The clonal expansion of chronic lymphocytic leukemia (CLL) cells requires the interaction with the microenvironment and is under the control of several cytokines. Here, we investigated the effect of IL-15 and IL-21, which are closely related to IL-2 and share the usage of the common γ chain and of its JAK3-associated pathway. We found remarkable differences in the signal transduction pathways activated by these cytokines, which determined different responses in CLL cells. IL-15 caused cell proliferation and prevented apoptosis induced by surface IgM cross-linking. These effects were more evident in cells stimulated via surface CD40, which exhibited increased cell expression of IL-15Rα chain and, in some of the cases, also of IL-2Rβ. IL-21 failed to induce CLL cell proliferation and instead promoted apoptosis. Following cell exposure to IL-15, phosphorylation of STAT5 was predominantly observed, whereas, following stimulation with IL-21, there was predominant STAT1 and STAT3 activation. Moreover, IL-15 but not IL-21 caused an increased phosphorylation of Shc and ERK1/2. Pharmacological inhibition of JAK3 or of MEK, which phosphorylates ERK1/2, efficiently blocked IL-15–induced CLL cell proliferation and the antiapoptotic effect of this cytokine. The knowledge of the signaling pathways regulating CLL cell survival and proliferation may provide new molecular targets for therapeutic intervention.
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in adults, is characterized by the progressive accumulation of CD5+ neoplastic B cells,1,–3 which results from a dynamic balance between cell death and proliferation.3,4 The finding that purified CLL cells do not proliferate and tend to undergo spontaneous apoptosis in vitro suggests that factors inducing CLL cell survival and proliferation might be present in the microenvironment of lymphoid organs or of the bone marrow.5,6 Several cytokines such as the TNF family members BAFF and APRIL,7,8 the chemokine SDF-1,9,10 and interleukin-2 (IL-2)11,12 and IL-1513 stimulate CLL cell proliferation and/or survival in vitro. IL-2 and IL-15 share the usage of the IL-2Rβ (CD122) and of the common γ chain (γc; CD132)14,15 and thus have similar functional effects on IL-2Rβ/γc+ cells.16,17 The IL-2Rβ and γc chains signal through the JAK1 and JAK3 tyrosine kinases, which activate downstream STAT pathways.18,19 In addition to the IL-2Rβ/γc complex, specific IL-2Rα or IL-15Rα20,21 molecules are required for high-affinity binding of each cytokine. Unlike IL-2Rα, IL-15Rα is a high-affinity receptor also when expressed as an isolated chain, and is present on both lymphoid and nonlymphoid cell types.20,21 IL-15Rα is crucial for IL-15 activity, since both IL-15– and IL-15Rα–deficient mice display similar defects in the development of natural killer (NK) cells, of intraepithelial T lymphocytes, and of CD8+ memory-type T cells.22,23 IL-15 gene is expressed in several tissues, and IL-15 can be either secreted in low amounts or displayed as a membrane-bound cytokine by several cell types.24,,,–28 Therefore IL-15 is considered a microenvironmental factor, which supports lymphoid cell homeostasis and survival.29,–31 IL-15 is a growth or survival factor not only for CLL cells13 but also for cells of other lymphoid neoplasias,32,–34 at least in vitro. Moreover, in IL-15–transgenic mice, IL-15 overexpression induces fatal T or NK lymphoproliferative disorders.35
We and others recently demonstrated that IL-21, the last identified member of the IL-2 family,36,–38 is unable to trigger CLL cell proliferation, but rather induces their apoptosis.39,40 This effect is potentiated by cell activation with CD40L,39 with CpG synthetic oligonucleotides, or by BCR cross-linking.40
IL-21 induces the JAK1 and JAK3 and the STAT1, STAT3, and STAT5 tyrosine phosphorylation in CLL B cells.39 Similar early signaling events are also activated by IL-21, IL-2, or IL-15 in normal lymphocytes.41,,–44 In addition, IL-15 or IL-2, through the IL-2Rβ, induces the recruitment of the adaptor protein Shc,45,46 which regulates cell survival and proliferation via the Ras and the downstream MAPK p38 and/or MEK/ERK1/2 pathways.47,48 IL-21R displays structural similarities with the IL-2Rβ36 and weakly induces phosphorylation of Shc.44
To shed light on the mechanisms underlying the opposite effects of IL-15 and IL-21 on CLL cell survival and proliferation, we compared the early signaling events triggered by the 2 cytokines. Indeed, relevant differences in the signaling cascade activated by the 2 cytokines on CLL cells were observed. IL-15 induced Shc, ERK1/2, and STAT5 phosphorylation. IL-21, instead, predominantly activated the JAK1, STAT1, and STAT3 pathways. Pharmacological blockade of MEK or of JAK3 inhibited IL-15–mediated mitogenic and survival signals, thus suggesting that these molecules represent suitable targets to interfere with CLL cell proliferation and survival.
Methods
Cells and culture conditions
This study was approved by the review board of the University of Genoa, Italy. Upon informed consent, according to institutional procedure and in accordance with the Declaration of Helsinki, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood obtained from 20 patients diagnosed with CLL on the basis of clinical and immunophenotypic criteria. All patients were untreated and their prognostic parameters are summarized in Table 1. CD38 and ZAP-70 expression and IgVH gene rearrangement were studied as previously described.39 PBMCs were isolated by density gradient centrifugation (Ficoll; Biochrom, Berlin, Germany) and subjected to a preliminary phenotypic characterization. When residual non–B cells exceeded 10%, B cells were enriched by negative selection with antibody-coated magnetic beads (CD2-beads; Dynal, Oslo, Norway) to obtain a more than 95% pure CD19+/CD5+ B-cell population.
Prognostic markers of the CLL cases studied
| Case no. . | CD38, % . | ZAP70, % . | IgVH . |
|---|---|---|---|
| 1 | 53 | 24 | U |
| 2 | 67 | 64 | U |
| 3 | 47 | 99 | U |
| 4 | 37 | 90 | U |
| 5 | 12 | 32 | M |
| 6 | 5 | 25 | M |
| 7 | 30 | 55 | U |
| 8 | 3 | 5 | M |
| 9 | 96 | 89 | U |
| 10 | 9 | 95 | M |
| 11 | 98 | 3 | M |
| 12 | 2 | 1 | M |
| 13 | 57 | 63 | U |
| 14 | 1 | 1 | M |
| 15 | 1 | 11 | M |
| 16 | 3 | 15 | U |
| 17 | 1 | 16 | M |
| 18 | 1 | 1 | M |
| 19 | 6 | 52 | U |
| 20 | 2 | 20 | M |
| Case no. . | CD38, % . | ZAP70, % . | IgVH . |
|---|---|---|---|
| 1 | 53 | 24 | U |
| 2 | 67 | 64 | U |
| 3 | 47 | 99 | U |
| 4 | 37 | 90 | U |
| 5 | 12 | 32 | M |
| 6 | 5 | 25 | M |
| 7 | 30 | 55 | U |
| 8 | 3 | 5 | M |
| 9 | 96 | 89 | U |
| 10 | 9 | 95 | M |
| 11 | 98 | 3 | M |
| 12 | 2 | 1 | M |
| 13 | 57 | 63 | U |
| 14 | 1 | 1 | M |
| 15 | 1 | 11 | M |
| 16 | 3 | 15 | U |
| 17 | 1 | 16 | M |
| 18 | 1 | 1 | M |
| 19 | 6 | 52 | U |
| 20 | 2 | 20 | M |
*U indicates unmutated, M, mutated IgVH genes; Unmutated had less than 2% mutations; mutated had more than 2%.
