A multicenter retrospective analysis was performed to estimate the frequency of thrombosis and hemorrhage after surgical procedures in patients with polycythemia vera (PV) and patients with essential thrombocythemia (ET). Data from 105 patients with PV and 150 patients with ET were analyzed, for a total of 311 surgical interventions. An emergency procedure was performed in 25 (8.1%) patients; 194 surgeries were done under general anesthesia, and 21 (23%) of 91 abdominal interventions were done under laparoscopy; 155 (50.1%) were major surgeries. Subcutaneous heparin was administered in 169 (54.3%) of 311 cases and antiplatelet therapy in 48 (15.4%) of 311 case interventions. One hundred eighty-eight (74%) of 255 patients were on cytoreductive therapy before surgery. No events were observed in 259 (83.2%) of 311 procedures during 3 months of follow-up; there were 12 arterial and 12 venous thrombotic events, 23 major and 7 minor hemorrhages, and 5 deaths. Arterial thromboses were more frequent in ET (5.3% vs 1.5%; P = .08), venous events were more frequent in PV (7.7% vs 1.1%; P = .002). There was not a correlation between bleeding episodes and the type of diagnosis, use of antithrombotic prophylaxis, or type of surgery. A high proportion of PV and ET surgeries was complicated by vascular occlusion (7.7%) or by a major hemorrhage (7.3%). Prospective investigations analyzing the optimal prophylaxis in these patients are suggested.
Introduction
Polycythemia vera (PV) and essential thrombocythemia (ET) are 2 chronic myeloproliferative diseases (cMPDs)1 with clinical courses that are characterized by a low rate of transformation into acute myeloid leukemia and myelofibrosis,2,–4 a long median survival, and an increased risk of venous and arterial thrombosis and of hemorrhage.5,6 There is a common belief that surgery could present an important circumstantial risk factor for thrombosis and bleeding,7,8 although only limited data are available.9,–11 We performed a retrospective evaluation of a cohort of patients with PV and patients with ET with the following aims: to estimate the incidence of thrombosis, hemorrhage, and fatality after surgical procedures; to identify additional risk factors for adverse outcomes in these patients and to evaluate a possible effect of different perioperative management strategies (control of the disease and prophylaxis for cardiovascular events and bleeding) on the frequency of an adverse outcome.
Methods
This study was approved by institutional review boards and ethics committees in each participating institution. The ethics committees stated that written consents were not required by patients to report the encrypted anonymous data; however, oral consent was given.
A retrospective chart review was performed for all patients with PV and ET who were consecutively diagnosed from January 1, 1985, to July 31, 2005, at 6 major Italian hematology departments that had an electronic database for clinical records and belonged to the GIMEMA (Gruppo Italiano Malattie Ematologiche dell'Adulto) network. Diagnoses of PV and ET were made locally according to the criteria of the PVSG12,–14 and the World Health Organization (WHO).15
Patient selection
Patients were eligible for the study if they had undergone at least one surgical procedure from diagnosis to July 30, 2005, and if they had clinical and laboratory follow-ups from diagnosis to December 31, 2005. The following information was extracted from the chart for each eligible patient: personal data; individual risk factors for arterial thrombosis (treated diabetes mellitus; treated hypercholesterolemia; treated arterial hypertension; smoking; cardiovascular diseases, such as atrial fibrillation and valvular or coronary disease; previous arterial thrombosis); previous history of venous or arterial thrombosis; date of diagnosis; laboratory characteristics at diagnosis; thrombosis and hemorrhagic complications during follow-up before surgery; specific therapy for the myeloproliferative disease; date and type of surgery and antithrombotic and antihemorrhagic perioperative prophylaxis procedures; thrombosis, hemorrhagic events, and fatality occurring within 3 months after surgery.
