Fibrinogen BβArg448Lys is a common polymorphism, positioned within the carboxyl terminus of the Bβ-chain of the molecule. Studies suggest that it is associated with severity of coronary artery disease and development of stroke. The effects of the amino acid substitution on clot structure remains controversial, and the aim of this study was to investigate the effect(s) of this polymorphism on fibrin clot structure using recombinant techniques. Permeation, turbidity, and scanning electron microscopy showed that recombinant Lys448 fibrin had a significantly more compact structure, with thin fibers and small pores, compared with Arg448. Clot stiffness, measured by means of a novel method using magnetic tweezers, was significantly higher for the Lys448 compared with the Arg448 variant. Clots made from recombinant protein variants had similar lysis rates outside the plasma environment, but when added to fibrinogen-depleted plasma, the fibrinolysis rates for Lys448 were significantly slower compared with Arg448.
This study demonstrates for the first time that clots made from recombinant BβLys448 fibrinogen are characterized by thin fibers and small pores, show increased stiffness, and appear more resistant to fibrinolysis. Fibrinogen BβArg448Lys is a primary example of common genetic variation with a significant phenotypic effect at the molecular level.
Introduction
An arginine to lysine substitution in the coding region of the β-chain of fibrinogen, BβArg448Lys, is a relatively common polymorphism, of which the Lys448 allele has a frequency of 15% to 20% in whites.1,2 A previous study has shown an association between BβArg448Lys polymorphism and the severity of coronary artery disease as assessed by angiography,3 with an increased frequency of the Lys448 allele in patients with triple-vessel disease compared with patients having single-vessel or double-vessel disease. The polymorphism has also been implicated in predisposition to stroke in female patients, an effect that was independent of fibrinogen levels, suggesting a functional role for this polymorphism.2 We have previously shown that genetic factors contribute to the ultrastructure of the fibrin clot and that more than one-third of the variation in fibrin structure is determined by genes.4 Clot structure has a role in determining the predisposition to atherothrombotic disease, as clots composed of tightly packed, thin fibers and small pores are associated with increased risk of cardiovascular disease.5 Furthermore, changes in clot structure have been reported to affect interactions with endothelial cells and fibroblasts, whereby dense fibrin structures with small pores impaired reorganization of these cells into microtubules,6 implicating effects on angiogenesis, wound healing, and atherosclerosis.7
Fibrin clot formation initiates with cleavage of fibrinopeptide (Fp)A from the fibrinogen Aα-chain by thrombin, exposing a binding site in the E-region that interacts with a complementary binding site on the γ-chain in the D-region, allowing formation of protofibrils. Thrombin cleavage of fibrinopeptide (Fp)B from the Bβ-chain occurs at a slower rate and is thought to also expose a binding site, this time for a complementary region on the β-chain, again located in the D-region. The precise function of this latter interaction is still a matter for debate. Interactions of the β-chain have been proposed to play a role in conformational changes in α-chain C-terminal region and lateral aggregation of fibrin protofibrils, which determines fiber thickness and final ultrastructure of the clot.8,9 The location of the BβArg448Lys polymorphism is relatively close to 3 areas of interest: the proposed β-chain polymerization site, a β-chain interaction site for the C-terminal region of the α-chain, and a β-chain calcium-binding site. We postulate that because of this location, BβArg448Lys may have a role in modulating fibrin polymerization, potentially affecting the final ultrastructure of the clot. Previous work on the effect of this polymorphism on fibrin structure, however, has led to conflicting results. We previously observed that BβLys448 is associated with a tighter clot structure when compared with BβArg448 in plasma samples, while others failed to detect such differences.10,11 This discrepancy may have been caused by the use of plasma samples and plasma-purified fibrinogen, respectively, in these studies. Noninherited posttranslational modifications that occur during synthesis and circulation in plasma could obscure the functional effect of a fibrinogen polymorphism in plasma samples.12 In addition, plasma-purified samples may contain other genetic variations such as the AαThr312Ala polymorphism, which has been shown to alter fibrin function,13 potentially resulting in inconsistent or contradictory data.
The aim of the current work was to investigate the role of BβArg448Lys on fibrin clot structure and function using recombinant BβArg448 and BβLys448 variants to reduce to a minimum any other potential heterogeneity (genetic or posttranslational), which may be associated with plasma-purified fibrinogen. We found that BβArg448Lys polymorphism is associated with significant changes in fibrin clot structure and rigidity, which is in agreement with a model that supports a role for the C-terminal region of the fibrinogen β-chain in fibrin formation and structure.
Methods
Vector construction and expression of recombinant fibrinogen
The expression vector pMLP-β, which contains the entire fibrinogen β-chain cDNA, was a gift from Prof Susan Lord (University of North Carolina at Chapel Hill) and has been described elsewhere.14,15 Codon 448 was changed from Arg (AGG) to Lys (AAG) using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The following primers (Invitrogen, Poole, United Kingdom) were used (mutated base in bold): forward: 5′ GGAAGGGGTCATGGTACTCAATGAAGAAGATGAGTATG 3′; reverse: 5′ CATACTCATCTTCTTCATTGAGTACCATGACCCCTTCC 3′.
