Hematopoietic stem cells (HSCs) are historically the most thoroughly characterized type of adult stem cell, and the hematopoietic system has served as a principal model structure of stem-cell biology for several decades. However, paradoxically, although HSCs can be defined by function and even purified to near-homogeneity, the intricate molecular machinery and the signaling mechanisms regulating fate events, such as self-renewal and differentiation, have remained elusive. Recently, several developmentally conserved signaling pathways have emerged as important control devices of HSC fate, including Notch, Wingless-type (Wnt), Sonic hedgehog (Shh), and Smad pathways. HSCs reside in a complex environment in the bone marrow, providing a niche that optimally balances signals that control self-renewal and differentiation. These signaling circuits provide a valuable structure for our understanding of how HSC regulation occurs, concomitantly with providing information of how the bone marrow microenvironment couples and integrates extrinsic with intrinsic HSC fate determinants. It is the focus of this review to highlight some of the most recent developments concerning signaling pathways governing HSC fate.
Introduction
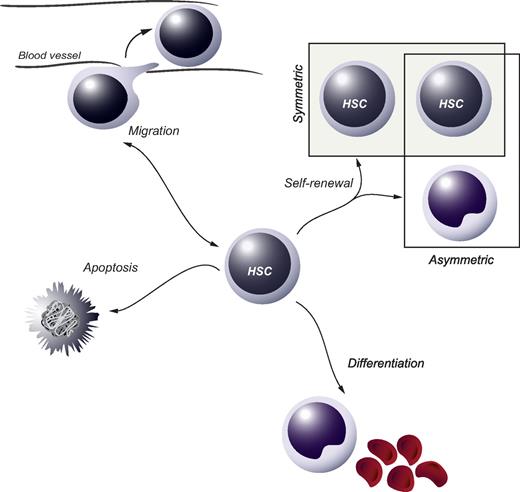
In tissues with a high cellular turnover, stem-cell populations are essential for lifelong maintenance of organ function. At present, somatic stem cells have been identified in several self-renewing organs, including intestinal and epidermal epithelia as well as the blood cell system. Residing in the bone marrow (BM) of adults, hematopoietic stem cells (HSCs) are ultimately responsible for the continuous daily production of all mature blood cell lineages.1 HSCs are functionally defined at the single-cell level by their dual capacity for self-renewal and multipotential differentiation. Self-renewal pertains to the process whereby at least one daughter cell of a dividing HSC retains stem-cell fate and may be either symmetric or asymmetric in its nature (Figure 1). A symmetric self-renewing division refers to the process whereby both daughter cells retain stem cell properties. Theoretically, this type of cell division expands the stem-cell pool and is therefore thought to be important after transplantation or after hematopoietic injury. In an asymmetric self-renewing division, the 2 daughter cells adopt different fates, resulting in one cell maintaining stem-cell properties. On a population basis, this allows for steady-state main-tenance of the HSC compartment. Self-renewal is critical for sustaining the HSC compartment and thus is a prerequisite for lifelong hematopoiesis.
HSC fate decisions. During or after cell division, the 2 daughter cells of an HSC are faced with several options, or fate decisions. These include the possibility to remain a HSC (the process of self-renewal), which can be either symmetric or asymmetric in its nature, to commit along the path of differentiation or to go through apoptosis. In addition, the option to move to other anatomic sites in or outside the BM (migration) can be regarded as a fate decision, which is particularly important during specific stages of ontogeny when HSCs seed the fetal liver or later the BM.
HSC fate decisions. During or after cell division, the 2 daughter cells of an HSC are faced with several options, or fate decisions. These include the possibility to remain a HSC (the process of self-renewal), which can be either symmetric or asymmetric in its nature, to commit along the path of differentiation or to go through apoptosis. In addition, the option to move to other anatomic sites in or outside the BM (migration) can be regarded as a fate decision, which is particularly important during specific stages of ontogeny when HSCs seed the fetal liver or later the BM.
Hematopoietic hierarchy
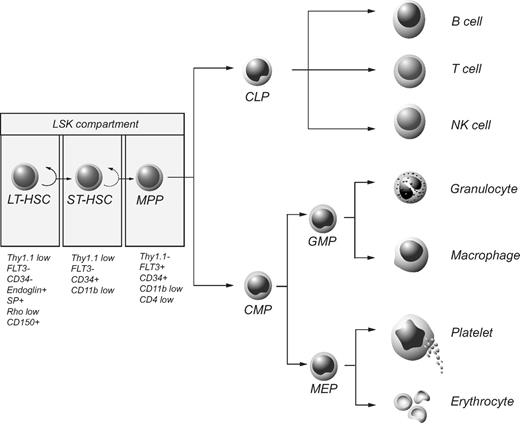
The hematopoietic system is hierarchically organized, with a rare population of HSCs giving rise to progeny that progressively lose self-renewal potential and successively become more and more restricted in their differentiation capacity, finally generating functionally mature cells (Figure 2). Phenotypic characterization of HSCs has allowed for their prospective isolation, and several groups have reported the successful regeneration of all blood cell lineages after transplantation of a single HSC into lethally irradiated mice.2,3 All functional HSCs are associated with lack of expression of a range of cell surface markers normally found on differentiating or mature blood cells while displaying high levels of Sca1 and c-kit.4,5 This population of cells is commonly referred to as the LSK compartment (lineage−/Sca1+/c-kit+) and 100 LSK cells have been shown to be sufficient for long-term multilineage repopulation of lethally irradiated hosts.5 Based on their ability to self-renew, HSCs can be divided into long-term and short-term reconstituting HSCs (LT-HSCs and ST-HSCs, respectively). LT-HSCs have extensive self-renewal capacity and can sustain lifelong hematopoiesis, whereas ST-HSCs are restricted in self-renewal potential, sustaining hematopoiesis only for a limited time in vivo.6,–8 With respect to self-renewal, the LSK compartment is heterogeneous, containing a mixture of LT-HSCs, ST-HSCs, and multipotent progenitor cells (MPPs). Further fractionation based on differential expression of CD34 and Flt3 has been shown to correlate with distinct functional properties, with LT-HSCs being negative for both CD34 and Flt3.3,6,7 In addition to cell-surface staining, exclusion of fluorescent dyes, such as Hoechst and rhodamine, has been shown to correlate with HSC activity.9 The side population defines a small and distinct subset of cells with LT-HSC characteristics.10 More recently, Kiel et al identified the SLAM family of cell surface receptors that were shown to be differentially expressed among stem and progenitor populations.11
The hematopoietic hierarchy and phenotypic markers associated with HSCs. The LSK compartment contains LT-HSCs, ST-HSCs, and MPPs, each subpopulation of which is associated with distinct phenotypic features as indicated. CLP indicates common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; and MEP, megakaryocyte/erythrocyte progenitor.