Endpoint definitions
Major thrombotic events were defined according to Landolfi et al16 Diagnostic procedures for establishing thrombosis included cerebral computed tomography (CT) or magnetic resonance imaging for stroke; characteristic neurologic symptoms for transitory ischemic attack; electrocardiography or increased cardiac enzymes or both for acute myocardial infarction (AMI); angiography for peripheral arterial thrombosis and ultrasonography of the arms or the legs, performed in patients with a clinical suspicion of thrombosis; or pulmonary ventilation-perfusion scan or CT scan for deep venous thrombosis (DVT) or pulmonary embolism.
Major hemorrhages were defined as intracranial (documented by imaging), ocular, articular, or retroperitoneal bleeding; episodes that require surgery or angiographic intervention and any bleeding that causes a hemoglobin reduction of 20 g/L or more that requires transfusion of 2 or more blood units.17 Minor hemorrhages included all cases of bleeding not classified as major, such as bruising, small ecchymoses or epistaxis, or microscopic hematuria.
Surgical bleeding was defined as minor if it was of severity grade 1 or 2 according to the National Cancer Institute Common Terminology Criteria for Adverse Events18 and major if it was of grade 3, 4, or 5.
In all centers, chart review and data collection were performed by the physician(s) specifically caring for patients with PV and ET. Confirmation of the diagnosis of myeloproliferative disease and of every vascular events or bleeding episodes by an independent senior researcher was however required for each center, with a careful review of the original chart and the laboratory data.
Surgical definitions
Surgeries that involved the thorax, abdomen, or pelvis lasting longer than 30 minutes in general surgery; hip or knee replacement or hip fracture in orthopedic surgery; valvular replacement and coronary by-pass in cardiovascular surgery; and intracranial intervention in neurosurgery were defined as major interventions; all other cases, including diagnostic procedures, were defined as minor interventions. Data about the type of anesthesia (general or local) and type of operation (elective or emergency procedure; laparoscopy or laparotomy for abdominal surgery) were also collected.
Statistical analysis
Logistic regression was used to model the factors possibly influencing treatment options by the attending physicians at baseline or before surgery. In this model, use of cytoreduction, antiplatelet therapy (aspirin or thienopyridine) or oral anticoagulant therapy at baseline was used as a dependent variable of age, sex, presence of diabetes, hypercholesterolemia, arterial hypertension, smoking, previous cardiovascular disease, and previous history of venous or arterial thrombosis. Cox regression analysis was used to model the probability of developing an endpoint during the follow-up period.
Results
Enrolled subjects
During the study interval, PV was diagnosed in 716 patients and ET in 1462 patients; 4.8% of patients in the whole cohort of patients were lost at follow-up; 255 patients (105 PV and 150 ET; Table 1) underwent at least one surgery, for a total of 311 interventions (212 patients, 1 intervention; 43 patients, 2-5 interventions).
Clinical and laboratory characteristics of surgical patients with ET and PV at diagnosis
| Characteristic . | PV, n = 105 . | ET, n = 150 . | P . |
|---|---|---|---|
| Male/female, no./no. | 66/39 | 62/88 | .001* |
| Age, y; median (range) | 61 (28-85) | 59 (20-84) | .13 |
| Presence of at least 1 cardiovascular risk factor, no. (%)† | 62 (59) | 66 (44) | .02* |
| Previous arterial thrombosis, no. (%) | 14 (10.7) | 22 (11.7) | .79 |
| Previous venous thromboembolism, no. (%) | 3 (2.8) | 6 (4) | .62 |
| Symptoms at presentation, no. (%) | |||
| None | 72 (68.5) | 107 (71.3) | .36 |
| Microvascular disturbances | 28 (26.6) | 33 (22.0) | .45 |
| Arterial thrombosis | 2 (1.9) | 6 (4.0) | .47 |
| Venous thromboembolism | 1 (0.9) | 3 (2) | .64 |
| Hemorrhage | 2 (1.9) | 1 (0.7) | .57 |
| Hemoglobin level, g/L, mean plus or minus SD | 180 ± 19.3 | 140 ± 14 | <.001* |
| Hematocrit level, %, mean plus or minus SD | 55.1 ± 6.1 | 41.7 ± 3.6 | <.001* |
| Leukocyte, × 109/L, mean plus or minus SD | 10.2 ± 4 | 10.2 ± 3.8 | .90 |
