when the cells were not permeabilized (lower right). Pale line denotes background control. (C) Temporal kinetics of tetCINT and tetCHIGH cells. The percentages of circulating tetCINT and tetCHIGH CD19+ CD38HIGH cells were obtained from day 3 to day 8 after booster. Results represent the mean plus or minus SEM (n = 7).
Detection of tetC− and 2 different subsets of tetC+ PCs in the peripheral blood of volunteers, 6 days after tet immunization. (A) PCs were identified as CD19+ CD38HIGH cells in a CD19/CD38 dot plot obtained by FACS (left panel). The presence of tetCINT and tetCHIGH cell subsets was established by labeling permeabilized CD19 CD38HIGH cells with increasing quantities of FITC-tetC. Right panel shows a representative experiment. FITC-tetC concentrations of 500 ng/mL or less only distinguished 2 cell subsets (tetC− and tetC+; top left), while the use of higher concentrations revealed the presence of 3 different cell subsets (tetCNEG, tetCINT, and tetCHIGH; bottom left). (B) Representative control experiments showing the expression histograms of FITC-tetC staining (2 μg/mL) when the cells were coincubated with increasing quantities of unlabeled tet (left panels) or with 5% BSA (middle right), and when the cells were not permeabilized (lower right). Pale line denotes background control. (C) Temporal kinetics of tetCINT and tetCHIGH cells. The percentages of circulating tetCINT and tetCHIGH CD19+ CD38HIGH cells were obtained from day 3 to day 8 after booster. Results represent the mean plus or minus SEM (n = 7).
Circulating tetCHIGH, tetCINT, and tetCNEG cell subsets consisted of PCs
The nature of these cells was investigated in blood samples obtained at the peak of the response (sixth day after booster). Figure 2 shows that tetCHIGH, tetCINT, and tetCNEG cells exhibited a similar high expression of CD38 and CD27, clear features of PCs.23,24,31,43 In addition, the 3 cell subsets showed positive and similar staining for CD19, CD31, CD40, and CD45, and identical negative expression for CD20 (data not shown). This phenotypic profile identified these cells as human circulating PCs.23,24,31 Despite these similarities, tetCNEG cells could be distinguished from both tetCHIGH and tetCINT cell subsets, as the former exhibited heterogeneous expression of CD138 and HLADR while the 2 latter subsets were clearly positive for these markers31 (Figure 2).
Comparative flow cytometric phenotypic analysis of blood tetCNEG, tetCINT, and tetCHIGH PCs obtained 6 days after tet immunization. A representative experiment is presented in the top row (top series of dot plots) showing the expression of isotypic control, CD27, CD38, CD138, and HLA-DR, respectively, by tetCNEG, tetCINT, and tetCHIGHCD19 CD38HIGH cells. The bottom row summarizes the results observed in several donors. Values are expressed as the MFI of expression for each molecule in positive PCs. Results represent the mean plus or minus SEM (n = 6). *Statistically significant differences.
Comparative flow cytometric phenotypic analysis of blood tetCNEG, tetCINT, and tetCHIGH PCs obtained 6 days after tet immunization. A representative experiment is presented in the top row (top series of dot plots) showing the expression of isotypic control, CD27, CD38, CD138, and HLA-DR, respectively, by tetCNEG, tetCINT, and tetCHIGHCD19 CD38HIGH cells. The bottom row summarizes the results observed in several donors. Values are expressed as the MFI of expression for each molecule in positive PCs. Results represent the mean plus or minus SEM (n = 6). *Statistically significant differences.
To further characterize the 3 cell subsets under study, they were isolated by FAC sorting. Figure 3A shows that tetCHIGH, tetCINT, and tetCNEG cell subsets exhibited morphologic features of PCs. Moreover, the expression of the relevant master regulator of the PC differentiation process BLIMP-144,45 was also evaluated in these cells. To this end, the quantity of mRNA for this factor in the 3 cell subsets was estimated by real-time PCR. When the amount of the factor was normalized to that expressed by tetCNEG PCs in each experiment, the results obtained for tetCHIGH and tetCINT cell subsets did not show significant differences (0.6 ± 0.4 and 0.7 ± 0.5, respectively; arbitrary units, mean ± SEM; n = 6).