The hematopoietic hierarchy and phenotypic markers associated with HSCs. The LSK compartment contains LT-HSCs, ST-HSCs, and MPPs, each subpopulation of which is associated with distinct phenotypic features as indicated. CLP indicates common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; and MEP, megakaryocyte/erythrocyte progenitor.
Efficient isolation of human HSCs has proven more difficult, primarily as a consequence of lack of appropriate in vivo assays. As of today, the rhodaminelow fraction of the Lin−CD34+CD38− population of human cord blood (CB), containing putative HSCs at a frequency of 1 in 30 cells, provides the most stringent isolation of human HSCs.12 Populations containing putative human HSCs can be functionally analyzed in nonobese diabetic/severe combined immune deficient (NOD/SCID) mouse strains.13 SCID repopulating cells (SRCs) represent the most primitive hematopoietic population assessable, but NOD/SCID assays are hampered by the generally low engraftment of human cells and the difficulty in keeping NOD/SCID mice for long-term analyses. This predicament can be overcome by serial NOD/SCID transplantations, allowing for analysis of even more primitive populations. However, such assays keep HSCs under constant stress, which is why caution should be taken when interpreting obtained data.
Regulation of hematopoietic stem cells, a dynamic state of balance
To meet the need of a variety of situations, ranging from normal homeostasis to acute blood loss or infection, hematopoiesis must be rapidly yet meticulously controlled. Whether regarded as stochastic mainly regulating probabilities or deterministic providing more precise instructions, both intrinsic and extrinsic signals undoubtedly participate in the regulation of HSCs.1,14,–16 Through reductionistic strategies, a wide variety of factors critical for HSC regulation have been identified, including cytokines, growth factors, transcription factors, chromatin modifiers, and cell-cycle regulators. Delineating the signaling pathways that integrate these diverse components remains an enduring challenge of foremost importance, which would have tremendous impact on regenerative medicine. During the course of development, a handful of signaling pathways shape the structures of the embryo and determine cell fate and identity. In the adult organism, these signaling pathways are reiterated, and it is becoming increasingly clear that most somatic stem-cell compartments are under the influence of developmentally conserved signals.
Regulation of HSCs by classic hematopoietic cytokines
Numerous attempts have been made to use classic hematopoietic cytokines for the purpose of expanding HSCs in vitro. Many of the interleukins, particularly, interleukin (IL)-3, IL-6, and IL-11, as well as Flt-3 ligand, thrombopoietin (TPO) and stem-cell factor (SCF) have been investigated. In most cases, efforts to expand HSCs have failed because of differentiation of HSCs and subsequent loss of reconstitution capacity. In one study, a modest expansion of repopulating murine HSCs was observed using a 10-day culture protocol in the presence of IL-11, Flt-3 ligand, and SCF.17 However, the same conditions failed to expand HSCs derived from the fetal liver and later studies have found that the Flt-3 receptor is not expressed on LT-HSCs.7,18 Furthermore, the gp130 protein, which is a part of the receptor for IL-6 and IL-11 was suggested to play a role in the self-renewal of HSCs.19 However, elimination of the IL-11 receptor does not affect hematopoiesis, making conclusions of the in vivo relevance uncertain.20 Detailed studies of purified murine HSCs in recent years have shown that the receptors for TPO and SCF, c-mpl and c-kit, respectively, are both expressed on repopulating HSCs,6,7,21,,–24 and it has been shown that mice with genetic mutations in TPO or c-mpl have a reduction in HSCs.21,22 Moreover, TPO has been reported to support viability25 and suppressed apoptosis of HSCs.26 Thus, the main function of TPO may be to counteract apoptosis of HSCs rather than promote their expansion.24
A further role for TPO in regulating HSC self-renewal was recently suggested through the association with Lnk, an intracellular adaptor molecule functioning as a broad inhibitor of several cytokine signaling pathways, including TPO, SCF, erythropoietin, IL-3, and IL-7.24,27,,–30 Mice deficient in Lnk have been reported to exhibit increased HSC numbers and their repopulation function was elevated, a phenotype attributable to augmented proliferation and self-renewal.24,27,31 Interestingly, the increased self-renewal capacity of Lnk−/− HSCs was dependent on TPO, and Lnk-negative HSCs were shown to be hypersensitive to stimulation by TPO.24,27 Thus, TPO and Lnk serve opposing roles in the regulation of HSC self-renewal and Lnk acts as a powerful inhibitor of TPO signaling in HSCs. To conclude, of the classic hematopoietic cytokines, the most important positive regulators of HSCs are TPO and SCF.