| Platelet, × 109/L, mean plus or minus SD | 516 ± 239 | 911 ± 376 | <.001* |
| Characteristic . | PV, n = 105 . | ET, n = 150 . | P . |
|---|---|---|---|
| Male/female, no./no. | 66/39 | 62/88 | .001* |
| Age, y; median (range) | 61 (28-85) | 59 (20-84) | .13 |
| Presence of at least 1 cardiovascular risk factor, no. (%)† | 62 (59) | 66 (44) | .02* |
| Previous arterial thrombosis, no. (%) | 14 (10.7) | 22 (11.7) | .79 |
| Previous venous thromboembolism, no. (%) | 3 (2.8) | 6 (4) | .62 |
| Symptoms at presentation, no. (%) | |||
| None | 72 (68.5) | 107 (71.3) | .36 |
| Microvascular disturbances | 28 (26.6) | 33 (22.0) | .45 |
| Arterial thrombosis | 2 (1.9) | 6 (4.0) | .47 |
| Venous thromboembolism | 1 (0.9) | 3 (2) | .64 |
| Hemorrhage | 2 (1.9) | 1 (0.7) | .57 |
| Hemoglobin level, g/L, mean plus or minus SD | 180 ± 19.3 | 140 ± 14 | <.001* |
| Hematocrit level, %, mean plus or minus SD | 55.1 ± 6.1 | 41.7 ± 3.6 | <.001* |
| Leukocyte, × 109/L, mean plus or minus SD | 10.2 ± 4 | 10.2 ± 3.8 | .90 |
| Platelet, × 109/L, mean plus or minus SD | 516 ± 239 | 911 ± 376 | <.001* |
indicates Mann-Whitney test was adopted to calculate P values of continuous variables.
Diabetes on treatment, hypercholesterolemia on treatment, arterial hypertension on treatment, previous AT, cardiovascular diseases (atrial fibrillation, valvular or coronary disease), or smoking.
At least one risk factor for arterial thrombosis was present in 128 (50.1%) of 255 patients; risk factors were more prevalent in patients with PV than in patients with ET (58.5% versus 46.8%; P = .02). An excess of men and older age at surgery explained this latter finding in a multivariate analysis. Previous arterial thrombosis was present in 23 of 255 patients (9.0%; 9.5% in PV and 8.7% in ET; P = .81); previous DVT was present in 9 (3.5%) of 255 patients, with a similar distribution between patients with PV and patients with ET. After diagnosis, antiplatelet drugs were given to 211 (82.7%) of 255 patients; cytoreductive treatments to 188 (74%) of 255 patients, and warfarin to 16 (6.2%) of 255 patients; all patients with PV received a phlebotomy to reduce their hematocrit levels.
Surgical characteristics and antithrombotic prophylaxis
The clinical features of patients at the time of surgery and by surgery type are reported in Table 2. Antithrombotic prophylaxis was significantly different between major and minor surgeries, with low-molecular-weight heparin (LMWH) administered at dosages above 3000 anti-Xa units almost exclusively in patients undergoing major surgery. No prophylaxis was given to 41% of patients undergoing minor surgery. Twenty-six patients started a short course of chemotherapy or phlebotomy just before intervention. Because of the retrospective nature of the study, the choice of antithrombotic prophylaxis, if any, and of chemotherapy or phlebotomy before intervention was based on each center policy. Administration of LMWH once a day or of unfractionated heparin (UH) twice daily or thrice daily, starting before intervention and until days 7 to 15, was the usual antithrombotic prophylaxis. In a minority of cases, antiplatelet prophylaxis was chosen, initiating it 3 to 5 days before surgery until days 7 to 15 (Table 2). To assess potential factors influencing the choice of antithrombotic prophylaxis and cytoreduction, we modeled with logistic regression the odds of being treated with heparin, antiplatelet drugs, or both and of phlebotomy or chemotherapy, considering significant those factors with a P value below .05. Patients who were given heparin had a lower chance of being given antiplatelet prophylaxis at surgery (and conversely); heparin was preferentially given to those patients undergoing major surgery and those having a history of venous or arterial thrombosis. Antiplatelet treatment was preferred in patients with PV rather than in those with ET. Hematology intervention (ie, phlebotomy or cytoreduction with either busulfan or hydroxyurea) was preferentially given in those patients with a platelet count at surgery higher than 1000 × 109/L with PV, with an age at surgery older than 60 years, a thrombotic event during follow-up, undergoing major surgery, and with a white blood cell count higher than 10 × 109/L.