Murine L cells stably transfected with the human CD40L (CD154) cDNA49,50 were cultured in RPMI 1640 (Cambrex Bio Science, Verviers, Belgium) supplemented with penicillin/streptomycin, l-glutamine, and 10% heat-inactivated FCS (Cambrex Bio Science). Isolated CLL B cells were cocultured with CD40L-transfected cells in 24-well plates for 72 hours.50
Immunofluorescence analysis of cell-surface antigens
Flow cytometric analyses of total CLL PBMCs and of B cell–enriched fractions were performed by 2-color immunofluorescence. Briefly, 1 × 105 cells/sample were incubated at 4°C, for 30 minutes with the following mAbs: FITC-labeled anti-CD19, -CD4, -CD8, -CD3, -CD2 -CD23, or -CD122 mAbs and PE-labeled anti-CD3, -CD5, -CD38, (Immunotools, Friesoythe, Germany), or – IL-21R (R&D Systems, Minneapolis, MN). FITC- and PE-labeled isotype-matched Igs were used as negative controls. After 2 washes with 2% FCS in PBS, cells were analyzed by flow cytometry (FACScan; BD Biosciences, San Jose, CA). IL-15Rα expression was determined through indirect staining with a specific anti–IL-15Rα Ab (Santa Cruz Biotechnology, Santa Cruz, CA) as primary antibody and antigoat FITC as secondary Ab.
Reverse-transcription–polymerase chain reaction analysis of IL-2Rβ, IL-15Rα, and γc mRNA expression
Total RNA was isolated by the NucleoSpin RNA II kit (Macherey-Nagel, Duren, Germany) according to the manufacturer's instructions. Total RNA (1 μg) was then reverse-transcribed using the SuperScript II Reverse transcriptase (Invitrogen, Milano, Italy) in a final volume of 20 μL. cDNA (2 μL) was separately amplified, in a final volume of 25 μL, with 2.5 IU Taq polymerase (Genecraft, Lüdinghausen, Germany), in the presence of 1 μM of the primers specific for IL-2Rβ, IL-15Rα, or the γc51 , and for the housekeeping gene β-actin. The amplifications were carried out in a polymerase chain reaction (PCR) Sprint thermal cycler (Hybaid, Ashford, United Kingdom) for 30 cycles or 25 cycles for β-actin (15 seconds at 94°C, 15 seconds at 60°C, 30 seconds at 72°C) with a final extension at 72°C for 5 minutes. PCR products (10 μL) were then analyzed on 1% to 2% agarose gel containing ethidium bromide.
For quantitative reverse transcription (RT)-PCR analysis, the following primers were used: IL-2Rβ upper, GGTGCGATGGAGGGTGATGG and lower, GTAGTGGGAGGCTTGGGAGATT; IL-15Rα upper, CCAGCCGCCAGGTGTGTATC and lower, GGTTTCCCCGAGTTTCATAGGTG; and RNA polymerase II A subunit upper, GACAATGCAGAGAAGCTGG and lower, GCAGGAAGACATCATCATCC. Amplification was carried out in an iCycler instrument (Biorad, Hercules CA) using the SYBRgreen super mix system (Biorad) according to the manufacturer's protocol. Relative quantification of mRNA was calculated by the delta-delta Ct method.
Cell treatments and determination of apoptosis, mitochondrial depolarization, and DNA synthesis
Resting or CD40-activated CLL B cells were cultured with recombinant human IL-21 (rIL-21; Biosource, Camarillo, CA) or IL-15 (PeproTech EC, London, United Kingdom) (concentration range: 1 to 80 ng/mL) and then tested for apoptosis or DNA synthesis. In selected experiments, rIL-21 was used together with rIL-15 to determine potential synergism or inhibition between the 2 cytokines. Some experiments were performed in presence of 10 μg/mL goat anti–human IgM antiserum (Caltag, Burlingame, CA) to enhance the apoptotic rate of CLL B cells. The MEK inhibitor UO126, the JAK3 inhibitor AG490, and the JAK-inhibitor I (all from Calbiochem/Merck, Darmstadt, Germany) were diluted in DMSO and used at concentrations ranging from 1 to 30 μM (for UO126 and AG490) or from 0.2 to 0.6 μM (for JAK-inhibitor I). Appropriate controls using DMSO were always included. In another set of blocking experiments, azide-free antibodies directed against the anti–IL-2Rβ (CD122; Immunotools) or the anti–IL-2Rγ (CD132; BD Biosciences) were added at 1 or 10 μg/mL, together with rIL-15.
Apoptosis was determined after 4 days of culture by annexin-V–FITC and propidium iodide (PI) double staining (Bender MedSystem, Vienna, Austria), by fluorescence-activated cell sorting (FACS) analysis according to the manufacturer's instructions. Changes in the inner mitochondrial membrane potential (Δψm) were evaluated by staining CLL B cells at 37°C for 15 minutes with 40 nM DiOC6 (3,3′ dihexylocarbocyanine iodide; Sigma, Milano Italy), a green fluorescent dye, as described.39 For the determination of cell proliferation, cells were cultured at 105 cells/well in triplicate wells of 96-well microtiter plates, and DNA synthesis was determined by 3H-thymidine uptake, as described.51
Western blotting and densitometric analyses
For the analysis of phosphorylated proteins, 107 CLL cells were incubated 10 and 20 minutes at 37°C with or without 80 ng/mL rIL-21 (Biosource) in 1 mL RPMI 1640 supplemented with 10% FCS. Cells, washed twice in ice-cold PBS containing 400 μM Na orthovanadate, 5 mM EDTA, and 1 mM Na pyrophosphate, were then solubilized in 100 μL lysis buffer containing 1 mM Na orthovanadate, incubated 30 minutes on ice and centrifuged 10 minutes at 10 000g in a microfuge. Of the lysates, 20 μL was resolved under reducing conditions by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting using the following primary Abs: rabbit anti–phospho-STAT1 (pY701; Cell Signaling Technology, Beverly, MA), mouse anti-STAT1 (Santa Cruz Biotechnology), mouse anti–phospho-STAT3 (pY705; Upstate-Millipore, Lake Placid, NY), mouse anti–phospho-STAT5 (pY694; BD Biosciences), rabbit anti–phospho-JAK1 (pY1022/1023), rabbit anti–phospho-JAK2 (pY1007/1008) (both from Biosource), rabbit anti–phospho-JAK3 (pY980; Santa Cruz Biotechnology), mouse anti–phospho-ERK1/2 (Santa Cruz Biotechnology), rabbit anti–phospho-Shc (Cell Signaling Technology), and rabbit anti–β-actin (Sigma). After washings with T-TBS, the blots were then incubated in the presence of the appropriate secondary HRP-conjugated Abs. Bands were visualized by the enhanced chemiluminescence (ECL) system (GE Healthcare, Little Chalfont, United Kingdom).