Notch pathway: at the interface of osteoblasts and HSCs
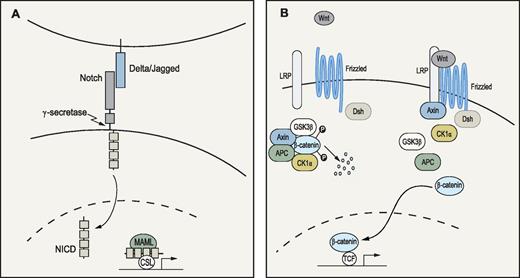
In humans, Notch was discovered 15 years ago as an oncogene that, after chromosomal translocation, gave rise to T-cell leukemia.32 Since then, several loss- and gain-of-function studies have demonstrated a critical role for Notch in lineage specification of lymphopoiesis, where active Notch signaling (Figure 3A) drives differentiation toward T cells.33 It was soon suggested that Notch could have a role in HSC biology as primitive hematopoietic cells as well as cells in the putative HSC microenvironment expressed both Notch and Notch-ligand mRNA.34,35 Indeed, through various approaches, several groups have shown that members of the Notch ligand families, Delta and Jagged, can expand both murine and human hematopoietic progenitors in vitro as measured by reconstitution assays.35,,,,,–41 Importantly, enforced activation of the Notch signaling pathway or the downstream target Hes-1, have been demonstrated to increase the self-renewal capacity of long-term in vivo repopulating HSCs42,43 or even to immortalize primitive hematopoietic progenitor cells.44 When Calvi et al established osteoblasts as putative members of the HSC niche, they demonstrated how constitutively active parathyroid hormone receptor expanded these cells and stimulated their expression of Jagged-1.45 The activated osteoblasts were able to increase self-renewal of primitive hematopoietic cells as measured by LTC-ICs and competitive repopulation assays.45 Moreover, LSK cells from these transgenic mice had increased levels of Notch intracellular domain (NICD), and the elevated LTC-IC numbers could be normalized with a γ-secretase inhibitor.45 Supporting these data were the observation by Duncan et al that a Notch reporter was active in c-kit+ hematopoietic cells in the trabecular bone and isolated LSK and side population cells.46
Notch and Wnt signaling pathways. (A) Notch pathway: When ligands of the Delta (Delta1-3) or Jagged (Jagged 1-2) families bind to the Notch receptor, proteolytic events involving γ-secretase lead to release and translocation of the intracellular domain of the receptor (NICD) to the nucleus. Subsequently, NICD will form a complex with the transcription factor CSL and cofactors of the Mastermind-like (MAML) family to activate transcription of target genes.138 (B) Wnt pathway: Wnt signaling is initiated when a ligand binds to the Frizzled and lipoprotein receptor-related protein (LRP) receptors at the cell surface. In the absence of Wnt ligands, the downstream signal transducer β-catenin is trapped by adenomatous polyposis coli (APC) and Axin in a destruction complex, where it is phosphorylated by casein-kinase 1α (CK1α) and glycogen synthase kinase (GSK3β). Phosphorylation ultimately leads to ubiquitination and degradation of β-catenin. On ligand binding, Frizzled forms a complex with disheveled (Dsh), whereas LRP is phosphorylated, resulting in Axin relocation to the cell membrane. Subsequently, the destruction complex is dispersed and β-catenin accumulates and translocates to the nucleus where it interacts with the T-cell factor/lymphoid enhancer factor (TCF) transcription factors to regulate gene expression.139,140
Notch and Wnt signaling pathways. (A) Notch pathway: When ligands of the Delta (Delta1-3) or Jagged (Jagged 1-2) families bind to the Notch receptor, proteolytic events involving γ-secretase lead to release and translocation of the intracellular domain of the receptor (NICD) to the nucleus. Subsequently, NICD will form a complex with the transcription factor CSL and cofactors of the Mastermind-like (MAML) family to activate transcription of target genes.138 (B) Wnt pathway: Wnt signaling is initiated when a ligand binds to the Frizzled and lipoprotein receptor-related protein (LRP) receptors at the cell surface. In the absence of Wnt ligands, the downstream signal transducer β-catenin is trapped by adenomatous polyposis coli (APC) and Axin in a destruction complex, where it is phosphorylated by casein-kinase 1α (CK1α) and glycogen synthase kinase (GSK3β). Phosphorylation ultimately leads to ubiquitination and degradation of β-catenin. On ligand binding, Frizzled forms a complex with disheveled (Dsh), whereas LRP is phosphorylated, resulting in Axin relocation to the cell membrane. Subsequently, the destruction complex is dispersed and β-catenin accumulates and translocates to the nucleus where it interacts with the T-cell factor/lymphoid enhancer factor (TCF) transcription factors to regulate gene expression.139,140
Together these findings indicate not only that osteoblasts are part of the so-called HSC niche but also that Jagged/Notch signaling activated by these cells might be important in the extracellular regulation of HSC self-renewal.45 However, HSCs deficient in Notch-1 could reconstitute Jagged-1 deficient hosts in a normal fashion, suggesting that Notch signaling through mechanisms including these molecules is dispensable for in vivo HSC function.47 It is probable that the lack of phenotype observed in this setting is the result of compensatory mechanisms mediated by other Notch receptors and ligands, which is why approaches to broadly eliminate Notch signaling would be informative. Regardless of what the case may be, manipulation of the Notch signaling pathway holds great promise for ex vivo expansion of HSCs in future clinical protocols.