Primers were designed according to manufacturer's guidelines and were purified by polyacrylamide gel electrophoresis (PAGE). The mutation was confirmed by sequencing using an ABI PRISM312 automated sequencer (Applied Biosystems, Warrington, United Kingdom). Chinese hamster ovary (CHO) cells expressing the human Aα and γ fibrinogen chains were cotransfected with the Lys448 β-chain vector and a plasmid containing a selection gene, pMSV his.16 Individual clones selected for high fibrinogen expression were grown in serum-free medium in roller bottles. Harvested medium was supplemented with 100 mM phenylmethylsulfonyl fluoride and stored at −20°C until purification. Wild-type (BβArg448) fibrinogen was obtained from CHO cells capable of expressing all 3 chains as previously described.14 Both CHO cell clones were donated by Prof Susan Lord. Fibrinogen was precipitated from culture medium by ammonium sulfate and further purified by affinity chromatography using the IF-1 antibody.17 Integrity of the samples was analyzed by sodium dodecyl sulfate (SDS)–PAGE. Fibrinogen concentration was determined at 280 nm using the extinction coefficient ε280 = 1.506 and adjusted to a dilution of 1 mg/mL. To determine clottability, subsamples (100 μL) from each preparation were clotted by adding 0.5 U/mL thrombin and 2.5 mM CaCl2 (final concentration). Samples were incubated for 2 hours at ambient temperature, and clotted fibrin was removed by centrifugation at 13 000g for 10 minutes. Fibrin present in the supernatant was measured at 280 nm. Clottability was calculated as (A280 at 0 time − A280 of the supernatant) ÷ (A280 at 0 time) × 100%. No correction was made for negligible increase in absorbance as a result of adding thrombin.
Preparation of factor XIII
A plasma-based concentrate of FXIII, used clinically for the treatment of patients with FXIII deficiency (FibrogamminP, ZLBBehring, Sussex, United Kingdom), was separated from contaminating albumin and glucose using gel filtration on sepharose 6B (40 × 2.5 cm).
Clot permeation experiments
Recombinant fibrinogen at 1.3 μM was incubated with 0.5 U/mL human thrombin (Sigma, St Louis, MO) and 2.5 mM CaCl2 in open tubes for 2 hours at room temperature in a wet chamber to allow for full clot formation. Permeation of 50 mM Tris, 100 mM NaCl, pH 7.4, was measured as described previously, with a pressure drop of 2 cm, and the Darcy constant Ks was subsequently calculated.18,19 Permeation experiments were also conducted by adding 3 volumes of recombinant fibrinogen variants at 1.3 μM to 1 volume of plasma immunodepleted of fibrinogen (Diagen Diagnostic Reagents, Thame, United Kingdom). Fibrinogen-depleted plasma was prepared by passing a pool of normal control plasma over immobilized monoclonal antifibrinogen antibody. The resulting plasma is depleted to less than 0.5% of fibrinogen, without significantly affecting levels of other clotting factors. In the preparation used, plasma levels of FII, FV, FVII, FIX, and FX were 89% to 90% the levels detected before fibrinogen depletion.
Thrombin-catalyzed fibrin polymerization
Fibrinogen variants, at a final concentration of 1.3 μM, were mixed with 0.5 U/mL thrombin and 2.5 mM calcium chloride in 100 mM NaCl, 50 mM Tris, pH 7.4, at ambient temperature in a 96-well plate. Four replicates were performed for each variant. Increase in turbidity at 350 nm was continuously monitored every 12 seconds on a FLX-800 multiwell plate reader (Biotek Instruments, Winooski, VT) over a period of 60 minutes. Recombinant fibrinogen variants were also added to fibrinogen-depleted plasma as described above, and turbidity analysis was repeated.
Scanning electron microscopy of fibrin
Recombinant fibrinogen (1.3 μM) was clotted upon addition of 0.5 U/mL thrombin and 2.5 mM CaCl2 in specially devised small, perforated plastic vessels. Samples were incubated at room tempature in a moist chamber for 2 hours then washed with sodium cacodylate buffer and subsequently fixed overnight in 2% glutaraldehyde. Clots were recovered and further processed by a stepwise dehydration with an acetone gradient and sputter coated with platinum palladium. Samples were viewed and photographed using a field-emission scanning electron microscope (LEO1530 FEGSEM, Leo Electron Microscopy, Cambridge, United Kingdom) in 10 different areas of each clot. Images were captured using Leo 32 version 03.0210 software (Leo Electron Microscopy) and cropped using Paintshop Pro version 8.0 (Corel, Minneapolis, MN). Fiber diameters of all clots were measured with image analysis software package ImageJ 1,23y (National Institutes of Health, Bethesda, MD). In all, 600 fibrin fibers per genotype in fibrin clots were measured. To exclude bias, the micrographs were analyzed by an operator blind to the genotype of the sample in examination.
Elastic properties of clots made from recombinant fibrinogen
Stiffness of clots made from recombinant fibrinogen was measured by magnetic tweezers, which operate by exerting a force to a micron-scale magnetic particle using an external magnetic field gradient. A custom-built magnetic tweezer device was used to exert 100 pN force pulses of 5 seconds' duration every 30 seconds for either 60 or 180 minutes. This technique has been applied previously to actin networks,20 where the particle is free to flow through the sample. In the case of fibrin, however, the particle is restricted by the fibrous network of the fibrin sample. The octapolar design of Huang et al21 was used, allowing 3-dimensional control over the particle, although particle movement was restricted to 1 dimension in this study. The tweezers were used in conjunction with an Olympus IX71 optical microscope (Olympus, Tokyo, Japan) incorporating a 40× ultra-long working distance objective lens (numeric aperture 0.55). Force calibration was achieved by applying Stokes' law to particles held in 100% glycerol. All particle tracking, electromagnet control, and image analysis was performed in real time and achieved using custom-written software (Labview 7.1; National Instruments, Austin, TX).