Band intensity was evaluated by densitometric analysis with the KODAK ID image analysis software (Kodak, Rochester, NY) and expressed as mean-fold change in cytokine-treated relative to untreated CLL B cells. Statistical significance was determined using the Student t test or the Mann-Whitney test, as appropriate, and P values of .05 or less were considered statistically significant.
Results
IL-15 but not IL-21 induces proliferation and survival of CLL B cells
To assess the mitogenic activity of IL-15, we tested 3H-thymidine uptake by purified B cells from 8 different untreated CLL patients in the presence of various amounts of cytokine. IL-15 induced the proliferation of freshly isolated CLL cells in all cases tested, although relatively high concentrations were required to detect substantial effects (a representative case is shown in Figure 1). The mitogenic effect of IL-15 was enhanced when CLL cells were previously activated via CD40 by coculture with CD40L-transfected murine fibroblasts (Figure 1A). A similar behavior was observed for the cells of all cases studied (nos. 1, 11, 12, 13, 14, 15, 18, and 20; data not shown and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), irrespective of their IgVH mutational status and the presence or absence of other risk parameters, such as CD38 and ZAP70 (Table 1). By contrast, IL-21 was unable to induce proliferation of both resting and CD40-activated CLL cells and inhibited the IL-15–driven proliferation when the 2 cytokines were present in equimolar amounts (Figure 1A and de Totero et al39 ).
IL-15 and IL-21 show opposite effects on DNA synthesis and CLL cell survival. (A) Resting or CD40-activated CLL cells were cultured in triplicate wells of 96-well plates in the presence of different concentrations of IL-15 for 48 hours and then pulsed with 3H-thymidine (1 μCi [0.037 MBq]/well) for an additional 18 hours of culture. DNA synthesis by cells cultured in the presence of IL-21 (50 ng/mL) both alone and in combination with IL-15 (50 ng/mL) is also shown. *P < .05 with respect to IL-15 alone. Error bars represent SD. (B) Freshly isolated CLL cells were cultured for 4 days with or without IL-15, IL-21, or a combination of the 2 cytokines (all at 50 ng/mL), and then apoptosis was assessed by the DiOC6 staining method, which determines mitochondrial depolarization. The percentage of apoptotic cells is indicated in each panel.
IL-15 and IL-21 show opposite effects on DNA synthesis and CLL cell survival. (A) Resting or CD40-activated CLL cells were cultured in triplicate wells of 96-well plates in the presence of different concentrations of IL-15 for 48 hours and then pulsed with 3H-thymidine (1 μCi [0.037 MBq]/well) for an additional 18 hours of culture. DNA synthesis by cells cultured in the presence of IL-21 (50 ng/mL) both alone and in combination with IL-15 (50 ng/mL) is also shown. *P < .05 with respect to IL-15 alone. Error bars represent SD. (B) Freshly isolated CLL cells were cultured for 4 days with or without IL-15, IL-21, or a combination of the 2 cytokines (all at 50 ng/mL), and then apoptosis was assessed by the DiOC6 staining method, which determines mitochondrial depolarization. The percentage of apoptotic cells is indicated in each panel.
Since unstimulated CLL cells are prone to spontaneous apoptosis in vitro, we studied whether the 2 cytokines had opposite effects on cell survival. Figure 1B shows a representative experiment on a CLL case. The cells displayed a 64% spontaneous apoptosis after 4-day culture, and this value was lowered to 35% in the presence of IL-15. In contrast, IL-21 slightly increased the apoptotic rate to 70%, and counteracted the antiapoptotic effects of IL-15 by bringing the apoptotic cell rate back to 55%, when the 2 cytokines were added to the culture in equimolar amounts. Similar data were consistently found on the cells from 5 additional cases (nos. 8, 9, 10, 12, and 16; data not shown) treated in the same fashion.
Expression of IL-2/IL-15R on CLL B cells and role of IL-2Rβ/γ in IL-15–mediated mitogenic and antiapoptotic effects
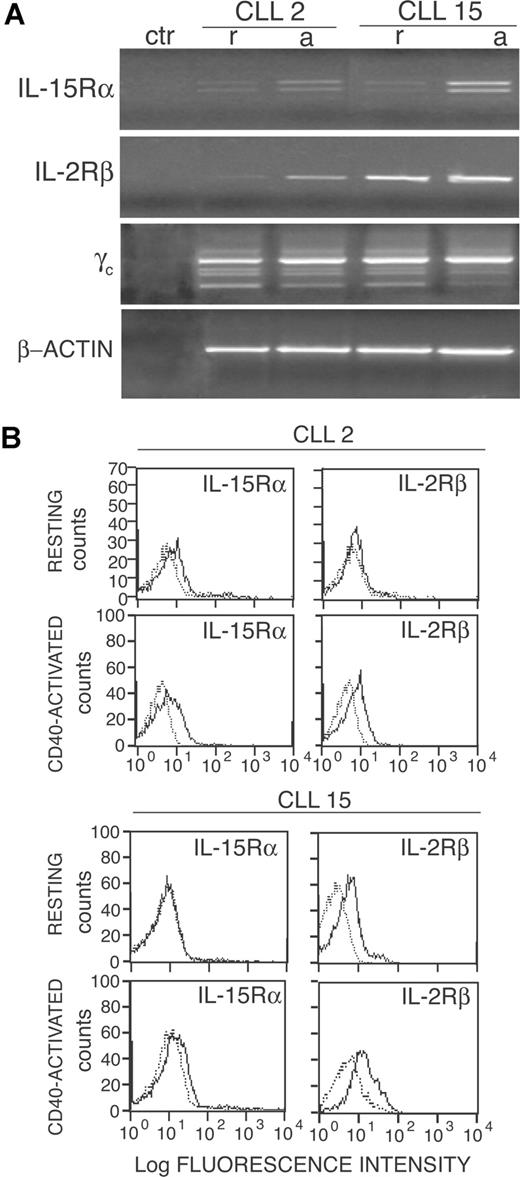
The increased sensitivity of CD40-activated CLL cells to the IL-15 mitogenic effects also could be related to an increased expression of the private IL-15Rα and/or of the IL-2Rβ. Indeed, IL-15Rα mRNA, which was barely detectable in resting cells of all (6/6) cases tested (nos. 2, 5, 6, 7, 9, and 15) was up-regulated after CD40 activation (2 representative cases in Figure 2A). The 2 amplification products detected by RT-PCR analysis in CLL cells correspond to 2 mRNA isoforms of IL-15Rα, generated by the alternative splicing of exon 3.20 These data were also confirmed by quantitative RT-PCR analyses, which showed an average increase of IL-15Rα mRNA of 64-fold in CD40-activated CLL cells of 6 patients (range from 24- to 28-fold, data not shown). Consistent with these findings, a slight up-regulation of IL-15Rα surface protein was also observed in CD40-activated cells (6/6) by the use of flow cytometry (Figure 2B shows 2 representative cases). The IL-2Rβ and the γc genes were instead constitutively expressed in all cases tested, although the IL-2Rβ mRNA and protein appeared to increase in some cases (3/6) upon CD40 triggering (2 representative cases are shown in Figure 2). An average increase of IL-2Rβ mRNA expression of approximately 9-fold was confirmed also by quantitative RT-PCR (range from 2- to 24-fold; data not shown).