Clinical features at the time of surgery, according to the type
| Feature . | Type of surgery . | P . | |
|---|---|---|---|
| Minor, n = 156 . | Major, n = 155 . | ||
| PV/ET, no./no. | 63/93 | 64/91 | .87 |
| Male/female, no./no. | 95/61 | 69/86 | .004* |
| Age at surgery, y; median (range) | 66 (21-87) | 56 (25-90) | .57 |
| Presence of at least 1 cardiovascular risk factor, no. (%)† | 77 (49.3) | 70 (45.2) | .45 |
| History of vascular disease, no. (%)‡ | 50 (32.1) | 50 (32.3) | .96 |
| Emergency procedure, no. (%) | 6 (3.8) | 19 (12.3) | .006* |
| General anesthesia, no. (%) | 49 (31.4) | 145 (92.9) | <.001* |
| Hemoglobin level, g/L, mean plus or minus SD | 141 ± 16 | 133 ± 18 | .001* |
| Hematocrit level, %, mean plus or minus SD | 42.7 ± 5.0 | 41.1 ± 5.2 | .004* |
| Leukocyte count, × 109/L, mean plus or minus SD | 8.1 ± 4.0 | 9.5 ± 5.1 | .007* |
| Platelet count, × 109/L, mean plus or minus SD | 494 ± 221 | 506 ± 231 | 0.64 |
| Type of prophylaxis at surgery, no. (%) | |||
| None | 64 (41.0) | 30 (19.3) | — |
| Antiplatelet | 32 (20.5) | 16 (10.3) | <.001* |
| Heparin lower dosage§ | 59 (37.8) | 30 (19.3) | — |
| Heparin higher dosage‖ | 1 (0.6) | 79 (50.9) | — |
| Chemotherapy or phlebotomy | 9 (5.7) | 17 (10.9) | .09 |
| Feature . | Type of surgery . | P . | |
|---|---|---|---|
| Minor, n = 156 . | Major, n = 155 . | ||
| PV/ET, no./no. | 63/93 | 64/91 | .87 |
| Male/female, no./no. | 95/61 | 69/86 | .004* |
| Age at surgery, y; median (range) | 66 (21-87) | 56 (25-90) | .57 |
| Presence of at least 1 cardiovascular risk factor, no. (%)† | 77 (49.3) | 70 (45.2) | .45 |
| History of vascular disease, no. (%)‡ | 50 (32.1) | 50 (32.3) | .96 |
| Emergency procedure, no. (%) | 6 (3.8) | 19 (12.3) | .006* |
| General anesthesia, no. (%) | 49 (31.4) | 145 (92.9) | <.001* |
| Hemoglobin level, g/L, mean plus or minus SD | 141 ± 16 | 133 ± 18 | .001* |
| Hematocrit level, %, mean plus or minus SD | 42.7 ± 5.0 | 41.1 ± 5.2 | .004* |
| Leukocyte count, × 109/L, mean plus or minus SD | 8.1 ± 4.0 | 9.5 ± 5.1 | .007* |
| Platelet count, × 109/L, mean plus or minus SD | 494 ± 221 | 506 ± 231 | 0.64 |
| Type of prophylaxis at surgery, no. (%) | |||
| None | 64 (41.0) | 30 (19.3) | — |
| Antiplatelet | 32 (20.5) | 16 (10.3) | <.001* |
| Heparin lower dosage§ | 59 (37.8) | 30 (19.3) | — |
| Heparin higher dosage‖ | 1 (0.6) | 79 (50.9) | — |
| Chemotherapy or phlebotomy | 9 (5.7) | 17 (10.9) | .09 |
indicates includes 1 patient treated with rituximals who obtained CR.