Clots were made from 1.3 μM recombinant fibrinogen Arg448 or Lys448 as described in “Clot permeation experiments” in the presence of 4.5 μm superparamagnetic particles (Dynal, Oslo, Norway). The particles, dispersed in a small amount of distilled water, were first added to the fibrinogen, which was then mixed with 0.5 U/mL thrombin and 2.5 mM calcium. The resulting mix was quickly placed into a thin glass capillary tube. As the fibrin polymerization took place, the magnetic particles were caught in the resulting network. A suitable particle was then found, away from the capillary walls, and the force pulses applied. Experiments with both recombinant variants were repeated in the presence of FXIII (0.068 μM final concentration). For each sample, the time development of the clot stiffness was measured for up to 180 minutes. After this period, several other particles were displaced with force pulses to test the elasticity of different sample regions.
Macroscopic lysis of recombinant fibrin clots
Macroscopic lysis velocity of recombinant fibrin clots was assessed directly after completion of polymerization at 60 minutes in the microtiter plates used for turbidity analysis. All wells were overlaid with 100 μL of a mixture of 10 nmol/L tissue plasminogen activator (Technoclone, Vienna, Austria) and 0.05 mg/mL Glu-plasminogen (Calbiochem, San Diego, CA). Changes in turbidity at 350 nm were monitored every 12 seconds for 60 minutes, as described in “Thrombin-catalyzed fibrin polymerization.” Lysis experiments were also conducted by adding the recombinant variants to plasma immunodepleted of fibrinogen, as described in “Clot permeation experiments.” One volume of fibrinogen-depleted plasma was added to 3 volumes of recombinant fibrinogen at 1.3 μM, and lysis experiments were conducted as described for 2 hours; these were further extended to 7 hours because of incomplete lysis of the clot at 2 hours.
Microscopic lysis of recombinant fibrin clots and fibrin structure by confocal microscopy
Real-time microscopic lysis was determined using laser confocal scanning microscopy as previously detailed by Collet et al.22 Recombinant fibrinogen was used at 1.3 μM with calcium at 5 mM and thrombin at 1 U/mL. After clot formation, tissue plasminogen activator (tPA) and plasminogen were added at 10 nmol/mL and 0.1 mg/mL final concentrations, respectively, to the border of the clot. Fibrinolysis rates were observed and recorded every 2 minutes for each of the fibrinogen variants using Leica TCS SP-2 laser scanning confocal equipment (Leica Microsystems, Wetzlar, Germany) on an inverted DM IRE 2 microscope with 63× water-immersion objective (numeric aperture 1.2), with characterization of at least 4 areas of 240 × 240 × 40 μm characterized per sample.
Molecular modeling
Preliminary molecular modeling calculations were performed to investigate the possibility of changes in molecular flexibility, both at the domain level and at the residue level, caused by the BβArg448 → Lys mutation.
Four models in total were constructed based on atomic coordinates from the x-ray crystallographic structure of the fibrinogen D-fragment.23,24 The Arg448 I model had polar hydrogen atoms added on using the HBUILD facility in CHARMM.25 A first Lys variant model (Lys I) was prepared from Arg I by changing the side-chain of BβArg448 into that of a lysine with the same conformation. A second Lys variant model (Lys II) was prepared from the first by positioning the BβLys448 side-chain in a low-energy conformation as determined by a rigid body mapping of the side-chain dihedral space. The 3 models were subjected to severe energy minimization (one phase of restrained minimization and one of unrestrained) until the potential energy gradient for the current minimization step was less than 10−5. The minimized models were subjected to normal modes analysis using a Diagonalisation in a Mixed Basis (DIMB) approach as implemented in CHARMM.26 The lowest frequency modes (100 in all) were computed for each model system.
A further Arg variant model was constructed (Arg II) from the atomic coordinates of the fibrinogen D-fragment23,24 with polar hydrogens added. The system was left unrestrained and then subjected to 10 ps of equilibration at 100 K followed by 10ps of production dynamics at 100 K. This structure (root mean square [RMS] deviation in atomic coordinates = 2.7Å) was subjected to the same protocol of normal modes analysis. This second wild-type model was prepared to eliminate the possibility that the hydrogen bonding between BβArg448 and BβGlu315 is an artifact of some fortuitous positioning of the arginine side chain in the crystal structure.