Activation via CD40 up-regulates IL-2Rβ and IL-15Rα expression by CLL cells. (A) RT-PCR analysis of IL-15Rα, IL-2Rβ, and γc mRNA expression on freshly purified (r) or on CD40-activated (a) CLL cells. A negative control (ctr) without RNA was also included. Amplification of the same cDNAs with β-actin–specific primers is also shown. (B) Immunofluorescence analysis of IL-2Rβ and IL-15Rα expression (heavy line) on freshly isolated or CD40-activated CLL cells from 2 representative cases. Light lines represent background fluorescence obtained by the use of irrelevant isotype-matched control Ig.
Activation via CD40 up-regulates IL-2Rβ and IL-15Rα expression by CLL cells. (A) RT-PCR analysis of IL-15Rα, IL-2Rβ, and γc mRNA expression on freshly purified (r) or on CD40-activated (a) CLL cells. A negative control (ctr) without RNA was also included. Amplification of the same cDNAs with β-actin–specific primers is also shown. (B) Immunofluorescence analysis of IL-2Rβ and IL-15Rα expression (heavy line) on freshly isolated or CD40-activated CLL cells from 2 representative cases. Light lines represent background fluorescence obtained by the use of irrelevant isotype-matched control Ig.
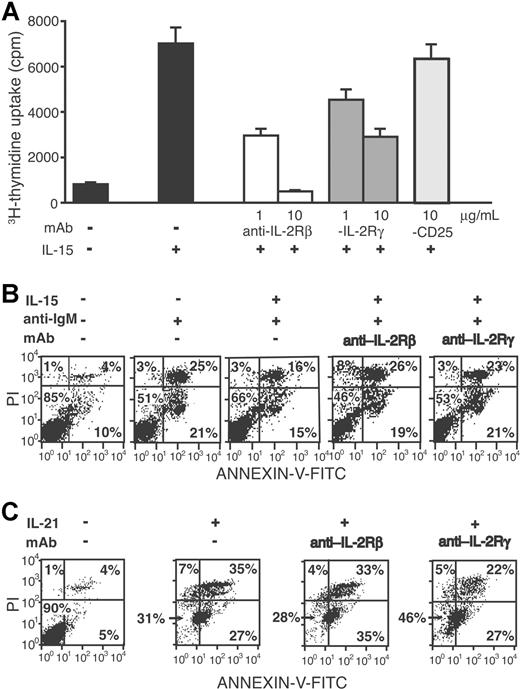
To verify the role of the IL-2Rβ and γc in the IL-15–induced proliferation, we performed blocking experiments using specific antibodies. As shown in Figure 3A, anti–IL-2Rβ or anti-γc significantly inhibited the IL-15–driven proliferation.
Anti–IL-2Rβ or anti–IL-2Rγ antibodies inhibit the mitogenic activity and antiapoptotic effect of IL-15. (A) CD40-activated CLL cells (case no. 1) were cultured in triplicate wells of 96-well plates in the presence of IL-15 (50 ng/mL) with or without anti–IL-2Rβ or anti–IL-2Rγ antibodies or with an irrelevant antibody (anti-CD25) for 48 hours and then pulsed with 3H-thymidine for additional 18 hours. Error bars represent SD. (B) CLL cells from a representative patient (no. 12) were cultured for 4 days in the presence or absence of anti-IgM antiserum, IL-15 (50 ng/mL), and anti–IL-2Rβ or anti–IL-2Rγ antibody (5 μg/mL), as indicated. Apoptosis was evaluated by 2-color fluorescence analysis of propidium iodide (PI) and annexin-V staining. (C) CD40-activated CLL cells (no. 12) were cultured in the presence or absence of IL-21 (50 ng/mL) and anti–IL-2Rβ or anti–IL-2Rγ antibody (5 μg/mL), as indicated and apoptosis was evaluated as described in panel B. The numbers on the plots are the percentages of total cells in the respective quadrants.
Anti–IL-2Rβ or anti–IL-2Rγ antibodies inhibit the mitogenic activity and antiapoptotic effect of IL-15. (A) CD40-activated CLL cells (case no. 1) were cultured in triplicate wells of 96-well plates in the presence of IL-15 (50 ng/mL) with or without anti–IL-2Rβ or anti–IL-2Rγ antibodies or with an irrelevant antibody (anti-CD25) for 48 hours and then pulsed with 3H-thymidine for additional 18 hours. Error bars represent SD. (B) CLL cells from a representative patient (no. 12) were cultured for 4 days in the presence or absence of anti-IgM antiserum, IL-15 (50 ng/mL), and anti–IL-2Rβ or anti–IL-2Rγ antibody (5 μg/mL), as indicated. Apoptosis was evaluated by 2-color fluorescence analysis of propidium iodide (PI) and annexin-V staining. (C) CD40-activated CLL cells (no. 12) were cultured in the presence or absence of IL-21 (50 ng/mL) and anti–IL-2Rβ or anti–IL-2Rγ antibody (5 μg/mL), as indicated and apoptosis was evaluated as described in panel B. The numbers on the plots are the percentages of total cells in the respective quadrants.
The possible protective effect of IL-15 on CLL cell apoptosis induced by surface IgM cross-linking52 was then addressed. Indeed, treatment with anti-μ Abs increased the CLL cell apoptosis (from 15% to 49% in a representative case shown in Figure 3B), while 50 ng/mL IL-15 partially inhibited this effect (from 49% to 34%, Figure 3B). Such antiapoptotic effect was reversed by the addition of anti–IL-2Rβ or anti-γc antibodies (53% and 47% of apoptotic cells, respectively; Figure 3B). Similar results were obtained with 3 additional cases (nos. 1, 10, 16; data not shown), thus demonstrating that both IL-15–induced proliferation and its antiapoptotic effects require IL-2Rβ and IL-2Rγ signaling.