Diabetes on treatment, hypercholesterolemia on treatment, arterial hypertension on treatment, previous AT, cardiovascular diseases (atrial fibrillation, valvular or coronary disease), or smoking.
Defined as history of cardiovascular disease or venous thromboembolism before or after diagnosis.
Less than 3000 U anti-Xa/day for low-molecular-weight heparin or 5000 U twice daily or three times daily for unfractionated heparin (38 surgeries).
More than 3000 U anti-Xa/day for low-molecular-weight heparin.
Vascular outcomes and risk factors
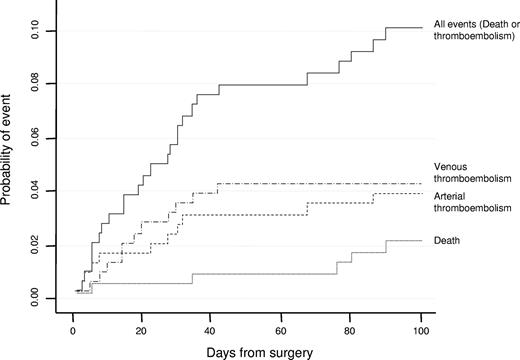
Clinical outcomes were recorded within a 3-month follow-up after surgery. No events were observed in 259 (83.2%) of 311 procedures; there were 12 arterial and 12 venous thrombotic events, 23 major and 7 minor hemorrhages, and 5 deaths (2 AMI, 2 ischemic stroke, 1 multiorgan failure). The incidence of major vascular outcomes and death during the follow-up is shown in Figure 1. The incidence of all thromboembolic events was higher in the first 2 weeks, whereas it decreased throughout the remaining period of time (Table 3). Major surgery showed a slightly higher, but not statistically significant, risk of DVT (HR, 2.0; 95% confidence interval [CI], 0.6-6.6). Arterial thromboembolism (AT) was more frequent in patients with ET (HR, 3.3; 95% CI, 0.7-15.3), whereas venous events were more frequent in patients with PV (HR, 7.3; 95% CI, 1.6-33.4). There was a strong risk gradient for arterial thrombosis associated with the presence of one or more arterial risk factors (HR for 3 or more risk factors, 49.7; 95% CI, 9.0-273.2).
Probability of death, venous thromboembolism, or arterial thromboembolism after surgery in the studied cohort.
Probability of death, venous thromboembolism, or arterial thromboembolism after surgery in the studied cohort.
Incidence of thromboembolic events, at end of period, by postoperative period and hazard ratios by specific risk factors in the observed cohort
| . | Event . | |||||
|---|---|---|---|---|---|---|
| Venous thromboembolism . | Arterial thrombosis . | Death . | ||||
| Cases per 1000 patent-day . | Cumulative % . | Cases per 1000 patient-day . | Cumulative % . | Cases per 1000 patient-day . | Cumulative % . | |
| Incidence at days | ||||||
| 0-15 | 1.4 (0.7-2.8) | 2.3 (0.8-5.0) | 1.4 (0.7-2.8) | 2.3 (0.8-5.0) | 0.2 (0.0-1.2) | 0.3 (0.02-1.8) |
| 15-30 | 1.0 (0.4-2.2) | 3.9 (2.1-6.5) | 0.7 (0.3-1.9) | 3.5 (1.8-6.0) | 0 | 0.3 (0.02-1.8) |
| 30 or more | 0.1 (0-0.4) | 4.7 (2.7-7.5) | 0.2 (0.1-0.5) | 4.7 (2.7-7.5) | 0.3 (0.1-0.6) | 1.9 (0.7-4.0) |
| Overall | 0.5 (0.3-0.8) | — | 0.5 (0.3-0.8) | — | 0.2 (0.1-0.4) | — |
| Event-specific hazard ratios | ||||||
| Major versus minor surgery | 2.0 (0.6-6.6) | — | 1.0 (0.3-3.1) | — | 1.5 (0.2-9.2) | — |
| PV versus ET | 7.3 (1.6-33.4) | — | 0.3 (0.1-1.3) | — | 0.4 (0.04-3.4) | — |