For each model, 100 ps of unrestrained molecular dynamics was then performed. This allowed exploration of the shifts in hydrogen bonding and side-chain conformations with time. The starting model for each simulation was a partially minimized model corresponding to the restrained model from the described normal modes analyses. The dynamics were performed using the Verlet algorithm molecular dynamics as implemented in CHARMM and the SHAKE algorithm on hydrogen bearing bonds.27 The systems were subjected to 20 ps of equilibration at 300 K followed by 100 ps of production dynamics at 300 K. Analysis was performed using CHARMM and the graphical visualization tool SQUID.28
Results
Expression of recombinant Bβ448 fibrinogen variants
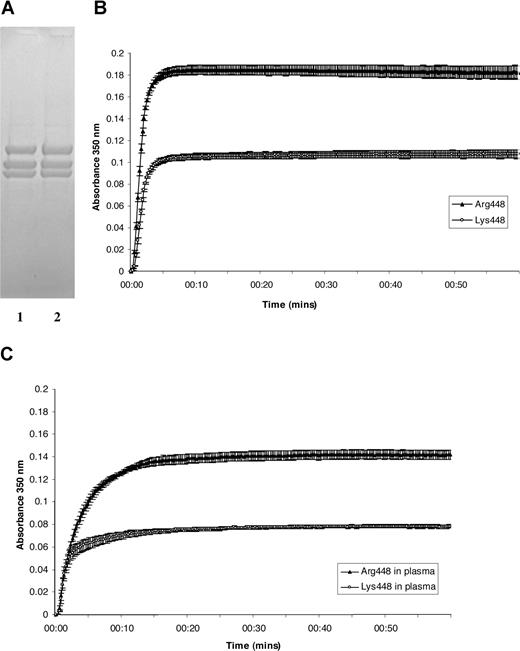
We synthesized both Bβ448 fibrinogen with arginine and lysine at codon 448 in the Bβ chain to mimic the naturally occurring BβArgLys448 and BβArg448 variants of fibrinogen. The entire cDNA from both variants was sequenced, and the only difference was Arg-to-Lys substitution at position 448. SDS-PAGE under reducing conditions showed that both recombinant variants contained the expected bands representing the Aα, Bβ, and γ chains of fibrinogen and that the mutation from Arg to Lys did not affect the migration of the Bβ chain (Figure 1A). Clottability of samples was 97% (± 1.1%) for Arg448 and 95.4% (± 1.2%) for Lys448.
Recombinant BβArg448 and BβLys448 fibrinogen, permeation, and polymerization curves of fibrin formed from the 2 variants. (A) Recombinant fibrinogen resolved on 12% to 24% SDS-PAGE: lane 1 Lys448, lane 2 Arg448. (B) Mean turbidity curves (± SEM) of 4 experiments with each Arg448 and Lys448 fibrin variants at fibrinogen concentration of 1.3 μM, 2.5 mmol CaCl2, and 0.5 U/mL thrombin. The final maximum absorbency at 350 nm was lower in the less common variant Lys448 than in the more common Arg448, indicating the presence of thinner fibers in Lys448 fibrin clots. (C) Mean turbidity curves (± SEM) of 4 experiments with each Arg448 and Lys448 fibrin variants after addition to fibrinogen-depleted plasma (1 volume of plasma was added to 3 volumes of recombinant fibrinogen at 1.3 μM concentration), 2.5 mmol CaCl2, and 0.5 U/mL thrombin.
Recombinant BβArg448 and BβLys448 fibrinogen, permeation, and polymerization curves of fibrin formed from the 2 variants. (A) Recombinant fibrinogen resolved on 12% to 24% SDS-PAGE: lane 1 Lys448, lane 2 Arg448. (B) Mean turbidity curves (± SEM) of 4 experiments with each Arg448 and Lys448 fibrin variants at fibrinogen concentration of 1.3 μM, 2.5 mmol CaCl2, and 0.5 U/mL thrombin. The final maximum absorbency at 350 nm was lower in the less common variant Lys448 than in the more common Arg448, indicating the presence of thinner fibers in Lys448 fibrin clots. (C) Mean turbidity curves (± SEM) of 4 experiments with each Arg448 and Lys448 fibrin variants after addition to fibrinogen-depleted plasma (1 volume of plasma was added to 3 volumes of recombinant fibrinogen at 1.3 μM concentration), 2.5 mmol CaCl2, and 0.5 U/mL thrombin.
Permeability of fibrin clots formed from recombinant fibrinogens
The Darcy constant (Ks) is a direct measure of the clot surface area available for flow and gives information on the size of pores within the fibrin gel. The mean of 3 experiments (± SEM) showed that clots made from recombinant Arg448 had a Ks of 2.96 (± 0.59), whereas the Ks for Lys448 variant was 1.54 (± 0.24), almost a 2-fold difference that was statistically significant (P < .05). A similar pattern was observed when recombinant protein was added to fibrinogen-depleted plasma. For Arg448 and Lys448 in plasma environment, the Ks was 1.19 (± 0.15) and 0.51 (± 0.04), respectively (P < .05).
Thrombin-catalyzed fibrin polymerization of recombinant fibrinogens
We observed pronounced differences in the polymerization rate between both variants. Curves that represent average values of 4 experiments for each recombinant variant (Figure 1B) showed a lower rate of fiber aggregation (reduced slope of the curve) and smaller fiber size (final maximum absorbency at 350 nm) for Lys448 when compared with Arg448 fibrin. The difference in final turbidity agrees with the magnitude of the difference observed in permeation. A similar pattern was observed when recombinant fibrinogen variants were added to fibrinogen-depleted plasma (Figure 1C).