The proapoptotic effect of IL-21 was also partially inhibited by anti–IL-2Rγ antibody (from 69 to 54%; Figure 3C), whereas no inhibition was observed by anti–IL-2Rβ antibody (a representative experiment of the 4 cases studied is shown in Figure 3C). The latter finding was expected since IL-2Rβ is not a component of the IL-21R complex.
Differential activation of the JAK/STAT pathways by IL-21 and IL-15
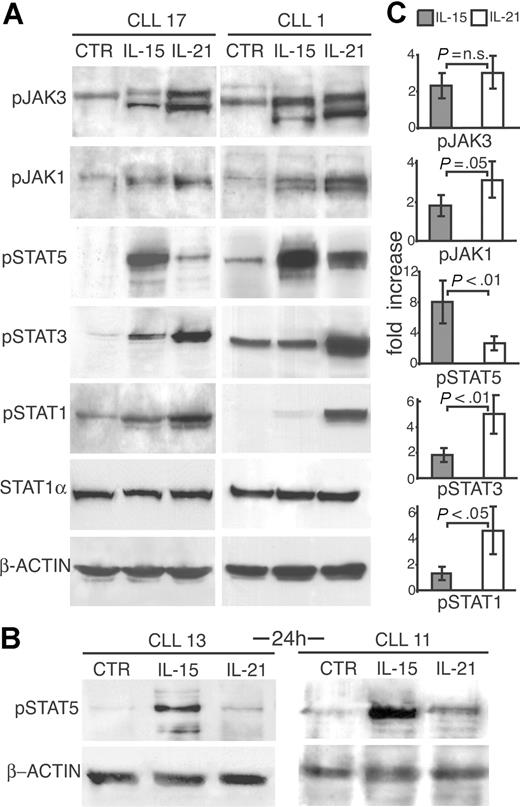
To clarify the mechanisms involved in the opposite effects exerted by IL-15 and IL-21 on CLL cells, we studied the early signaling events triggered by these 2 cytokines. CD40-activated CLL B cells were stimulated for different time intervals with IL-15 or IL-21 (both at 80 ng/mL) and tested for phosphorylation of JAK/STAT molecules by Western blotting using specific anti–phospho-protein antibodies. Both cytokines activated JAK3 phosphorylation. However, IL-15 induced stronger STAT5 phosphorylation than IL-21 both at 10-minute (Figure 4A,C) and 20-minute (not shown) time points in cells from 2 representative CLL cases (nos. 1 and 17), which differed in their main prognostic markers. Moreover, STAT5 phosphorylation induced by IL-15 was still evident after 24 hours from IL-15 addition to the cultures (Figure 4B). On the other hand, IL-21 induced a stronger STAT1 and STAT3 phosphorylation than IL-15, as also indicated by normalization to unphosphorylated STAT1 or to β-actin levels (Figure 4A). In addition, JAK1 phosphorylation was also more evidently increased by IL-21 than by IL-15. Data similar to those reported in the figure were obtained in 5 additional cases (nos. 3, 11, 13 19, and 20; data not shown and Figure S2) and were independent of the types of prognostic markers expressed by the leukemic clone. Densitometric analyses confirmed that IL-15 and IL-21 show significant differences in their ability to activate STAT molecules in CLL cells (Figure 4C), in accordance with different functional effects.
Comparison of JAK/STAT signaling pathways activated by IL-15 or by IL-21 shows remarkable differences. (A) CLL cells from 2 representative cases were stimulated with IL-15 or IL-21 (80 ng/mL) or medium only (CTR) for 10 minutes, lysed in appropriate buffers and analyzed by Western blotting using mAbs specific for tyrosine-phosphorylated (JAK1, JAK3, STAT1, STAT3, and STAT5) or unphosphorylated (STAT1) forms of signal transducer proteins, or for β-actin. (B) CLL cells stimulated by IL-15 for 24 hours show enhanced STAT5 phosphorylation compared with IL-21–treated or untreated cells. (C) Densitometric analysis of Western blots to quantify p-JAK1 and p-JAK3 and p-STAT1, p-STAT3, and p-STAT5 levels following treatment with IL-21 or IL-15. Densitometric measurements of phosphoproteins were normalized to unphosphorylated STAT1 or to β-actin levels, and expressed as fold of increase relative to the untreated control. The mean values (± SD) of 7 different patients and statistical significance are shown.
Comparison of JAK/STAT signaling pathways activated by IL-15 or by IL-21 shows remarkable differences. (A) CLL cells from 2 representative cases were stimulated with IL-15 or IL-21 (80 ng/mL) or medium only (CTR) for 10 minutes, lysed in appropriate buffers and analyzed by Western blotting using mAbs specific for tyrosine-phosphorylated (JAK1, JAK3, STAT1, STAT3, and STAT5) or unphosphorylated (STAT1) forms of signal transducer proteins, or for β-actin. (B) CLL cells stimulated by IL-15 for 24 hours show enhanced STAT5 phosphorylation compared with IL-21–treated or untreated cells. (C) Densitometric analysis of Western blots to quantify p-JAK1 and p-JAK3 and p-STAT1, p-STAT3, and p-STAT5 levels following treatment with IL-21 or IL-15. Densitometric measurements of phosphoproteins were normalized to unphosphorylated STAT1 or to β-actin levels, and expressed as fold of increase relative to the untreated control. The mean values (± SD) of 7 different patients and statistical significance are shown.
IL-15 but not IL-21 triggers Shc and ERK1/2 phosphorylation
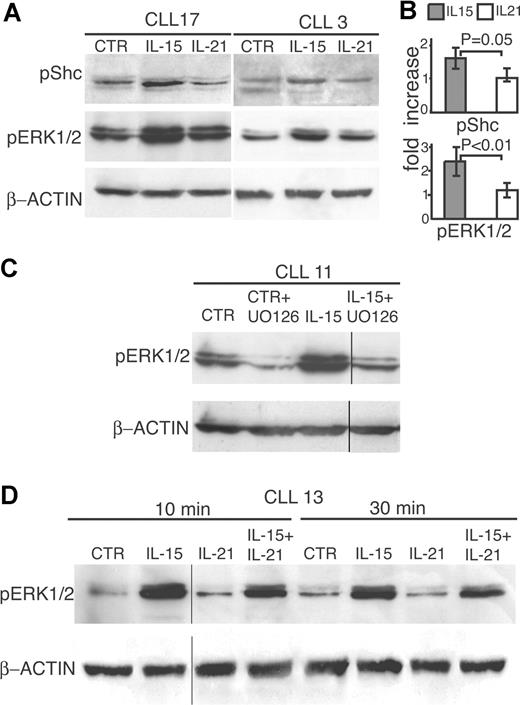
Upon ligand binding by the IL-2Rβ/γc complex, IL-2Rβ recruits the adaptor molecule Shc through a phosphotyrosine docking site at position 338 in its cytoplasmic domain.45,46 Since IL-21R shares structural homologies with IL-2Rβ, including an intracellular domain with potentially phosphorylated tyrosine residues, we tested whether IL-21 and IL-15 induce Shc phosphorylation in CLL cells. Experiments performed on CD40-activated CLL cells showed that treatment with IL-15 resulted in increased phosphorylation of the p50 Shc isoform, as detected by Western blot (Figure 5A,B). Two representative tests on cases, nos. 3 and 17, of the 5 showing consistent results, are shown in Figure 5A. By contrast, no significant increase in Shc phosphorylation was observed after IL-21 stimulation (Figure 5A,B), even at different time points (not shown).