Isolation of tetCNEG, tetCINT, and tetCHIGH PCs and comparison of the quantity of cytoplasmic Ig. (A) The 3 PC subsets were purified from peripheral blood by FAC sorting. A May-Grunwald-Giemsa staining is shown in the top row; immunofluorescence confocal microscopy for FITC-tetC and IgG are shown in the middle and bottom rows, respectively (original amplification 40×/0.75 NA). (B) Top row: representative experiments showing the flow cytometry dot plots analysis of the intracellular expression of Igγ, Igκ, and Igλ light chains by tetCNEG, tetCINT, and tetCHIGH PCs. Bottom row: Bar histograms summarize the results obtained in different donors. Values are expressed as the MFI change (MFI of positive PCs minus MFI of negative PCs) for IgG and Igκ light-chain expression, and as MFI of positive PCs for Igλ light chain. Results represent the mean plus or minus SEM (n = 6). (C) Quantification of the IgG contained in purified tetCINT and tetCHIGH PCs. Whole-cell lysates were prepared in Triton X-100, and the lysed cells were centrifuged to remove the cell debris. The quantity of IgG released was assessed by ELISA. Results are expressed as picograms of IgG per cell obtained in 2 different experiments. (D) Comparison of the quantity of IGγ1 mRNA expressed by blood isolated tetCINT and tetCHIGH PCs. Values were normalized using as endogenous calibrators those obtained for tetCNEG PCs in each experiment, which were taken as 1. Results represent the mean plus or minus SEM (n = 6).
Isolation of tetCNEG, tetCINT, and tetCHIGH PCs and comparison of the quantity of cytoplasmic Ig. (A) The 3 PC subsets were purified from peripheral blood by FAC sorting. A May-Grunwald-Giemsa staining is shown in the top row; immunofluorescence confocal microscopy for FITC-tetC and IgG are shown in the middle and bottom rows, respectively (original amplification 40×/0.75 NA). (B) Top row: representative experiments showing the flow cytometry dot plots analysis of the intracellular expression of Igγ, Igκ, and Igλ light chains by tetCNEG, tetCINT, and tetCHIGH PCs. Bottom row: Bar histograms summarize the results obtained in different donors. Values are expressed as the MFI change (MFI of positive PCs minus MFI of negative PCs) for IgG and Igκ light-chain expression, and as MFI of positive PCs for Igλ light chain. Results represent the mean plus or minus SEM (n = 6). (C) Quantification of the IgG contained in purified tetCINT and tetCHIGH PCs. Whole-cell lysates were prepared in Triton X-100, and the lysed cells were centrifuged to remove the cell debris. The quantity of IgG released was assessed by ELISA. Results are expressed as picograms of IgG per cell obtained in 2 different experiments. (D) Comparison of the quantity of IGγ1 mRNA expressed by blood isolated tetCINT and tetCHIGH PCs. Values were normalized using as endogenous calibrators those obtained for tetCNEG PCs in each experiment, which were taken as 1. Results represent the mean plus or minus SEM (n = 6).