Ultrastructure of fibrin clots formed from recombinant fibrinogens
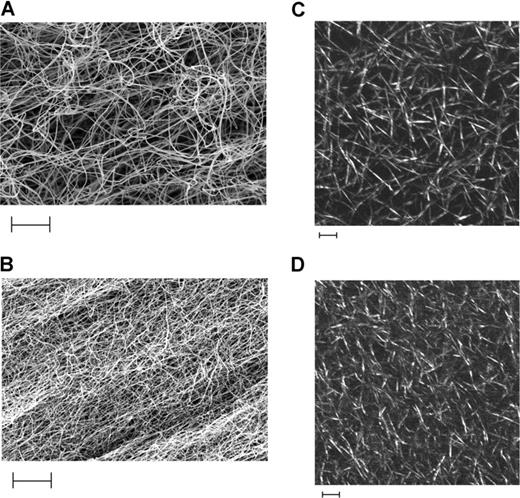
Clots prepared from recombinant Lys448 fibrinogen showed a dense and thin fibrin network with small interstitial pores by both scanning electron microscopy and laser scanning confocal microscopy (Figure 2). In contrast, clots made using the Arg448 variant were characterized by fibrin fibers with larger diameter and bigger interstitial pores. In Lys448 fibrin clots, the average fiber diameter was 80 (± 22) nm compared with 126 (± 35) nm in Arg448 clots (P < .05) as measured on the scanning electron micrographs.
Scanning electron micrographs (EM) and laser scanning confocal microscopy (LSCM) of BβArg448 and BβLys448 fibrin clots. Panels A and B represent EM of clots prepared from recombinant fibrinogen Arg448 and Lys448, respectively, at a concentration of 1.3 μM in the presence of 2.5 mmol CaCl2 and 0.5 U/mL thrombin (magnification, ×10 000). Panels C and D represent LSCM of clots prepared from recombinant fibrinogen Arg448 and Lys448, respectively, at 1.3 μM using 5 mmol CaCl2 and 0.5 U/mL thrombin. Scale bars on all micrographs indicate 5 μm. Complete microscopy information can be found in “Methods.”
Scanning electron micrographs (EM) and laser scanning confocal microscopy (LSCM) of BβArg448 and BβLys448 fibrin clots. Panels A and B represent EM of clots prepared from recombinant fibrinogen Arg448 and Lys448, respectively, at a concentration of 1.3 μM in the presence of 2.5 mmol CaCl2 and 0.5 U/mL thrombin (magnification, ×10 000). Panels C and D represent LSCM of clots prepared from recombinant fibrinogen Arg448 and Lys448, respectively, at 1.3 μM using 5 mmol CaCl2 and 0.5 U/mL thrombin. Scale bars on all micrographs indicate 5 μm. Complete microscopy information can be found in “Methods.”
Elastic properties of the fibrin clot formed from recombinant fibrinogen variants
The compliance J of the samples was measured using the formula J(t) = [6πrx(t)] / F, where r = radius of particle, x = displacement of particle, and F = force. The elastic modulus was then calculated using the following equation below, which is obeyed for a purely elastic material: G′ = 1/J. At the low forces and low-pulse durations used in this work, the viscous component of the fibrin fibers was negligible.
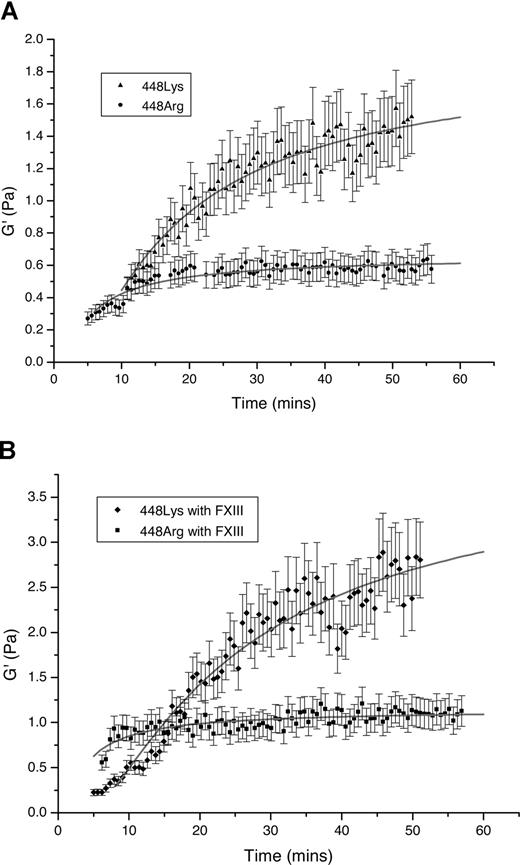
G′ was measured throughout clot formation from 5 to 45 minutes in both variants in the absence and presence of FXIII. Experiments were repeated at least 3 times, and mean values are presented in Figure 3. In clots formed from fibrinogen Arg448, G′ reached a plateau in less than 30 minutes. In contrast, clot stiffness of the Lys448 clots continued to increase after 30 minutes and G′ was almost 3-fold higher than in Arg448 variant (P < .001). In the presence of FXIII, clot stiffness increased markedly, manifested by more than doubling of G′ in both variants. We also measured G′ in clots formed in the presence of FXIII over an extended period of time. In the Arg448 variant, G′ reached plateau at approximately 1 hour, whereas in the Lys448 variant, G′ was still increasing after 3 hours (data not shown).
Rigidity of recombinant BβArg448 and BβLys448 fibrin clots assessed by magnetic tweezers. Shown are G′ values in different time points (up to 45 minutes) of clots prepared from recombinant Arg448 and Lys448 variants (1.3 μM fibrinogen, 2.5 mmol CaCl2, and 0.5 U/mL thrombin) in the absence (A) and presence (B) of FXIII at concentrations of 0.068 μM. Error bars represent SD.