IL-15 but not IL-21 increases Shc and ERK1/2 phosphorylation. (A) CD40-activated CLL cells from 2 representative cases were stimulated with IL-15 or IL-21 (80 ng/mL) or medium (CTR) for 10 minutes, lysed in appropriate buffers, and analyzed by Western blotting using antibodies specific for phosphorylated forms of Shc or of ERK1/2 or for β-actin. (B) Densitometric analysis of Western blots performed on different cases to quantify p-Shc (5 cases) and p-ERK1/2 (9 cases) levels following treatment with IL-21 or IL-15. The data are expressed as normalized average fold of increase relative to the untreated control, as for Figure 4B. Error bars represent SD. (C) The MEK inhibitor UO126 blocks IL-15–mediated phosphorylation of ERK1/2 in CD40-activated CLL cells. Cells were pretreated with UO126 (10 μM) or DMSO (CTR) for 2 hours before the addition of IL-15 (80 ng/mL). After an additional 10 minutes of culture, cells were processed for Western blot analysis. Vertical lines have been inserted to indicate a repositioned gel lane. (D) Freshly isolated CLL cells from a representative case were stimulated for 10 or 20 minutes with IL-15 and/or IL-21, lysed, and analyzed by Western blotting using antibodies specific for phosphorylated ERK1/2 or for β-actin. Vertical lines have been inserted to indicate repositioned gel lanes.
IL-15 but not IL-21 increases Shc and ERK1/2 phosphorylation. (A) CD40-activated CLL cells from 2 representative cases were stimulated with IL-15 or IL-21 (80 ng/mL) or medium (CTR) for 10 minutes, lysed in appropriate buffers, and analyzed by Western blotting using antibodies specific for phosphorylated forms of Shc or of ERK1/2 or for β-actin. (B) Densitometric analysis of Western blots performed on different cases to quantify p-Shc (5 cases) and p-ERK1/2 (9 cases) levels following treatment with IL-21 or IL-15. The data are expressed as normalized average fold of increase relative to the untreated control, as for Figure 4B. Error bars represent SD. (C) The MEK inhibitor UO126 blocks IL-15–mediated phosphorylation of ERK1/2 in CD40-activated CLL cells. Cells were pretreated with UO126 (10 μM) or DMSO (CTR) for 2 hours before the addition of IL-15 (80 ng/mL). After an additional 10 minutes of culture, cells were processed for Western blot analysis. Vertical lines have been inserted to indicate a repositioned gel lane. (D) Freshly isolated CLL cells from a representative case were stimulated for 10 or 20 minutes with IL-15 and/or IL-21, lysed, and analyzed by Western blotting using antibodies specific for phosphorylated ERK1/2 or for β-actin. Vertical lines have been inserted to indicate repositioned gel lanes.
It is known that phosphorylated Shc recruits Grab2 and Sos, leading to activation of MEK through the Raf/Ras pathway. Upon activation, MEK in turn phosphorylates ERK1/2. Therefore, we studied the effects of the 2 cytokines on ERK1/2 phosphorylation in CD40-activated CLL cells. These cells already exhibited a baseline ERK1/2 phosphorylation, which was further increased by cell exposure to IL-15 (Figure 5A). In contrast, IL-21 did not induce significant changes of ERK1/2 phosphorylation (Figure 5A, case nos. 17 and 3). Similar findings were obtained on 7 additional CLL cases (nos. 2, 4, 11, 13, 18, 19, and 20; data not shown and Figure S2) and were confirmed by statistical analyses of densitometric data (Figure 5B), which showed a significantly higher ability of IL-15 to activate ERK1/2 phosphorylation. In addition, the ability of IL-15 to activate the ERK1/2 pathway was similar in CLL with favorable or unfavorable prognostic markers. Pretreatment of CLL cells with the MEK inhibitor UO126 blocked the ERK1/2 phosphorylation triggered by IL-15, thus confirming the role of MEK (Figure 5C).
Since the levels of pERK1/2 were relatively high in CD40-activated cells, the same analysis was performed on CLL cells that were not preactivated via CD40. These CLL cells showed low constitutive ERK1/2 phosphorylation, which was strongly increased in response to IL-15 but not to IL-21 stimulation (Figure 5D). The ability of IL-15 to augment ERK1/2 phosphorylation in CLL B cells may support its antiapoptotic and mitogenic effects. When IL-21 and IL-15 were added simultaneously at equimolar amounts, the resulting ERK1/2 phosphorylation was reduced (approximately 40% as detected by densitometric analyses) compared with IL-15 treatment alone (Figure 5D).
Pharmacological blockade of MEK or of JAK3 inhibits proliferative and survival signals delivered by IL-15 to CLL B cells
In view of the involvement of the JAK3/STAT5 and MEK/ERK1/2 pathways in IL-15 signaling, we investigated whether pharmacological inhibitors of these 2 pathways could block IL-15–mediated proliferation and survival of CD40-activated CLL cells.
The mitogenic effect of IL-15 on CLL B cells was blocked by the MEK inhibitor UO126, in a dose-dependent manner, and a complete inhibition was observed at a 10-μM concentration (Figure 6A). Also the JAK-inhibitor AG490 significantly blocked the IL-15 mitogenic effect with a complete inhibition at 30 μM, while DMSO (used as solvent for inhibitors) showed no effect, even at the maximal concentration used (Figure 6A).
Pharmacological inhibitors of JAK3 or of MEK kinases block IL-15–mediated proliferative and antiapoptotic effects. (A) CD40-activated CLL cells were cultured in triplicate wells of 96-well plates in the presence of IL-15 (50 ng/mL) and of different concentrations of the MEK inhibitor UO126 or of the JAK3 inhibitor AG490 for 48 hours, and then pulsed with 3H-thymidine (1 μCi [0.037 MBq]/well) for an additional 18 hours of culture. Controls using the maximal DMSO concentration used as solvent for the inhibitors were also included. (B) Apoptosis was measured by annexin-V/PI staining of CD40-activated CLL cells after 72 hours of culture in the presence or absence of IL-15 (50 ng/mL) with our without AG490 (30 μM) or UO126 (10 μM). (C) CD40-activated CLL cells were left untreated or treated with IL-21 (50 ng/mL) for 72 hours in the presence or absence of AG490 (30 μM), UO126 (10 μM), or JAK-inhibitor I (0.2 to 0.6 μM) and apoptosis was detected by annexin-V/PI staining. Error bars represent SD.
Pharmacological inhibitors of JAK3 or of MEK kinases block IL-15–mediated proliferative and antiapoptotic effects. (A) CD40-activated CLL cells were cultured in triplicate wells of 96-well plates in the presence of IL-15 (50 ng/mL) and of different concentrations of the MEK inhibitor UO126 or of the JAK3 inhibitor AG490 for 48 hours, and then pulsed with 3H-thymidine (1 μCi [0.037 MBq]/well) for an additional 18 hours of culture. Controls using the maximal DMSO concentration used as solvent for the inhibitors were also included. (B) Apoptosis was measured by annexin-V/PI staining of CD40-activated CLL cells after 72 hours of culture in the presence or absence of IL-15 (50 ng/mL) with our without AG490 (30 μM) or UO126 (10 μM). (C) CD40-activated CLL cells were left untreated or treated with IL-21 (50 ng/mL) for 72 hours in the presence or absence of AG490 (30 μM), UO126 (10 μM), or JAK-inhibitor I (0.2 to 0.6 μM) and apoptosis was detected by annexin-V/PI staining. Error bars represent SD.
In addition, the antiapoptotic effect of IL-15 on anti-IgM–stimulated CLL cells was efficiently counteracted by UO126 at the same 10-μM concentration (Figure 6B) capable of completely inhibiting proliferation. AG490 at the concentration of 30 μM had a weaker effect on the IL-15 antiapoptotic effect (Figure 6B). Consistent findings were recorded in 3 additional CLL cases (nos. 2, 3, and 19). Altogether, these data indicate that the MEK/ERK1/2 pathway is involved in both the antiapoptotic and the mitogenic effects of IL-15, and that the JAK3/STAT5 pathway seems more relevant to the mitogenic effect than to the protection from apoptosis. By contrast, UO126 did not inhibit, but rather enhanced, the proapoptotic effect of IL-21 on CD40-activated CLL B cells, and AG490 had only a limited inhibitory effect (Figure 6C). Nonetheless the JAK-inhibitor I efficiently blocked STAT1 phosphorylation (Figure S3) and significantly inhibited apoptosis induced by IL-21 in a dose-dependent fashion (Figure 6C). Inhibition was reproducible, as consistent data were obtained in additional CLL cases (nos. 3, 12, and 19; data not shown), suggesting that the JAK1/STAT1 pathway is involved in IL-21 proapoptotic effect.
Discussion
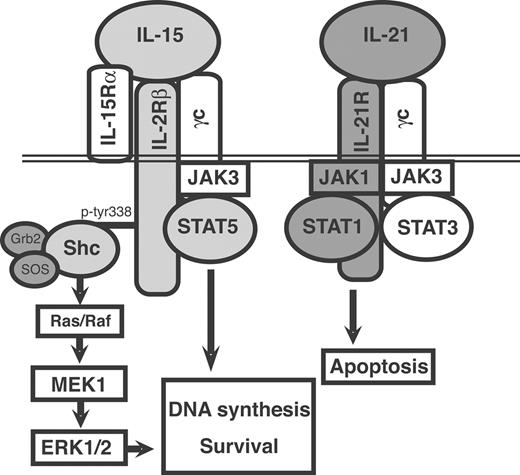
This study demonstrates that IL-15 and IL-21 exert opposite effects on CLL cell proliferation and survival, and that there are remarkable differences in the signal transduction pathways activated by the 2 cytokines, as summarized in Figure 7.
Summary of the signaling pathways predominantly activated by IL-21 or by IL-15 in activated CLL cells and of their biologic effects.
Summary of the signaling pathways predominantly activated by IL-21 or by IL-15 in activated CLL cells and of their biologic effects.
The mitogenic effect of IL-15 was potentiated by prior stimulation of the cells via CD40, a finding that may be, in part, related to the up-regulation of IL-15Rα and IL-2Rβ expression in CLL cells. Previous data suggested that CD40L is an important microenvironmental stimulus for CLL cell survival and expansion since activation via CD40 induces expression of the antiapoptotic protein survivin in vitro. Survivin+ CLL cells coexpress Bcl-2 in vivo and proliferate within lymph node pseudofollicles and bone marrow clusters, where CD40L+ T cells are also found.53 IL-15 is produced by stromal cells of the bone marrow and of lymphoid organs,26 follicular dendritic cells (DCs),25 and activated monocytes.24 Therefore, IL-15 may support the proliferation of CLL cells in synergy with CD40L in vivo. Besides CD40 activation, other signals, such as the redox-regulatory protein thyoredoxin, enhance the sensitivity of CLL B cells to IL-15.54 However, CD40L enhances the sensitivity of CLL B cells to proapoptotic molecules, such as IL-21.39 Thus, stimulation via surface CD40 may activate and render CLL cells more prone to progression into cell cycle or to apoptosis, the final outcome eventually dictated by the presence of additional stimuli such as IL-15 and IL-21.
The opposite functional effects of IL-15 and IL-21 on CLL B cells prompted us to search for possible differences in their early signal transduction pathways. Indeed, here we report remarkable differences in the activation of the JAK/STAT and the Shc/MEK/ERK1/2 pathways by the 2 cytokines (Figure 7). While both of them activated JAK3, IL-15 predominantly induced STAT5 phosphorylation and weakly activated the STAT1 and STAT3 pathways. IL-21 predominantly triggered STAT1 and STAT3 phosphorylation and induced a limited STAT5 activation. A similar differential pattern of STAT molecule activation by IL-15 and IL-21 was observed in murine CD8+ T cells.43 However, an important difference between CD8+ T cells and CLL cells is that IL-21 cooperates with IL-15 in inducing CD8+ T-cell proliferation and function,55 while IL-21 counteracts the antiapoptotic and proliferative effects of IL-15 in human CLL cells. The predominant activation of STAT3 by IL-21 may depend on the presence of an YXXQ STAT3 consensus binding motif56 in the IL-21R intracytoplasmic domain, whereas STAT1 phosphorylation could be related to the activation of JAK1. The differential pattern of STAT activation by IL-21 and IL-15 in CLL cells is consistent with the opposite biologic effects of these cytokines. Thus, STAT5 activation by IL-15 may be related to its mitogenic activity, since pSTAT5 is known to induce cyclin D2 expression and mitogenic effects in lymphoid cells,57,–59 and STAT5 is required for lymphoid cell development.60 Conversely, STAT1 activation has been linked to apoptosis in lymphoid61 as well as in other cell types.62 In addition, the JAK-inhibitor I prevents the inhibition of c-myc expression and the proapoptotic effects of IL-21 on Epstein-Barr virus (EBV)–transformed B cells by blocking STAT1 phosphorylation,63 a finding that is consistent with our data on CLL cells.