| Antiplatelet versus no active treatment | 0.5 (0.1-4.0) | — | 0.5 (0.1-4.0) | — | 1.4 (0.2-12.8) | — |
| Heparin versus no active treatment | 0.8 (0.3-2.6) | — | 1.7 (0.5-5.6) | — | 0.6 (0.1-3.4) | — |
| . | Event . | |||||
|---|---|---|---|---|---|---|
| Venous thromboembolism . | Arterial thrombosis . | Death . | ||||
| Cases per 1000 patent-day . | Cumulative % . | Cases per 1000 patient-day . | Cumulative % . | Cases per 1000 patient-day . | Cumulative % . | |
| Incidence at days | ||||||
| 0-15 | 1.4 (0.7-2.8) | 2.3 (0.8-5.0) | 1.4 (0.7-2.8) | 2.3 (0.8-5.0) | 0.2 (0.0-1.2) | 0.3 (0.02-1.8) |
| 15-30 | 1.0 (0.4-2.2) | 3.9 (2.1-6.5) | 0.7 (0.3-1.9) | 3.5 (1.8-6.0) | 0 | 0.3 (0.02-1.8) |
| 30 or more | 0.1 (0-0.4) | 4.7 (2.7-7.5) | 0.2 (0.1-0.5) | 4.7 (2.7-7.5) | 0.3 (0.1-0.6) | 1.9 (0.7-4.0) |
| Overall | 0.5 (0.3-0.8) | — | 0.5 (0.3-0.8) | — | 0.2 (0.1-0.4) | — |
| Event-specific hazard ratios | ||||||
| Major versus minor surgery | 2.0 (0.6-6.6) | — | 1.0 (0.3-3.1) | — | 1.5 (0.2-9.2) | — |
| PV versus ET | 7.3 (1.6-33.4) | — | 0.3 (0.1-1.3) | — | 0.4 (0.04-3.4) | — |
| Antiplatelet versus no active treatment | 0.5 (0.1-4.0) | — | 0.5 (0.1-4.0) | — | 1.4 (0.2-12.8) | — |
| Heparin versus no active treatment | 0.8 (0.3-2.6) | — | 1.7 (0.5-5.6) | — | 0.6 (0.1-3.4) | — |
Values in parentheses are 90% confidence intervals.
— indicates no data.
Treatment with heparin or antiplatelet drugs had no apparent effect on adverse outcomes, with a potential trend for heparin treatment in the prevention of venous thromboembolism (HR, 0.55; 95% CI, 0.1-2.1). The effect of heparin in the prevention of venous thromboembolism was unmodified by the inclusion of variables influencing the choice of a prophylaxis in the Cox regression model. White blood cell count, platelet count, hematocrit level, and age at surgery; diagnosis (ET or PV); past history of DVT or AT; and sex were not associated with an increased risk of death, arterial thromboembolism, or venous thromboembolism in the multivariate Cox regression (data not shown).
Bleedings
The overall incidence of bleeding complications (major and minor) was 10.5% (30 episodes in 284 surgeries; 95% CI, 7.6-14.3). There was a clear trend for an increased bleeding risk in those subjects receiving antithrombotic prophylaxis versus no prophylaxis (heparin versus no prophylaxis; HR, 1.7; 95% CI, 0.7-7.7; antiplatelet therapy versus no prophylaxis: HR, 2.4; 95% CI, 0.8-7.7). Furthermore, the hemorrhagic risk was strongly related to the immediate postsurgical period with an incidence at periods 0 to 15 days, 15 to 30 days, and 30 to 60 days of 3.1, 0.8, and 0.2 cases per 1000 patient-days in patients receiving no prophylaxis; 9.8, 0, and 0 in patients receiving antiplatelet therapy; and 6.6, 0, and 0.2 in patients receiving heparin.
Discussion
Advanced age, a history of thrombosis, and leucocytosis are the major risk factors for cardiovascular events4,6,7,16,19,,,–23 in patients with PV and ET, whereas the contribution of hereditary and acquired thrombophilic states or of the other well-recognized risk factors for cardiovascular disease, such as hypertension, diabetes mellitus, smoking, and hypercholesterolemia, has not been firmly established.
Surgery is a strong circumstantial risk factor for venous thromboembolism, both in the general population and in patients with cancer, increasing the DVT risk by at least 2-fold.24 Antithrombotic prophylaxis with heparin (either LMWH or UH) is effective at reducing the rate of DVT in patients with cancer with a low incidence (3%) of bleeding complications.25,26 Unfortunately, there are no available data on the incidence of vascular complications, bleeding, or fatalities after surgical interventions in patients with PV and ET.
We performed a retrospective 20-year analysis of patients with PV and ET who underwent surgical interventions to collect clinical data on vascular outcomes, bleeding, and deaths. We tried to improve the quality of the data collection by enrolling consecutive patients only from Italian tertiary care hematology centers that had an electronic database for clinical records. Moreover, with the aim of reducing the unavoidable limitations of this study because of its retrospective nature, confirmation of the diagnosis of the myeloproliferative disease and of vascular events or bleeding episodes by a senior researcher was required for each center, with a careful review of the original chart and the laboratory data. Despite these efforts, the present study has some obvious limitations. First, the lack of a definite protocol for the assessment of thrombotic events and the ascertainment of only symptomatic thromboses may have led to an underestimation of thrombotic events. Thus, our reported incidence rates are not directly comparable with the results from randomized clinical trials that evaluated asymptomatic events. Second, the study could only analyze a small number of patients with cMPD. In a post hoc analysis, our study (enrolling 311 surgeries, with an incidence of arterial and venous thromboses of around 10%) had a power of only 0.36; thus, it is able to detect significant differences only if they are present in approximately one-third of the cases. Alternatively, given an observed incidence of thromboembolic complications of around 10%, approximately 1000 patients should be enrolled in a prospective study, with the expectation of a 50% relative reduction of risk. Even this latter figure is likely underestimated because it does not take the interaction between possible confounders (eg, the different thrombotic risk of each surgery type) into account.
Despite these limitations, the study shows an increased risk of both thrombotic and bleeding episodes in cMPD. The majority of cases in this large cohort of patients (188 of 255; 74%) were treated with cytoreductive therapy and phlebotomy before surgery, and, consequently, the platelet and white blood cell counts and the hematocrit level were above their respective normal ranges at the time of intervention in only a small number of patients. Moreover, the large majority of patients were treated with antithrombotic prophylaxis (heparin or antiplatelet agents), even in the case of a minor surgery or a diagnostic procedure. Despite this active approach, a significant proportion of surgeries (8 [5.1%] of 155 major surgeries and 4 [2.5%] of 156 minor surgeries) were complicated by an episode of DVT. This incidence could be compared with the 6.3% rate of DVT in patients with cancer receiving no prophylaxis and 1.8% in those receiving heparin, as detected by ultrasound evaluation.27,28 The rate of symptomatic DVT in patients receiving heparin after major surgeries is, however, much lower, usually in the range of 0.5% to 2%.29 Thus, we can estimate that the risk of DVT after major surgery in patients with PV and ET is at least 5-fold increased, confirming the predisposition to thrombosis in patients with cMPD. Unfortunately, other than the type of cMPD (DVT is more frequent in PV than in ET), no other personal characteristics, baseline clinical data, or type of treatment were found to be predictive of DVT occurrence. Given the retrospective design of this study, an evaluation of coagulation thrombophilic defects was not performed.