Rigidity of recombinant BβArg448 and BβLys448 fibrin clots assessed by magnetic tweezers. Shown are G′ values in different time points (up to 45 minutes) of clots prepared from recombinant Arg448 and Lys448 variants (1.3 μM fibrinogen, 2.5 mmol CaCl2, and 0.5 U/mL thrombin) in the absence (A) and presence (B) of FXIII at concentrations of 0.068 μM. Error bars represent SD.
Macroscopic and microscopic lysis of clots formed from recombinant fibrinogen
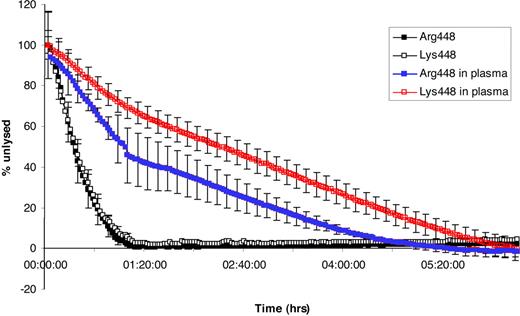
Macroscopic analysis of fibrinolysis by turbidity was largely similar in the 2 variants in 4 repeated experiments (P > .1; Figure 4). Time to 50% lysis was 24 minutes in each variant. Real-time lysis rates were also observed using laser scanning confocal microscopy, and experiments were repeated on 8 and 12 occasions with the Arg448 and Lys448, respectively. The mean lysis speed (± SEM) of clots formed from Arg448 variant was 13.6 μm/min (± 2.1), and 14.3 μm/min (± 2.5) for clots formed from the Lys448 variant (P > .1; data not shown).
Macroscopic lysis velocity of fibrin clots formed from recombinant BβArg448 and BβLys448. Fibrinolysis was assessed directly after completion of polymerization (1.3 μmol/L fibrinogen, 2.5 mmol CaCl2 and 0.5 U/mL thrombin) at 60 minutes, and results are corrected for maximum absorbancy at the time of addition of plasminogen and tPA at 0.05 g/L and 10 nmol/L, respectively. Fibrinolysis was observed every 2 minutes for 7 hours. There was no difference in lysis rates of Lys448 (□) compared with the Arg448 variant (■; P > .1). In contrast, the addition of the recombinant protein to fibrinogen-depleted plasma resulted in prolongation of lysis rate, which was more pronounced with the Lys448 variant (red □) compared with Arg448 (blue □), a difference that was statistically significant (P < .001). Error bars represent SEM.
Macroscopic lysis velocity of fibrin clots formed from recombinant BβArg448 and BβLys448. Fibrinolysis was assessed directly after completion of polymerization (1.3 μmol/L fibrinogen, 2.5 mmol CaCl2 and 0.5 U/mL thrombin) at 60 minutes, and results are corrected for maximum absorbancy at the time of addition of plasminogen and tPA at 0.05 g/L and 10 nmol/L, respectively. Fibrinolysis was observed every 2 minutes for 7 hours. There was no difference in lysis rates of Lys448 (□) compared with the Arg448 variant (■; P > .1). In contrast, the addition of the recombinant protein to fibrinogen-depleted plasma resulted in prolongation of lysis rate, which was more pronounced with the Lys448 variant (red □) compared with Arg448 (blue □), a difference that was statistically significant (P < .001). Error bars represent SEM.
In contrast, there was a significant difference between the 2 variants when analysis was repeated after adding the recombinant proteins to fibrinogen-depleted plasma. As expected, lysis time increased for both variants in plasma, but this increase was significantly more pronounced for the Lys448 compared with the Arg448 variant (P < .001). Results are summarized in Figure 4 and presented as the mean of 6 experiments (± SEM). Time to 50% lysis for Arg448 in plasma was 64 minutes, whereas time was more than double at 156 minutes for Lys448, indicating a significant effect of this polymorphism on fibrinolysis rate in the plasma milieu.
Molecular modeling
Inspection of the vibrational modes computed for the 2 BβArg448 and the 2 BβLys448 models showed that there were no discernible differences between the Arg 448 and the Lys 448 variants in the rigid body motions of the αC domain relative to the B domain.
Examination of the hydrogen bonding patterns around the mutation site revealed differences between the models for the 2 Bβ448 variants (Figure 5). The hydrogen bond between Bβ448 NH1 and BβGlu315 OE2 lasted 94% and 73% of the lifetime of simulations Arg I and Arg II, respectively. The corresponding hydrogen bond from BβLys448 NZ to BβGlu315 OE2 could not occur at all during the either of the Lys448 simulations. The mean distances between these atoms during the simulations were 2.9 Å and 3.0 Å (Arg448 simulation I and II, respectively) and 6.8 Å and 6.5 Å (Lys448 simulation I and II, respectively). It is possible that the “lost” hydrogen bonding from BβLys448 could be compensated for by hydrogen bonding to solvent molecules (not included explicitly in our simulations) as the Lys 448 side-chain is oriented more toward the exterior of the protein than for Arg 448 and thus the mutation would not necessarily destabilize the protein molecule.