HSC self-renewal under control by the Wnt signaling pathway
A role for Wnt signaling in the regulation of murine HSCs was suggested a decade ago when expression of different Wnts was discovered at sites of fetal hematopoiesis and Wnt5a was shown to stimulate proliferation and self-renewal of fetal HSCs/progenitors in vitro.48 Adult HSCs also appear to respond to Wnt treatment because exposure to purified Wnt3a was recently shown to increase self-renewal of murine HSCs in vitro, as determined by in vivo reconstituting assays.49 Moreover, Van den Berg et al demonstrated that the positive effects on HSCs were not exclusive to the murine setting because human Lin−CD34+ cells exposed to WNTs also expanded in vitro as measured by immunophenotype and colony assays.50
Not only is there evidence supporting the hypothesis that Wnts could be used as tools for expanding HSCs in culture, but WNT proteins have also been reported to be expressed in both human fetal BM stroma and adult BM, where WNT5A has been shown to be expressed in the primitive Lin−CD34+ population.50 This would suggest that Wnts are physiologically important and act on HSCs through both paracrine and autocrine mechanisms. In support of the latter statement was the finding that exposure of HSCs in vitro to a soluble Wnt binding antagonist reduced their proliferation capacity.51 However, the intracellular mechanisms through which Wnts exercise their effect on HSCs are somewhat inconclusive. Canonical Wnt signaling (Figure 3B) occurs through mechanisms involving the stabilization and nuclear translocation of β-catenin, followed by transcriptional regulation of target genes by a β-catenin/T-cell factor complex. Reya et al detected active canonical Wnt signaling, as measured by a T-cell factor reporter construct, in a large proportion of the HSC enriched LSK compartment in adult BM.51 Intriguingly, retroviral–mediated enforced expression of β-catenin resulted in a 102 to 103 fold increase in HSCs during long-term cultures of KTLS cells as defined by both phenotype and function.51 In accordance with the observed phenotype resulting from forced expression of β-catenin was an up-regulation in expression of the HSC self-renewal stimulating genes Notch1 and HoxB4.51 However, when β-catenin was conditionally expressed in vivo under the control of the ROSA26 locus, no increase in HSC self-renewal was observed.52 Adding to the contradiction, deletion of the β-catenin gene in HSCs failed to result in an in vivo phenotype.53
The reasons for these discrepancies are unclear but may reflect a stronger role for Wnt signaling in in vitro expansion of HSCs as opposed to its function in vivo. The more complex and enduring in vivo setting might allow for redundant mechanisms to develop. Alternatively, the HSC expansion observed when β-catenin was retrovirally overexpressed might be affected by the use of Bcl-2 transgenic HSCs in that particular study.51 Thus, even though Wnts may possibly be useful for expanding HSCs in vitro, the unresolved mechanisms behind the effect and the question pertaining to the role of canonical Wnt signaling remains to be determined.
Integration of Wnt and Notch signals in HSC regulation
Recently, an intriguing report presented evidence that Notch and Wnt pathways act in synergy to maintain the HSC pool as both signaling circuits were shown to be active simultaneously in a large fraction of cells in the trabecular bone.46 Even though Notch- and Wnt signaling both are implied to stimulate self-renewal of HSCs, Duncan et al propose that they do so through different mechanisms.46 As Wnt signaling seems to induce proliferation and support viability of LSK cells, inhibition of the Notch pathway had no effect on these functions.46 Instead, Notch signaling was demonstrated to be imperative in maintaining HSCs in an undifferentiated state, even if Wnt3a was present in the culture.46 These findings suggest that Wnt and Notch signaling together could play a role in self-renewal of HSCs. Moreover, observations that Wnt3a regulate expression of established Notch target genes46 and that inhibition of the Wnt signaling component GSK-3 affect HSC fate options through mechanisms involving regulation of both Wnt and Notch target genes54 suggest that the 2 pathways belong to a network of regulatory circuits controlling the HSC pool.
Smad signaling pathway
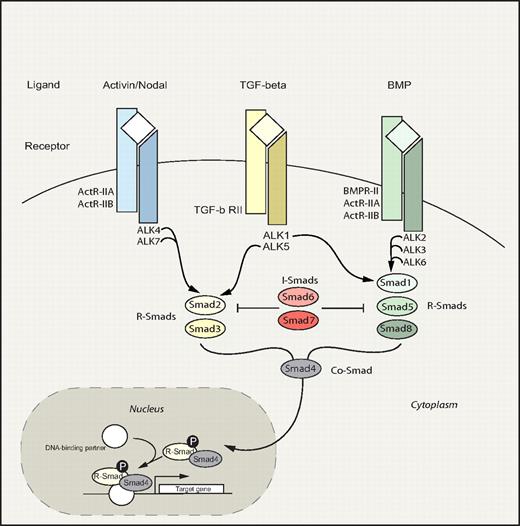
The Smad signaling pathway embodies an evolutionary ancient signaling circuitry, which functions to transduce signals downstream of the transforming growth factor-β (TGF-β) family of ligands (Figure 4). Apart from TGF-β, activins and bone morphogenetic proteins (BMPs) also belong to this superfamily, which regulate a bewildering array of fundamental biologic processes during development and postnatally. Because of the highly redundant nature of the Smad pathway and early embryonic lethality of most knockout mouse models, the precise role of the Smad circuitry in hematopoiesis has remained nebulous. However, recently several reports have shed new light suggesting that this pathway plays a pivotal role in the regulation of HSC fate decisions.
Smad signaling pathway. Smad pathway: TGF-β family members bind and signal through 2 types of serine/threonine kinase receptors, type I and type II, both of which are necessary for signal transduction. On ligand binding and receptor activation, the Smad proteins are activated through phosphorylation by type I receptors.141,142 Three groups of Smads have been identified: receptor-activated Smads (R-Smads), common- partner Smads (Co-Smads), and inhibitory Smads (I-Smads). In general, TGF-β and activin signal via R-Smad2 and 3, whereas BMP signals are transduced through R-Smad1, 5, and 8. Phosphorylated R-Smads subsequently associate with the Co-Smad4, creating a complex that translocates to the nucleus where target gene transcription is modified. In contrast to R- and Co-Smads, the I-Smads, Smad6 and Smad7, function in a negative feedback loop to prevent activation of R-Smads.143,–145 Divergence and convergence of the Smad signaling circuitry are depicted. Commonly used alternative names include: ALK2/Activin type I receptor, ALK3/BMP type IA receptor, ALK4/Activin type IB receptor, ALK5/TGF-β type I receptor, and ALK6/BMP type IB receptor. The shaded portion indicates the nucleus. P indicates phosphorylation.
Smad signaling pathway. Smad pathway: TGF-β family members bind and signal through 2 types of serine/threonine kinase receptors, type I and type II, both of which are necessary for signal transduction. On ligand binding and receptor activation, the Smad proteins are activated through phosphorylation by type I receptors.141,142 Three groups of Smads have been identified: receptor-activated Smads (R-Smads), common- partner Smads (Co-Smads), and inhibitory Smads (I-Smads). In general, TGF-β and activin signal via R-Smad2 and 3, whereas BMP signals are transduced through R-Smad1, 5, and 8. Phosphorylated R-Smads subsequently associate with the Co-Smad4, creating a complex that translocates to the nucleus where target gene transcription is modified. In contrast to R- and Co-Smads, the I-Smads, Smad6 and Smad7, function in a negative feedback loop to prevent activation of R-Smads.143,–145 Divergence and convergence of the Smad signaling circuitry are depicted. Commonly used alternative names include: ALK2/Activin type I receptor, ALK3/BMP type IA receptor, ALK4/Activin type IB receptor, ALK5/TGF-β type I receptor, and ALK6/BMP type IB receptor. The shaded portion indicates the nucleus. P indicates phosphorylation.
TGF-β, key negative modulator of HSCs in vitro
TGF-β is generally catalogued as one of the most potent inhibitors of HSC growth in vitro, and ample evidence from a variety of culture systems exist to support this fact.55,–57 A hallmark feature of HSCs is their relative quiescence, and given the strong growth inhibitory properties of TGF-β, it has naturally been hypothesized to be a cardinal regulator of HSC quiescence in vivo. In keeping with this, neutralization of TGF-β in vitro was shown to release early hematopoietic progenitor cells from quiescence.58,–60 Several molecular mechanisms have been proposed to account for TGF-β–mediated growth inhibition, including alterations in cytokine receptor expression and up-regulation of cyclin-dependent kinase inhibitors, such as p21 and p57.59,61,,,,,,,–69
TGF-β can affect most cell types throughout the hematopoietic hierarchy and depending on the context and differentiation stage of the target cell different biologic responses are ultimately elicited.70,–72 In vivo, TGF-β plays a principal role as regulator of immune cell homeostasis and function, as unambiguously evidenced by the development of a lethal inflammatory disorder of both TGF-β1- ligand and receptor knockout mice.73,–75 Furthermore, Tgf-β1 null mice exhibited enhanced myelopoiesis, suggesting that TGF-β acts as a negative regulator of myelopoiesis in vivo.76 However, despite the pronounced role of TGF-β in vitro, mice deficient of the TGF-β type I receptor displayed normal HSC self-renewal and regenerative capacity in vivo, even under extreme hematopoietic stress.77,78 The apparent discrepancy between in vitro and in vivo findings may reflect mechanisms of redundancy between other type I receptors or alternatively other ligands such as activin, which signals through the same R-Smad pathway.
BMP promotes maintenance of HSCs in culture
BMPs have been implicated as key regulators of hematopoietic development in a variety of species, from zebrafish to mouse.79,,,–83 In the context of adult hematopoiesis, BMP-4 was shown to promote maintenance of HSCs in culture, whereas lower concentrations of BMP-4 induced proliferation and differentiation of human hematopoietic progenitors.84 Furthermore, Shh induced proliferation of primitive human hematopoietic progenitors in vitro, apparently through a BMP-4–dependent mechanism.85 However, although BMP-4 has been shown to maintain human NOD/SCID repopulating cells in culture, it does not seem to cause an expansion, suggesting that Shh may act through additional mechanisms. In the murine system, BMP-4 does not appear to affect proliferation of purified HSCs in vitro, although it is currently unclear whether BMP-4 can extend the maintenance of murine HSCs in culture.86 Furthermore, BMPs function to regulate the HSC niche, thus indirectly controlling HSC numbers as discussed in “HSC niche.”
A role for Smad5, traditionally characterized as a mediator of BMP signals, in self-renewal was implicated through a series of in vitro studies. Interestingly, the number of colonies, derived from yolk sacs or ES cell differentiated embryoid bodies deficient of Smad5, were found to be increased.87 Smad5 deficient colonies also had an enhanced regenerative potential when secondary colony formation was assessed, indicative of increased self-renewal. However, conditional deletion of Smad5 in adult mice does not affect hematopoiesis, and HSCs lacking Smad5 have a normal differentiation and regenerative potential in vivo.88 It is possible that BMP–activated Smad signaling plays a more pronounced role early in hematopoietic development, as opposed to adult hematopoiesis. Alternatively, redundant mechanisms within the Smad pathway may explain the lack of phenotype in the adult setting.
Smad signaling: toward a deeper understanding
To block the entire Smad signaling pathway, thus sidestepping redundant mechanisms, the inhibitory Smad7 was overexpressed in murine HSCs using a retroviral gene transfer approach.89 Forced expression of Smad7 significantly increased the self-renewal capacity of HSCs in vivo, indicating that the Smad pathway negatively regulates self-renewal in vivo. In a similar approach using human SCID repopulating cells (SRCs), overexpression of Smad7 resulted in a shift from lymphoid dominant engraftment toward increased myeloid contribution.90 Thus, in the xenograft model system, forced expression of Smad7 modulates cell fate decisions of primitive multipotent human SRCs.
It is beyond doubt that the Smad signaling pathway lies at the very core of mediating signals from the TGF-β family of ligands. However, a considerably more diversified picture is now emerging, which indicates that the Smad circuitry is more varied than previously thought.91 Using a conditional knockout mouse model, disruption of the entire Smad pathway at the level of Smad4 was recently investigated. Intriguingly, Smad4 deficient HSCs displayed a significantly reduced repopulative capacity of primary and secondary recipients, indicating that Smad4 is critical for HSC self-renewal in vivo.92 Because overexpression of Smad7 versus deletion of Smad4 would be anticipated to yield similar hematopoietic phenotypes, it is conceivable that Smad4 functions as a positive regulator of self-renewal independently of its role as a central mediator of the canonical Smad pathway. The precise molecular mechanism for this is currently unknown, but it is possible that Smad4 participates in other signaling cascades such as Wnt or Notch.93,–95 Moreover, He et al proposed an elegant model for how TGF-β mediates erythroid differentiation concomitantly with balancing growth inhibition in human hematopoietic stem/progenitor cells.96 According to this model, transcriptional intermediary factor1γ (TIF1γ) selectively binds Smad2 and Smad3 in competition with Smad4. In response to TGF-β, the TIF1γ/Smad2/3 complex was shown to stimulate erythroid differentiation of human hematopoietic progenitors, whereas Smad2/3 in association with Smad4 inhibited proliferation of the same cells. Thus, the relative abundance of Smad4 and TIF1γ is likely to be important for determining the effect of TGF-β stimulation. Interestingly, the zebrafish homolog of TIF1γ, encoded by moonshine, has been shown to be essential for blood formation with mutants displaying severe red cell aplasia, indicating that the function of TIF1γ may be preserved across species.97
However, the mechanism through which TIF1γ cooperates with Smads is not entirely clear. Evidence from Xenopus has suggested that TIF1γ, known as Ectodermin, acts as a ubiquitin ligase for Smad4, thus functioning as a direct inhibitor of Smad4 downstream of TGF-β and BMP signaling.98 This dichotomy may reflect differences in cellular context or alternatively species or temporal differences. Another interesting observation is the association between Smad4 and Hox proteins. Homeobox (hox) genes encode transcription factors that function as paramount regulators of hematopoiesis and are frequently dysregulated in human leukemia, particularly acute myeloid leukemia. Enforced expression of HoxA9 immortalizes and blocks myeloid differentiation, eventually causing acute myeloid leukemia.99 Recently, Wang et al described a mechanism whereby TGF-β/BMP inhibited the bone marrow transformation capacity of HoxA9 and HoxA9-Nup98 fusion protein through a Smad4-dependent mechanism. Accordingly, Smad4 was shown to interact directly with HoxA9 and Nup98-HoxA9 fusion protein, thus precluding their DNA binding capacity and subsequent transcriptional activity.100 Whether Smad4 mutations facilitate leukemogenesis through increased Hox activity remains an unanswered question.
Angiopoietin-like proteins
Building on the fact that the fetal liver (FL) is a powerful site of HSC expansion during development, Lodish et al embarked on an approach to identify “stromal” cells and their expression pattern of molecules responsible for stimulation of HSC self-renewal in the murine FL. Ter119+CD3+ cells were recognized as a population of cells derived from the FL that could support expansion of HSCs in vitro. Insulin-like growth factor 2 (IGF-2) was identified on the basis of being expressed in CD3+ FL cells and was shown to modestly expand HSCs in vitro. More interestingly, they found that several angiopoietin-like (Angptl) proteins were expressed in these support cells for HSC growth. When highly enriched HSCs were cultured under serum free conditions in the presence of TPO, SCF, IGF-2, and Angptl protein 2 or 3, a robust increase in repopulating HSCs was observed after 10 days of in vitro culture.101 More specifically, a 24- to 30-fold increase in HSC number was observed. Angptl proteins 5 and 7 were also reported to expand murine HSCs, whereas Angptl protein 4 failed to show a similar effect. The molecular mechanisms and the signaling events downstream of Angptl proteins remain unidentified and their receptors are currently unknown, which is why it is not clear how the Angptl proteins expand HSCs. However, Angptl protein 2 has been shown to bind to a highly enriched population of HSCs. Therefore, the expansion effect attributable to these proteins is likely to be a result of a direct action on HSCs. It will be exciting to see whether the Angptl proteins can also be used for robust expansion of human HSCs, particularly HSCs derived from cord blood.
These findings are important especially in light of the convenience of adding these factors to cultures of HSCs in vitro, and they can therefore be used in future attempts to expand HSCs for the clinic and possibly also to increase the efficiency of gene delivery to HSCs.
HSC niche
On the role of osteoblasts
The spatial organization of hematopoietic cells within the BM, with primitive cells distributed closer to the bone surface and more differentiated cells positioned closer to the BM longitudinal axis of the femur, has long been recognized.102,103 As early as 1978, Schofield proposed the niche hypothesis of a physiologically limited microenvironment supporting stem cells in vivo.104 Because of the anatomic complexity of mammalian stem cell niches, the precise location as well as the cellular and molecular basis for these structures has only recently begun to be uncovered. Several lines of evidence now converge to suggest that the osteoblasts, cells of mesenchymal origin positioned at the endosteal surface of bone, are essential components of the HSC niche.105 Using a transgenic mouse model expressing a constitutively active Parathyroid hormone receptor under an osteoblast specific promoter (α1(I) collagen), Calvi et al demonstrated that an increase in trabecular osteoblasts was accompanied by an increase in HSC numbers.45 Importantly, Calvi et al45 propose a model in which increased parathyroid hormone signaling in osteoblasts results in elevated levels of the Notch1 ligand, Jagged1, ultimately generating increased activation of Notch1 signaling in HSCs. An independent study investigated the role of the BMP signaling pathway to regulate HSC numbers, by conditional inactivation of the BMP type IA receptor Alk3.106 In the absence of Alk3 the number of spindle shaped osteoblastic cells expressing N-cadherin were increased. Interestingly, this correlated with an increase in the number of HSCs, which were found attached to N-cadherin+ osteoblasts. Moreover, depletion of osteoblasts in vivo was associated with decreased BM cellularity and extramedullary hematopoiesis, further emphasizing the importance of osteoblasts for HSC function.107 In addition, Arai et al identified Tie2/Angiopoietin1 signaling to be critical for the maintenance of HSCs in a quiescent state in the BM niche.108 Tie2 is a receptor tyrosine kinase expressed on endothelial cells and HSCs, whereas Angiopoietin1 (Ang1) is expressed by osteoblasts. Tie2+ HSCs adhered to Ang1 expressing osteoblasts, supposedly through a mechanism involving N-cadherin.
Even though osteoblasts clearly constitute part of the HSC niche, they are probably not the only players. Apart from soluble factors, matrix components and other cellular constituents are likely to also have important functions. One example is osteopontin, a matrix glycoprotein synthesized by osteoblasts, which has been associated with negative regulation of the HSC compartment.109,110 Furthermore, HSCs deficient in the calcium-sensing receptor were profoundly defective in localizing anatomically to the endosteal niche, a behavior that correlated with diminished adhesion to the extracellular matrix protein collagen I.111 These data indicate that the local calcium gradient is involved in retaining HSCs in close physical proximity to the endosteal surface of bone. Furthermore, it was recently shown that a defective niche could result in HSC disorders further emphasizing the regulatory function of the HSC niche in vivo.112,113
Hypoxia and reactive oxygen species (ROS)
Another peculiar property of the BM microenvironment is its hypoxic nature, a fact recently highlighted by Parmar et al showing that HSCs are distributed predominantly at the lowest end of an oxygen gradient within the BM.114 This observation bears importance for several findings, which indicate that the level of oxidative stress influences HSC function. For example, mice deficient of the cell-cycle regulator Atm developed early onset BM failure, a phenotype accompanied by elevated levels of ROS in HSCs.115 Interestingly, ROS appear to activate the p38/MAPK pathway causing quiescent HSCs to cycle more frequently and eventually become exhausted.116 Moreover, members of the FoxO subfamily of forkhead transcription factors have been shown to protect HSCs from oxidative stress by up-regulating genes involved in their detoxification. Triple knockout mice of FoxO1, FoxO3, and FoxO4 exhibited defective long-term repopulating activity that correlated with increased cycling and apoptosis of HSCs as well as increased levels of ROS.117 Similarly, the HSC compartment of FoxO3a null mice suffers from augmented levels of ROS.146 As previously reported for mice deficient of Atm, the HSC defect resulting from loss of FoxOs could be rescued by administration of the antioxidant N-acetyl-L-cysteine. In light of these observations, it is conceivable that the hypoxic environment in which the HSCs reside may serve to protect them from oxygen radicals ultimately keeping them quiescent.
It's not all in the niche
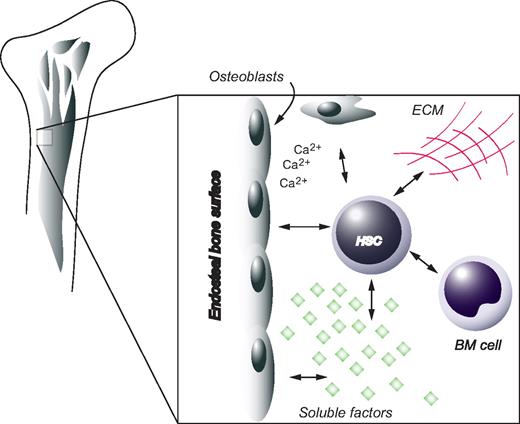
Despite the plethora of evidence pointing at the importance of the BM milieu, it would be premature to assume that the microenvironment is the sole regulator of HSC fate options. For instance, during ontogeny before the establishment of the BM niche, HSCs undergo extensive self-renewal in the fetal liver. Indeed, HSCs derived from fetal liver have been shown to encompass a more efficient regenerative potential compared with their adult counterparts when transplanted into irradiated recipients.118 Recent evidence uncovered by Bowie et al suggest the existence of an intrinsically regulated developmental switch, which abruptly changes the self-renewing properties of HSCs at the age of 3 to 4 weeks in the mouse.119 At this time the HSC compartment rapidly changes from a fully cycling population to a largely quiescent one.119 This change appears to be independent of the niche as it occurs well after the migration of HSCs to the BM and because it is not affected by transplantation into adult recipients.120 Interestingly, juvenile HSCs exhibit a greater sensitivity to SCF; thus, signaling events downstream of the c-kit receptor may be developmentally and intrinsically regulated, ultimately determining symmetric versus asymmetric self-renewal.121 To summarize, HSC behavior is regulated by joint efforts from intrinsic factors and environmental cues, both entities that are integrated in a more comprehensive and holistic view by the concept of the stem-cell niche (Figure 5).
The HSC niche. HSCs are positioned in close proximity to osteoblasts at the endosteal surface of bone. Other factors contributing to the in vivo microenvironment of HSCs include extracellular matrix (ECM), soluble compounds, as well as other cellular components.
The HSC niche. HSCs are positioned in close proximity to osteoblasts at the endosteal surface of bone. Other factors contributing to the in vivo microenvironment of HSCs include extracellular matrix (ECM), soluble compounds, as well as other cellular components.
Stem-cell expansion for cell and gene therapy
The process of finding donors that have compatible histocompatibility antigens for patients who need blood and marrow transplantation is often a challenge of significant magnitude. As of today, umbilical CB is being used increasingly as a source of HSCs because of the common availability of CB cells and the diversity of histocompatibility gene haplotypes that are available in banked CB samples.122 The number of HSCs in each CB sample is limited, and it would therefore greatly increase the applicability of this stem-cell source if the CB stem cells could be expanded ex vivo before transplantation. Stem-cell expansion can also theoretically be accomplished in vivo after engraftment of the transplanted cells, but this approach may involve greater risks compared with ex vivo expansion unless the growth stimuli used in vivo can be carefully controlled.123,124 Moreover, because culture conditions can be precisely defined and transient modulation of regulatory pathways more easily used in vitro, stem-cell expansion ex vivo presents an attractive approach. However, even this tactic has proven a great challenge because a successful stem-cell expansion involves symmetric self-renewal divisions of HSCs, where both daughter cells retain HSC properties.123 More commonly, HSCs grown in vitro undergo asymmetric divisions characterized by the production of one HSC and a more differentiated progenitor, or alternatively, a symmetric division where both progeny cells have lost their HSC potential.
Positive and negative regulators ultimately balance the transition from quiescence to proliferation of HSCs. Thus, the strategies for stem-cell expansion should involve activation of regulators that encourage HSC self-renewal and/or inhibition of pathways that mediate quiescence, differentiation, or apoptosis of HSCs. Because of the recent advances in understanding the mechanisms that control HSC self-renewal, novel methods to expand stem cells can now be developed.70,123,124 Some approaches require viral vector-mediated gene transfer to HSCs for efficient expansion. Ideally, the vectors should be nonintegrating and mediate transient gene expression for safety reasons.125,–127 However, the safest approaches would involve soluble factors, for example, cytokines, developmental cues or factors like the Angptl proteins.
When the transcription factor HoxB4 was expressed in HSCs ex vivo, a more than 40-fold net expansion of murine HSCs was generated over a period of 10 to 14 days.128 Furthermore, modified HoxB4 proteins have been used successfully to stimulate proliferation of human and murine HSCs ex vivo.129,130 The soluble form of the HoxB4 protein could actively be taken up by HSCs and could be used without concerns for insertional mutagenesis or the potential adverse effects that continuous production of HoxB4 in HSCs might generate in vivo. However, the HSC expansion achieved by the soluble HoxB4 was quite modest compared with overexpression of HoxB4 within HSCs. Developmental cues that activate Notch and Wnt signaling in HSCs may also be useful to increase HSC self-renewal ex vivo. As mentioned previously, Wnt3A has been shown to expand murine repopulating HSCs and Wnt5A injection into NOD/SCID mice repopulated with human hematopoietic cells increased the reconstitution and number of primitive hematopoietic cells. Similarly, a soluble form of the Notch ligand, Jagged 1, was reported to stimulate growth of human SRCs and may therefore be used to expand stem cells ex vivo.35 However, the Angptl proteins are by far the most promising soluble factors identified to date for expansion of murine HSCs.101 If the angiopoietin-like proteins would prove to expand human HSCs as efficiently as in the murine system, they may become a promising tool for future cell and gene therapy approaches. Furthermore, other positive regulatory factors, in combination with Angptl proteins, may be able to expand human HSCs by a factor of 10 during a relatively short and safe culture period, suitable for GMP conditions that will be required for the clinic. Worthy of mention are also fibroblast growth factors, which have been shown to sustain primitive murine hematopoietic cells in culture and to maintain their primitive phenotype.131,132 Interestingly, fibroblast growth factor-1 in conjunction with SCF, TPO, and IGF-2 resulted in a 20-fold increase of LT-HSCs during a 10-day culture period.133
It should be emphasized that most of the knowledge about stem- cell expansion has come from studies in mice. There is a difference between mouse and human HSCs with regards to cytokine receptors, telomere biology, and proliferative capacity, and there will therefore be both differences and similarities in the approaches used to expand mouse and human HSCs. Recently, the protein nephroblastoma overexpressed (NOV/CCN3) has been shown to expand primitive human HSCs, and this protein should therefore be carefully evaluated for use in clinical stem-cell expansion protocols.134 As more detailed knowledge is unearthed concerning the regulatory pathways that govern HSC self-renewal, it may even be possible to modulate these pathways with small molecule drugs as has been shown in the modulation of Wnt signaling and the expression of p21 and HoxB4.54,135,136 As an example, chemicals that enhance prostaglandin E2 synthesis were recently reported to increase HSC numbers in zebrafish and mice.137 Undoubtedly, however, a major challenge still ahead will be the integration of major signaling pathways into networks of control mechanisms ultimately coupling extrinsic with intrinsic regulatory fate determinants. Such knowledge would open up new avenues for maintaining and expanding HSCs in vitro.
Acknowledgments
This work was supported by the European Commission (INHERINET, Gene Therapy of Hematopoietic Stem Cells for Inherited Diseases, and CONSERT, Concerted Safety and Efficiency Evaluation of Retroviral Transgenesis for Gene Therapy of Inherited Diseases); Swedish Gene Therapy Program; Swedish Medical Research Council; Swedish Children Cancer Foundation; Clinical Research Award from Lund University Hospital; Joint Program on Stem Cell Research; Juvenile Diabetes Research Foundation; and Swedish Medical Research Council (S.K.), the Wenner-Gren Foundations (U.B.), and Kungliga Fysiografiska Sällskapet (G.K. and U.B.). The Lund Stem Cell Center is supported by a Center of Excellence grant in life sciences from the Swedish Foundation for Strategic Research.
Authorship
Contribution: All authors contributed to the writing of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Karlsson, Molecular Medicine and Gene Therapy, Lund University Hospital, BMC A12, 221 84 Lund, Sweden; e-mail: Stefan.Karlsson@med.lu.se.