A similar rate of AT (12 of 311; 3.8%) was observed after intervention. Surgery is not a risk factor for AT, apart from patients who underwent cardiovascular interventions, and this seems to be a specific characteristic of patients with cMPD. In contrast with DVT, 2 risk factors emerged for AT, namely ET diagnosis and the presence of more than one risk factor for cardiovascular thrombosis. Whether stratification of risk for these factors is required for optimal prophylaxis remains uncertain.
Interestingly, the risk of both DVT and AT dramatically decreases 1 month after surgery, the incidence of arterial events becomes similar to the incidence recently reported in the ECLAP study (0.2 versus 0.15 of arterial events).20 This observation could suggest that there is no apparent need for prolonged prophylaxis in these patients.
Recently, a somatic G>T conversion at nucleotide position 1849 in exon 14 of the Janus kinase 2 (JAK2) gene was identified in Philadelphia chromosome–negative chronic myeloproliferative disorders.30,–32 This mutation results in the substitution of a valine to phenylalanine at position 617 (JAK2 V617F mutation), and it can be detected in most patients with PV and approximately half of the patients with ET and idiopathic myelofibrosis. Whether this JAK2 mutation represents a risk factor for thrombosis in PV and ET remains questionable because the results from many studies are conflicting.33,,–36 A JAK2 assay was not performed in our cohort at surgery or at diagnosis; thus, a possible association with thrombotic complications could not be evaluated.
The evaluation of efficacy of prophylactic anticoagulation for patients with PV and ET before surgery must take the often unpredictable development of bleeding complications into account. In our cohort, 23 (7.3%) of 311 surgical interventions experienced major hemorrhage episodes that required transfusion treatment, prolonged hospital admission, and, in some cases, new interventions. This rate is higher than those observed in clinical trials evaluating heparin prophylaxis and in surgical patients with cancer,25 which are around 1%. Given the retrospective, nonrandomized study design, the actual rate of bleeding could not be accurately assessed, because it could be biased by the treating center strategies and should be considered with caution. However, it could confirm the apparent paradoxical predisposition to both bleeding and thrombosis in patients with cMPD. There was not a correlation between bleeding episodes and type of diagnosis, use of antithrombotic prophylaxis, or type of surgery. Five fatalities (1.6%) within 3 months after interventions were recorded, but it was not possible to show a clear association with intervention, because of the paucity of clinical data in the medical chart. However, only 1 of the 5 deaths occurred immediately after surgery (5 days), whereas all other deaths occurred 2 months after surgery.
In conclusion, this study shows that the rate of symptomatic DVT and AT after surgery remains high in patients with cMPD despite effective control of cell blood count with phlebotomy and cytoreduction and administration of standard antithrombotic prophylaxis. However, these patients exhibited an increased bleeding risk after surgery, with an unexpectedly high incidence of major bleeding. It seems appropriate to restrict the use of antithrombotic prophylaxis with LMWH in patients with PV undergoing major surgery. Conversely, antiplatelet drugs may be the optimal choice in patients with ET with several arterial risk factors. This approach should be weighed against the surgery-specific bleeding risk, eg, for surgeries involving mucous tissues. Prospective studies are needed to define optimal antithrombotic management in these patients. The high heterogeneity of bleeding and thrombotic risk observed will probably require a stringent stratification of prophylaxis by surgery and cMPD subgroups. Until such results are available, a very careful clinical approach to patients with cMPD is still essential.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.R., F.R., A.T., and T.B. designed the research, analyzed the data, and wrote the manuscript; G.C., F.S., G.F., F.D., C.M., A.M.V., E.A., V.D.S., T.Z., L.G., A.T., M.G.M., and C.S. contributed to patient selection, discussed the data, and gave final approval of the manuscript.
A complete list of the members of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) Chronic Myeloproliferative Diseases Working Party is found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Rodeghiero, Dipartimento di Ematologia e Terapie Cellulari, Divisione di Ematologia, Ospedale San Bortolo, via Rodolfi 37, 36100 Vicenza, Italy; e-mail: rodeghiero@hemato.ven.it.