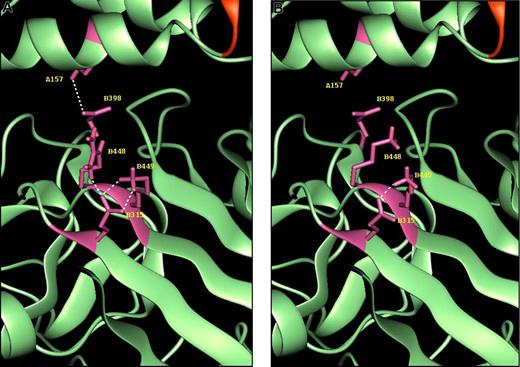
Molecular modeling showing the position of the fibrinogen Bβ448 residue in relation to BβAsp398, BβGlu315, and AαLys157. Modeled on Arg (A) and Lys (B) at position 448. Demonstrated are the potential hydrogen bonds (—) from residue Bβ448 and BβLys449 to BβGlu315 and from BβAsp398 to AαLys157 as observed in our simulations. The proximity of the mutation site to areas integral to fibrin polymerization and calcium binding suggests that the potential weakening of hydrogen bonding in this region may have important effects on fibrin structure and function.
Molecular modeling showing the position of the fibrinogen Bβ448 residue in relation to BβAsp398, BβGlu315, and AαLys157. Modeled on Arg (A) and Lys (B) at position 448. Demonstrated are the potential hydrogen bonds (—) from residue Bβ448 and BβLys449 to BβGlu315 and from BβAsp398 to AαLys157 as observed in our simulations. The proximity of the mutation site to areas integral to fibrin polymerization and calcium binding suggests that the potential weakening of hydrogen bonding in this region may have important effects on fibrin structure and function.
In addition, BβGlu315 was found to be capable of forming hydrogen bonds to BβLys449 in our simulations. In the 2 Arg448 models (I and II) there were 2 hydrogen bonds between these groups: NZ Bβ449 to OE1 Bβ315 and NZ Bβ449 to OE2 Bβ315. The hydrogen bonds to OE2 Bβ315 tended to be more persistent throughout the trajectories. The Arg448 simulations had NZ Bβ449-OE1 Bβ315 surviving 31% and 11% of the lifetime of the simulation (simulations Arg I and Arg II, respectively). The Lys448 variant showed 1% and 16% survival times for this interaction (simulations Lys I and Lys II, respectively); mean distances were 3.9 Å and 4.6 Å (Arg448 simulations I and II) and 6.8 Å and 5.4 Å (Lys448 simulations I and II). The interactions to OE2 Bβ315 persisted for 71% (Arg I) and 64% (Arg II) of the simulation duration and 37% (Lys I) and 23% (Lys II). Mean distances were 3.1 Å and 3.7 Å (Arg I and Arg II simulations, respectively) and 5.1 Å and 4.7 Å (Lys I and Lys II simulations, respectively). Hence the interaction between BβGlu315 and BβLys449 appears to be weakened as an effect of the amino acid variation at Bβ448.
Most crucially, the interaction between BβAsp398 and AαLys157 could also be weakened by the variation at Bβ448. This interaction is thought to be responsible for anchoring the C-terminal region of the β chain to the α chain in the coiled-coil in fibrinogen.24 The interaction survives for 99% and 97% of the simulation time for the 2 Arg448 simulations (Arg I and II). In the Lys448 simulations (Lys I and II), these survival times have been reduced to 15% (NZ A157-OD1 B398) and 41% (NZ A157-OD2 B398) in simulation Lys I and to a mere 1%(NZ A157-OD2 B398) in simulation Lys II, with the hydrogen bond NZ A157-OD1 B398 not occurring in this simulation. Although the simulations failed to show hydrogen bonds between BβArg448 and BβGlu397 or BβAsp398, these residues appeared more mobile in Lys448, suggesting that this variant enhances the flexibility of the molecule in this region by mechanisms that are unclear.
Discussion
We found that a common mutation in the fibrinogen β-chain, Arg448Lys, influenced fibrin structure, clot rigidity, fibrinolysis rate, and conformation of the D-region of fibrinogen by molecular modeling. These effects were observed with variants produced by recombinant technology, strongly suggesting that the amino acid substitution itself is responsible for the observed changes.
The formation of thinner fibrin fibers with BβArgLys448 suggests that lateral aggregation is affected in this variant, which may be caused by several mechanisms. The substitution of Arg with Lys may induce conformational changes in the Bβ-chain of fibrinogen, a hypothesis supported by previous work showing that a similar substitution in other proteins can induce significant changes in the molecule.29,30 In support of this hypothesis, dynamic molecular modeling based on crystallographic coordinates suggest that the Arg-to-Lys substitution could result in the weakening or loss of 3 hydrogen bonds in an area of the fibrinogen molecule close to the proposed b site that may be involved in lateral aggregation.31 The abolition of a hydrogen bond between Bβ448 and Bβ315 secondary to Arg-to-Lys substitution could potentially destabilize Bβ398 and Aα157 interaction in the coiled coil.32 This may result in altered αC domain conformation, in the process affecting lateral aggregation.33 Furthermore, the apparent increased mobility of residues BβGlu397 and BβAsp398 in Lys448, which have been shown to play a critical role in B-b interaction, may influence fibrin polymerization of this variant.34 Together, these conformational changes as indicated by molecular modeling could result in defective B-b or αC domain interactions and consequently impair lateral aggregation.