Another important difference relates to the capacity of IL-15, and not of IL-21, to activate the Shc and ERK1/2 signaling pathways, under the same experimental conditions. The reduced ability of IL-21 to recruit Shc may depend on the lack of appropriate docking sites in the cytoplasmic domain of the IL-21R chain. Although the IL-21R is structurally related to the IL-2Rβ,36,44 the amino acidic motif containing ptyr338 (SCFTNOGpYFF) in the IL2Rβ, which serves as Shc docking site,46 is not conserved in the IL-21R. Our present findings only partially differ from the recent observation that IL-21 induces signaling via the Shc molecule in mouse CD8+ T cells, albeit less efficiently than IL-15.43 The different ability of IL-15 and IL-21 to activate ERK1/2 phosphorylation could explain both the antiapoptotic and the mitogenic activity of IL-15. Indeed, the MEK inhibitor UO126 blocked both the mitogenic and antiapoptotic activity of IL-15 on CLL cells. Our present findings support the notion that activation of the ERK1/2 pathway is important for CLL cell survival9,10,64,–66 and provide a possible explanation as to why IL-21, by inhibiting ERK1/2 phosphorylation, counteracts the IL-15 effects. The CXCL12 (SDF-1) chemokine produced by stromal or by nurselike cells induces CLL cell survival and proliferation through ERK1/2 phosphorylation, an effect blocked by MEK inhibitors.9,10 Conversely, induction of CLL cell apoptosis by flavopiridol64 or by the vitamin-D analog EB108965 is associated with suppression of ERK1/2 activity. Moreover, inhibitors of MEK signaling potentiate the cytotoxic effects of 2-chloro-2′-deoxyadenosine on CLL cells.66
In conclusion our data suggest that IL-15 and IL-21 have opposite biologic roles in the regulation of CLL B-cell survival and proliferation through the differential activation of signaling pathways. No differences in the response to the 2 cytokines were noted in CLL cells from cases characterized by the expression of different prognostic markers, although the number of cases studied was limited. It has been proposed that IL-15 may represent a suitable tool for immunotherapy, because of its ability to stimulate CD8+ T cells and/or NK-cell responses to neoplastic cells. However, since IL-15 is a growth and survival factor for CLL neoplastic cells, the use of IL-15 for CLL immunotherapy67 should be considered with caution. IL-21 may represent an alternative candidate in view of both its immune-enhancing effects and its antiproliferative/proapoptotic activity on CLL cells. A better knowledge of the signaling pathways involved in the mitogenic and survival factors of CLL, such as IL-15, may provide new potential molecular targets for therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Italian Association for Cancer Research (AIRC), Italian Ministry of Health, ARC (3690).
Authorship
Contribution: D.d.T. and R.M. performed experiments, analyzed and discussed data, and wrote the paper; M.C. performed experiments, analyzed and discussed data, and prepared figures; M. Fabbi performed experiments and discussed data; B.A. provided reagents and participated in experimental design and discussion; E.B., M.G., and M. Ferrarini provided clinical cases, diagnosed CLL, studied patient characteristics, discussed data, and contributed to paper revision; G.C. studied CLL phenotypic and genotypic characteristics and contributed to experimental design; S.F. originally designed and discussed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvano Ferrini, Laboratory of Immunotherapy IST c/o CBA Largo R. Benzi, 10; 16132 Genova Italy; e-mail: silvano.ferrini@istge.it.

![Figure 1. IL-15 and IL-21 show opposite effects on DNA synthesis and CLL cell survival. (A) Resting or CD40-activated CLL cells were cultured in triplicate wells of 96-well plates in the presence of different concentrations of IL-15 for 48 hours and then pulsed with 3H-thymidine (1 μCi [0.037 MBq]/well) for an additional 18 hours of culture. DNA synthesis by cells cultured in the presence of IL-21 (50 ng/mL) both alone and in combination with IL-15 (50 ng/mL) is also shown. *P < .05 with respect to IL-15 alone. Error bars represent SD. (B) Freshly isolated CLL cells were cultured for 4 days with or without IL-15, IL-21, or a combination of the 2 cytokines (all at 50 ng/mL), and then apoptosis was assessed by the DiOC6 staining method, which determines mitochondrial depolarization. The percentage of apoptotic cells is indicated in each panel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/2/10.1182_blood-2007-04-087882/4/m_zh80040812610001.jpeg?Expires=1769091998&Signature=Zk4Sd5FFw~XgqaKBjmKmnmNw3RprSPJ2CPRNQzyJhO3fU2~NLQUaJVzA0D9v0a1kY5pr~0TaiY9P4Vao4OQI3BOBdmsDpR~~Ygo3kqb11uG3Gc1zzumVrha3ALMm~iCGBKmnfa-ci2CxQkFIx-vnoKWOahC4KsPtRV0rGb4GkV~usgwxrdCgn-aQvFPBySNMUFs6Ci2yRly3bXUfDAUUFM8M7L7b7bv~-tSyQOqZfICmUcHplZfnRfHcKIB75eL6BjGWVYbHHT-P7fYA5Sw~s2uWbtYEmw6Q5DDxf4gUWB9zfWcWAhYSco146ipih6t7OKgNNAao27iv-G2N1yMOwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Pharmacological inhibitors of JAK3 or of MEK kinases block IL-15–mediated proliferative and antiapoptotic effects. (A) CD40-activated CLL cells were cultured in triplicate wells of 96-well plates in the presence of IL-15 (50 ng/mL) and of different concentrations of the MEK inhibitor UO126 or of the JAK3 inhibitor AG490 for 48 hours, and then pulsed with 3H-thymidine (1 μCi [0.037 MBq]/well) for an additional 18 hours of culture. Controls using the maximal DMSO concentration used as solvent for the inhibitors were also included. (B) Apoptosis was measured by annexin-V/PI staining of CD40-activated CLL cells after 72 hours of culture in the presence or absence of IL-15 (50 ng/mL) with our without AG490 (30 μM) or UO126 (10 μM). (C) CD40-activated CLL cells were left untreated or treated with IL-21 (50 ng/mL) for 72 hours in the presence or absence of AG490 (30 μM), UO126 (10 μM), or JAK-inhibitor I (0.2 to 0.6 μM) and apoptosis was detected by annexin-V/PI staining. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/2/10.1182_blood-2007-04-087882/4/m_zh80040812610006.jpeg?Expires=1769091998&Signature=vyhkfcNwz64VhmJ2U1sGgSQD6NyM9G3~AOGMRx1kh8KC0eUX8g7~djYnxx9NevSmgfY-yr9g5CpKIorJc5xovU78oPRsm70Po2cBSEMbjU2-70KLP5nXv2OzCbydrirJW9NsQu78xjngrHO1m6ZEk-7viaW3HeivJTst4OSiQdRXTauLy6JkpLW-cqWsLwJcADiO2dWh4WA0cphUlzYsyEoXeXCm~HR8cJuqZze~OL7lMFvzS5rA4sAfMrL0SlvEv42jiBeH1nGX1zngf2RvtbeS09KIIVnIw2gqeS44IvxBn5qgv3WwdUCyx6~xssTC6t0SQjwbUt~MVRSQt6qJXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)