The quantity of cytoplasmic Ig contained by tetCHIGH, tetCINT, and tetCNEG PCs was similar
Intracytoplasmic staining with FITC-tetC of purified PCs revealed that each of the 3 cell subsets showed a distinctive staining capacity for FITC-tetC: tetCNEG PCs exhibited no staining, tetCINT PCs were weakly positive, and tetCHIGH PCs presented a brightly labeled cytoplasm (Figure 3A middle row), a result concordant with those obtained by FACS. To explore whether the distinctive FITC-tetC staining capacity of these cells was due to differences in IgG contents, intracytoplasmic staining of IgG was performed in isolated cells. In clear contrast with the staining obtained with FITC-tetC, the intensity of intracytoplasmic IgG labeling seemed to be equivalent in the 3 cell subsets (Figure 3A bottom row). A similar conclusion was reached from FACS detection of intracytoplasmic Igγ, Igκ, and Igλ chains: the 3 cell subsets showed identical fluorescence intensity for the 3 Ig chains (Figure 3B). It should be noted that most (> 90%) tetCHIGH and tetCINT PCs produced IgG, while the proportion of tetCNEG PCs synthesizing IgG was lower (approximately 60%; Figure 3B). The possibility that tetCHIGH and tetCINT PCs contained different quantities of IgG was directly assessed in lysates of identical numbers of purified cells of the 2 tetC-specific PC subsets. Figure 3C shows that the quantity of IgG per cell was similar in both cell subsets in 2 independent experiments. In addition, the quantity of mRNA for Igγ1 measured by real-time PCR was also the same (Figure 3D). These data clearly indicated that distinctive FITC-tetC staining was not due to differences in cytoplasmic IgG contents, nor did it represent the detection of differential accessibility for intracellular Ab-containing compartments distinctively present in tetCINT and tetCHIGH PC subsets, since internally labeled Igγ, Igκ, and Igλ chains performed with the same permeabilization procedure gave similar intensity results in tetCINT and tetCHIGH PCs (Figure 3B).
The Abs synthesized by tetCHIGH and tetCINT PC differed in their affinity for tetC
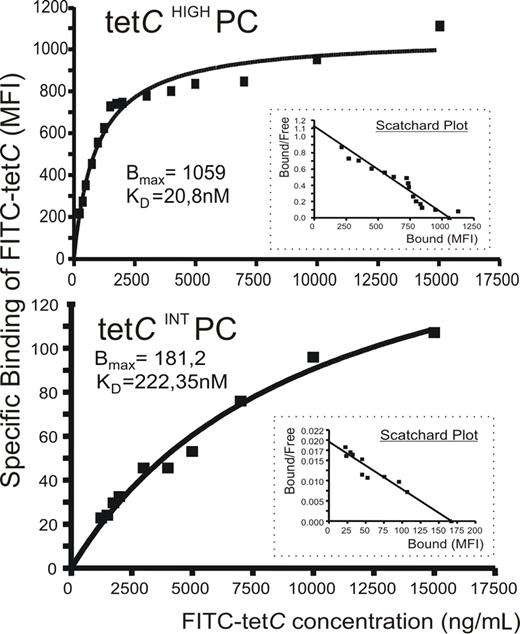
A possible explanation for the previous findings could be that IgG molecules produced by tetCHIGH and tetCINT PCs recognized the Ag with different affinity. To test this possibility, FITC-tetC saturation curves for tetCHIGH and tetCINT PC subsets were obtained by a FACS analysis assay in which detected MFI was estimated as specific binding for each FITC-tetC concentration,37,–39 and the corresponding Scatchard plots were derived. An example of this analysis is shown in Figure 4, and the relative KD obtained in 5 experiments for both PC populations are given in Table 1. As can be seen, Ab synthesized by tetCHIGH PCs exhibited, on average, an affinity for tetC 13 times higher than that of tetCINT PC Ab.
Anti-tetC Ab contained in tetCHIGH and tetCINT PC subsets differed in their affinity for tetC. A flow cytometric assay was used to assess the specific tetC binding of the Ab synthesized by these PC subsets in which MFI of FITC-tetC staining is plotted against FITC-tetC concentration. A representative experiment of the saturation curves of tetCHIGH PC (top panel) and tetCINT PC (bottom panel) for FITC-tetC is shown. Insets show the corresponding Scatchard plots. Bmax and relative KD data are also included.
Anti-tetC Ab contained in tetCHIGH and tetCINT PC subsets differed in their affinity for tetC. A flow cytometric assay was used to assess the specific tetC binding of the Ab synthesized by these PC subsets in which MFI of FITC-tetC staining is plotted against FITC-tetC concentration. A representative experiment of the saturation curves of tetCHIGH PC (top panel) and tetCINT PC (bottom panel) for FITC-tetC is shown. Insets show the corresponding Scatchard plots. Bmax and relative KD data are also included.
Affinity for FITC-tetC of cytoplasmic anti-tetC Ab synthesized by human circulating tetCHIGH and tetCINT PC subsets
| EXP . | KDTET CHIGH(nM) . | KDTET CINT(nM) . |
|---|---|---|
| 1 | 5.17 | 100.46 |
| 2 | 7 | 131.7 |
| 3 | 13.2 | 87.42 |
| 4 | 20.8 | 222.35 |
| 5 | 26.4 | 414.22 |
| Mean | 14.5 | 191.2 |
| SEM | 4.0 | 60.4 |
| EXP . | KDTET CHIGH(nM) . | KDTET CINT(nM) . |
|---|---|---|
| 1 | 5.17 | 100.46 |
| 2 | 7 | 131.7 |
| 3 | 13.2 | 87.42 |
| 4 | 20.8 | 222.35 |
| 5 | 26.4 | 414.22 |
| Mean | 14.5 | 191.2 |
| SEM | 4.0 | 60.4 |
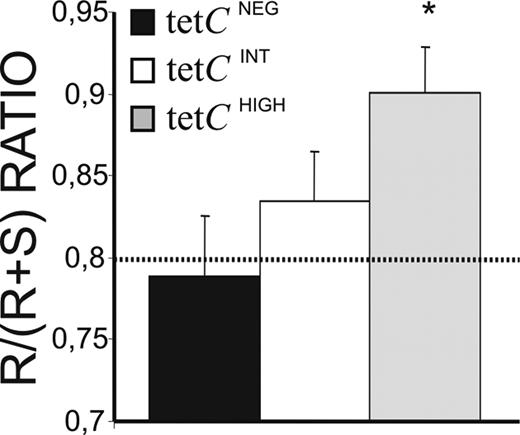
Differences in the affinity of Ab for the Ag have classically been associated with specific changes in the pattern of somatic mutations of IgV genes. Accordingly, IGVH3 genes from isolated tetCHIGH, tetCINT, and tetCNEG PCs were cloned and sequenced, and their corresponding mutations analyzed. This IGVH family was chosen for its high frequency of usage. In summary, all the sequences obtained showed considerable numbers of mutations, predominating in the complentarity-determining regions (CDRs), where many of the mutations gave rise to amino acid replacement (R mutations; Table S1 and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Distinctively, tetCHIGH and tetCINT PC sequences contained significantly higher numbers of total mutations, R mutations, and amino acid substitutions than those from tetCNEG PCs (Figure S1). Phylogenetic analysis revealed that a fraction of the obtained sequences was clonally related (27%, 28%, and 37.5% for tetCNEG, tetCINT, and tetCHIGH PCs, respectively). Clonally related sequences from tetCINT and tetCHIGH PCs, but not those from tetCNEG PCs, were clustered together in every donor (Figures S2Figure S3. Comparison of the clonally-related sequences obtained from experiments 1, 2, 3 and 4 (PDF, 32.0 KB)–S4). The ratio of R versus silent (S) mutations in the CDRs of the IGV sequences is considered an estimation of affinity maturation. In the present study, the R/R + S ratio had to be used because a high proportion of sequences, mainly those obtained from tetCHIGH PCs, lacked S mutations in the CDR (50% versus 32% for tetCHIGH and tetCINT PC sequences, respectively). Interestingly, the sequences obtained from tetCHIGH PCs showed R/R + S ratios significantly higher than the theoretical expected ratio46 (Figure 5 dotted line) and than those observed for tetCINT and tetCNEG PC (Figure 5).
Affinity maturation of IgG synthesized by tetCHIGH, tetCINT, and tetCNEG PCs. A total of 86 different IGVH3 gene sequences were obtained from isolated PC subsets of 4 different individuals, and the numbers of R and S mutations present in the CDR1 and CDR2, as well as the corresponding R/R + S ratio, were calculated. The theoretical R/R + S ratio value calculated for random mutations45 is shown as a dotted line. Results represent the mean plus or minus SEM of the different sequences obtained (n = 33, 32, and 16 for isolated tetCHIGH, tetCINT, and tetCNEG PCs, respectively). *Statistically significant differences.
Affinity maturation of IgG synthesized by tetCHIGH, tetCINT, and tetCNEG PCs. A total of 86 different IGVH3 gene sequences were obtained from isolated PC subsets of 4 different individuals, and the numbers of R and S mutations present in the CDR1 and CDR2, as well as the corresponding R/R + S ratio, were calculated. The theoretical R/R + S ratio value calculated for random mutations45 is shown as a dotted line. Results represent the mean plus or minus SEM of the different sequences obtained (n = 33, 32, and 16 for isolated tetCHIGH, tetCINT, and tetCNEG PCs, respectively). *Statistically significant differences.
Survival and migratory capacities of circulating tetCHIGH, tetCINT, and tetCNEG PC subsets
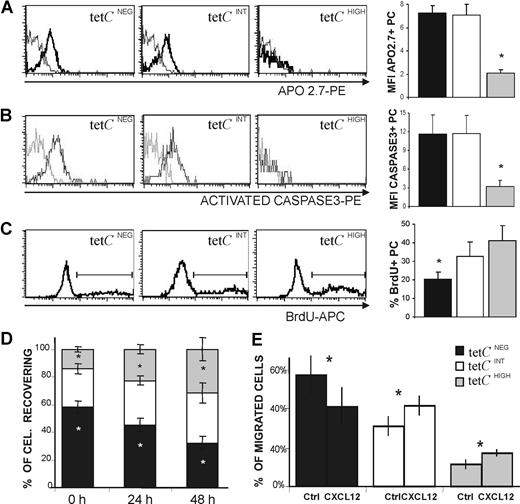
Human early-generated PCs show an enhanced tendency to undergo spontaneous apoptosis.7,31 This property was evaluated in the 3 PC subsets under consideration. Figure 6A shows that the tetCINT and tetCNEG PC subsets, but not the tetCHIGH PC subset, exhibited increased expression of APO2.7, a protein induced during apoptosis in human PCs.47 This was confirmed by the observation that activated caspase-3 was rapidly expressed by tetCINT and tetCNEG PCs after 24 hour of culture (Figure 6B); in contrast, tetCHIGH PCs were essentially negative for activated caspase-3 (Figure 6B), and this characteristic remained so even when analyzed after 72 hours of culture. It has been previously reported that human blood PCs induced after tet immunization go through a short, and apparently residual, proliferation period during the first 18 hours of culture.31 In consequence, this spontaneous activity was explored in the 3 cell subsets by detecting their respective uptake of BrdU. As can be seen in Figure 6C, tetCHIGH and tetCINT PCs showed a proliferating capacity higher than that of tetCNEG PCs. Similar results were obtained when this capacity was assessed by detecting the presence of the proliferation marker Ki67 (data not shown). Since cell recovery was probably the result of the combination of apoptosis and proliferation in each PC subset, this property was also assessed in blood cell cultures. Figure 6D shows that the tetCHIGH PC subset was the only subset that was increasingly detected over time, whereas tetCNEG PCs clearly decreased in these cultures.
Functional capacities of tetCHIGH, tetCINT, and tetCNEG PC subsets. (A-C) Left panels show a representative histogram of APO2.7 expression (A) and activated caspase-3 expression after a 24-hour culture period (B), and BrdU uptake after an 18-hour incubation period (C), by the 3 blood PC subsets. The corresponding bar histograms on the right summarize the results obtained from several donors. Values are expressed as the MFI of the expression of positive PCs (A,B) and as the percentage of positive PCs (C). Results represent the mean plus or minus SEM (n = 5 for panels A-C). (D) Recovery of tetCNEG, tetCINT, and tetCHIGH PCs in blood cell cultures. The percentage of tetCNEG, tetCINT, and tetCHIGH PCs recovered initially (0 hours), and after 24 and 48 hours of culture, were determined by flow cytometry. Results represent the mean plus or minus SEM (n = 6). (E) CXCL12-induced chemotaxis of tetCNEG, tetCINT, and tetCHIGH PCs. CXCL12 (1 μg/mL) was added to the bottom chamber of a transwell culture system. Values are expressed as the percentage of migrated PCs. Results represent the mean plus or minus SEM (n = 6). Asterisks in panels A through C indicate statistically significant differences among PC subsets, and in panels D and E indicate statistically significant differences within every PC subset among data from different times (D) or from different experimental conditions (E).
Functional capacities of tetCHIGH, tetCINT, and tetCNEG PC subsets. (A-C) Left panels show a representative histogram of APO2.7 expression (A) and activated caspase-3 expression after a 24-hour culture period (B), and BrdU uptake after an 18-hour incubation period (C), by the 3 blood PC subsets. The corresponding bar histograms on the right summarize the results obtained from several donors. Values are expressed as the MFI of the expression of positive PCs (A,B) and as the percentage of positive PCs (C). Results represent the mean plus or minus SEM (n = 5 for panels A-C). (D) Recovery of tetCNEG, tetCINT, and tetCHIGH PCs in blood cell cultures. The percentage of tetCNEG, tetCINT, and tetCHIGH PCs recovered initially (0 hours), and after 24 and 48 hours of culture, were determined by flow cytometry. Results represent the mean plus or minus SEM (n = 6). (E) CXCL12-induced chemotaxis of tetCNEG, tetCINT, and tetCHIGH PCs. CXCL12 (1 μg/mL) was added to the bottom chamber of a transwell culture system. Values are expressed as the percentage of migrated PCs. Results represent the mean plus or minus SEM (n = 6). Asterisks in panels A through C indicate statistically significant differences among PC subsets, and in panels D and E indicate statistically significant differences within every PC subset among data from different times (D) or from different experimental conditions (E).
A major fraction of human circulating PCs express CXCR4,22,–24,31 and increasing evidence supports the view that this chemokine receptor plays a relevant role in the homing of circulating PCs into suitable BM niches.21,,–24 Therefore, the capacity of tetCHIGH, tetCINT, and tetCNEG PC subsets to migrate to the CXCR4 ligand CXCL12 was tested. This chemokine (1 μg/mL) induced the migration of blood CD19+ CD38HIGH PCs in this chemotaxis assay by a factor of 2.5 (± 0.4) over the control (mean ± SEM; n = 6). This migration was specific, since the preincubation with anti-CXCR4 mAb, as well as the addition of anti-CXCL12 mAb to the chemotactic assay, blocked the activity (data not shown). Figure 6E shows that differences in migratory capacity were presented by the 3 PC cell subsets under study, as the percentage of migrated tetCNEG PCs exhibited a clear decrease, while tetCHIGH and tetCINT PC percentages were similarly increased, indicating that they seemed to possess similar migratory capacities in this system.
Discussion
The present study shows that, several days after tet-booster immunization, 2 discrete subsets of tetC-specific cells appear in the human blood; these subsets differ in their capacity for cytoplasmic staining with FITC-tetC. A first subset of tetC-specific cells, called tetCHIGH, is probably equivalent to the tetC-specific cells identified as PCs in previous studies.24,31 A second circulating subset of tetC-specific cells was also observed, and it was only detectable when high concentrations of FITC-tetC (> 2 μg/mL) were used in this FACS assay. This second cell subset, called tetCINT to denote the intermediate capacity of these cells for FITC-tetC staining, also consisted of tetC-specific PCs, as indicated by the following data: (1) FITC-tetC staining of these cells was specific since it could be blocked by tet, but not by unrelated proteins, even when used at much higher concentrations. (2) The tetCINT cell subset did not consist of surface IgG-tetC specific memory B lymphocytes, since tetCINT cells remained undetectable in the absence of cell permeabilization. Further, tet-specific memory B lymphocytes are not present in the blood as early as 6 days after tet booster24 (data not shown). (3) The tetCINT cell subset consisted of circulating PCs as demonstrated by their phenotype (CD19+CD20NEGCD38HIGHCD27HIGHCD31HIGH)23,24,31,43 and morphology. Moreover, distinctive FITC-tetC staining exhibited by these 2 PC subsets was not due to differences in the content of Ab, as several methods of measurement of these molecules, either at the protein or at the mRNA level, indicated that both tetC-specific PC subsets contained similar quantities of IgG. This observation indicates that tetCINT and tetCHIGH PCs had acquired similar levels of Ab synthesis and maturation. This notion is reinforced by the finding that both PC subsets equally expressed CD138 and HLA-DR, and contained similar quantities of BLIMP-1mRNA, all features indicative of their parallel progression into the PC maturational program.27,44,45 Taken together, these results support the view that tet immunization mobilizes into the blood 2 subsets of tetC-specific PCs exhibiting a similar maturity level, but differing in the FITC-tetC staining intensity of their cytoplasmic Ab.
Kinetics studies revealed that the human circulating tetCINT and tetCHIGH PC subsets were simultaneously detectable during the same period of time (from 5-8 days after boost), and that the tetCINT PC subset was slightly more abundant. In addition, both tetC-specific PC subsets showed similarly high HLA-DR expression, a feature indicative of their recent incorporation in the PC differentiation program,48 and contained a substantial proportion of cycling cells. These characteristics, as well as the common high expression of HLA-DR and CD138 mentioned earlier, clearly distinguished these 2 PC subsets from tetCNEG PCs, as has been discussed elsewhere,24,31 and indicate that tetCINT and tetCHIGH PCs had probably undergone in vivo a similar rapid activation, proliferation, and differentiation progression, presumably induced by the recent drainage of tet into the appropriate areas of the secondary lymphoid organs. The most probable origin of circulating tetCINT and tetCHIGH PCs is the differentiation of pre-existing tetC-specific memory B lymphocytes, recruited during the early extrafollicular tet response occurring in the lymphoid tissues. This assumption is also supported by the finding that IGVH3 sequences obtained from purified tetCINT and tetCHIGH PCs harbored large numbers of mutations, giving rise to frequent amino acid replacement and predominating in the CDR fragments, all properties of pre-existing and extensive Ag selection. In this context, the finding that about a third of tetCINT and tetCHIGH PC sequences appeared to be phylogenetically associated indicated that a part of both populations originate from shared intraclonal diversification. Alternatively, some of these PCs (the tetCHIGH PCs) could have been generated in tet-induced germinal centers. This seems less probable given the early, similar, and rapid kinetics exhibited by both PC subsets in the blood. Nevertheless, since little is known about the germinal center role in human humoral responses to booster immunization, this possibility cannot be excluded.
The occurrence of 2 discrete and stable subsets of tetC-specific PCs could be due to differences in the affinity for tetC shown by the anti-tetC Ab synthesized by the 2 PC subsets. This possibility was examined by using a FACS technique, as previously reported for other protein-protein interactions.37,–39 The results indicate that the affinity for tetC of the cytoplasmic Ab was on average more than 10 times higher in tetCHIGH PCs than in tetCINT PCs. In addition, IGVH3 sequences obtained from tetCHIGH PCs exhibited R/R + S ratios clearly higher than those from tetCINT PCs, a possible indication of a more precise Ag selection process and, in consequence, of higher affinity. It is well established that tet, as well as tetC, is a complex multiepitopic Ag that elicits an extensively polyclonal humoral response32,36,49 (data not shown). The present findings imply that tet booster generated not only PCs secreting a variety of high-affinity Abs, but also induced a considerable number of PCs whose Abs exhibited a certain range of intermediate affinity for this Ag. The recruitment into a specific humoral systemic response of B cells whose Abs exhibit a wide range of affinities for the Ag has been previously demonstrated at several B-cell levels, including the early PC level.12,34,50 In this context, the occurrence of 2 PC subsets exhibiting discrete ranges of intermediate and high affinities is a surprising observation. In this regard, it has recently been demonstrated that the selection of Ag-specific B lymphocytes into the rapid extrafollicular PC generation pathway does not only depend on the high Ag affinity of their surface (S)Ig, since B lymphocytes bearing intermediate-affinity SIg recognizing certain Ag epitopes can also be selected, provided that such epitopes are repeatedly exposed on the antigenic particle.51 This study concludes that the strength rather than the affinity of the Ag recognition by a SIg is the factor determining the rapid extrafollicular differentiation of B cells into the early PC compartment.51 Likewise, human tet booster might induce the formation of both tetCHIGH and tetCINT PC subsets, as a result of the selection of memory B cells with high-affinity SIg specific for unique tetC epitopes, and those expressing intermediate-affinity SIg recognizing epitopes more densely expressed on the tetC molecule, respectively. Furthermore, the selection of Ab with these 2 Ag affinity ranges might be consolidated by the reexposure to the same Ag, an event that has occurred from 6 to 11 times in the tet-immunized population analyzed in the present study.
As mentioned in the preceding paragraph, systemic humoral responses usually trigger B-cell clones that express Ab showing a variety of Ag affinities, a fact confirmed at the early PC level.12,34,50 However, the long-lived BM PC compartment is distinctively enriched by PCs that secrete Abs with high affinity for the Ag.10,–12,34 This selective step might be explained by a differential capacity to localize in suited BM niches. In this regard, the chemotactic capacity of tetCINT and tetCHIGH PCs to CXCL12, a chemokine involved in the homing of BM PCs,21 was similar for both human circulating PC subsets. A well-established feature of early PCs is the enhanced tendency to undergo apoptosis.5,–7,31,52 Interestingly, present data support the view that these human circulating tetC-specific PC subsets exhibit different survival capacity, a characteristic that appears associated with the affinity of their Ab for the Ag. This assumption was based on the following results: (1) a substantial proportion of tetCINT PC spontaneously exhibited apoptotic features, including APO2.7 expression and rapid caspase-3 activation—both features were similarly observed in tetCNEG PC as well, as previously reported,31 and were almost totally absent in tetCHIGH PCs; and (2) further experiments demonstrated that only tetCHIGH PCs showed augmented recovery in vitro. These results indicate that tetCHIGH PCs exhibit increased resistance to undergoing spontaneous apoptosis, a property that alone, or combined with others, such as the previously demonstrated IL-6 responsiveness,31 can give these cells a clear advantage for homing into the specialized BM survival niches and, accordingly, for becoming BM PCs. Therefore, a relevant factor determining that PCs synthesizing high-affinity Ab are enriched in the BM compartment seems to be their inherent superior survival capacity. Collectively, present data provide a reasonable explanation of how PCs synthesizing high-affinity Abs are predominantly selected into the long-lived PC compartment, a process of marked biological relevance in health and disease. Finally, it has been proposed that blood tetCNEG PCs represent senescent PCs removed from survival PC niches, which, in turn, are occupied by recently generated immigrant PCs, putatively, the tetC+ PCs.24,31 Our finding indicating that IGVH3 sequences from tetCNEG PCs exhibited significantly fewer mutations than tetC+ PCs might suggest that this removal mechanism appears to follow a certain affinity-associated hierarchy. This reinforces the notion that Ab affinity is a critical determinant of the PC fate.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. González and M. L. Villar for helpful discussion.
This work was supported in part by grants PI05/2357 and PI05/2406 from the Fondo de Investigaciones Sanitarias of Spain and 258/2005 from the Consejería de Salud de Andalucía, Spain.
Authorship
Contribution: I.G.-G. performed most laboratory work and helped in manuscript preparation; B.R.-B. contributed in PC purification procedures; F.M.-L. and A.C.-C. helped in genetic studies; and J.A.B. conducted the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: José A. Brieva, Servicio de Inmunología, Hospital Universitario Puerta del Mar, Avenida Ana de Viya 21, 11009 Cádiz, Spain; e-mail: josea.brieva.sspa@juntadeandalucia.es.