We demonstrated an increase in clot stiffness in the Lys448 variant even before crosslinking by FXIII. An association between tight clot structure and increased stiffness has been previously shown in dysfibrinogenemic states,35,–37 which is in agreement with our findings. Differences in clot stiffness between the 2 variants were even more pronounced after the addition of FXIII. In both variants, clot stiffness increased greatly in the presence of FXIII. However, stiffness in crosslinked Arg448 fibrin reached a plateau in less than 30 minutes, whereas that of crosslinked Lys448 fibrin was still increasing after 3 hours, with a G′ value almost 3-fold higher compared with Arg448 fibrin. The reason for the continued increase in stiffness of the Lys448 variant (even without FXIII) is unclear and may be related to continued structural rearrangement of the fibrin fibrils with this variant,38 which does not occur, or is less pronounced, with Arg448.
Outside the plasma environment we found lysis rates of the 2 variants to be comparable, despite the observed alterations in clot structure. In contrast, a major difference in fibrinolysis was detected when the recombinant proteins were added to fibrinogen-depleted plasma. This suggests that clot structure/rigidity per se do not necessarily affect fibrinolysis rates, and that the interaction between clot structure and fibrinolysis is more complex than previously thought, especially in the presence of other plasma proteins.22,39,40 The similar lysis rates of clots formed from the 2 variants could be explained by relatively quicker lysis of individual thin fibers “compensating” for the increased number of fibers in the Lys448 variant.22 However, when in plasma, different proteins are crosslinked or attached to the final clot (hence the prolongation in fibrinolysis rates in plasma with both variants), and as a result of the more compact structure of Lys448, it is possible that the composition of these fibrin-associated proteins is different quantitatively or qualitatively, thereby compromising the fibrinolytic process. Differences in the incorporation of α2-plasmin inhibitor into the clot, plasminogen binding, or conversion rates of plasminogen to plasmin could play a role, and therefore investigating the association of clot structure, protein interactions, and lysis rate in relation to BβArg448Lys remains an area for future research. Our data suggest that Arg448Lys polymorphism influences fibrinolysis through yet unidentified plasma factors, and clot structure may play an indirect role.
The association between a compact clot structure and premature coronary artery disease,5,40 together with our findings of a significant effect of fibrinogen Arg448Lys polymorphism on clot structure/fibrinolysis, may help to explain the presence of more extensive coronary artery disease in subjects with the Lys448 variant.3 Moreover, the association between increased clot stiffness and myocardial infarction41 further implicates fibrinogen Lys448 in predisposition to atherothrombotic disease. We also demonstrate in this study a role for plasma components in determining fibrinolysis rates, highlighting an interaction between plasma protein and genetic variations in clotting factors. This further emphasizes the importance of gene-environment interaction in determining fibrinolysis rate and predisposition to atherothrombotic disease.10 It should be noted, however, that others have shown a protective effect of Lys448 variant against myocardial infarction at a young age,11 but the number of subjects studied was too small to draw definitive conclusions. Clearly more studies are needed to fully elucidate the effect of this polymorphism on predisposition to cardiovascular disease.
In summary, the current work demonstrates that the Arg/Lys substitution at position 448 of the Bβ-chain of fibrinogen causes significant changes in clot structure, clot rigidity, and fibrinolysis. Fibrinogen Lys448 is associated with stiffer clots composed of thinner fibers and smaller pores, which are more difficult to lyse, suggesting an increased risk of atherothrombotic disease with this variant. The clinical impact of these findings will depend on the interaction between BβArgLys448 polymorphism and the different genetic makeup of each individual as well as the interaction between these fibrinogen variants and the diverse environmental factors.
R.A. and B.C.B.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors also thank Prof Susan Lord for the generous provision of CHO cells expressing fibrinogen and Dr Chandrasekaran Nagaswami for his kind and expert assistance with the scanning electron microscopy. We also thank Dr Tom Waigh for his contribution to the development of the magnetic tweezers.
R.A. is funded by a Department of Health Clinician Scientist Award. We further wish to express our gratitude to the British Heart Foundation (FS/2000023), the Medical Research Council (G9900904, G0000624), and the National Institutes of Health (HL30954) for their generous support.
National Institutes of Health
Authorship
Contribution: R.A. assisted in study design, supervised the work, analyzed data, and wrote the manuscript; B.C.B.L. completed preliminary work and assisted in study design; K.F.S. assisted in study design, helped with laboratory work and data analysis, and wrote the manuscript; R.H. developed a new methodology for the study of clot stiffness and analyzed the data; S.D. made the fibrinogen constructs and transfected CHO cells; F.P. completed the majority of the laboratory work; R.G. completed the molecular modeling work; R.H.A.-O., S.C., and D.A.M.S. assisted in designing and analyzing the clot stiffness data; J.W.W. assisted in study design and critically reviewed the manuscript; P.J.G. critically reviewed the manuscript and provided general advice during the study; R.A.S.A. assisted in study design, S.A. wrote the paper, performed confocal microscopy analysis, and provided overall supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Grant, MD, FRCP, Academic Unit of Molecular Vascular Medicine, The LIGHT Laboratories, University of Leeds, Leeds, LS2 9JT, United Kingdom; e-mail: p.j.grant@leeds.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal