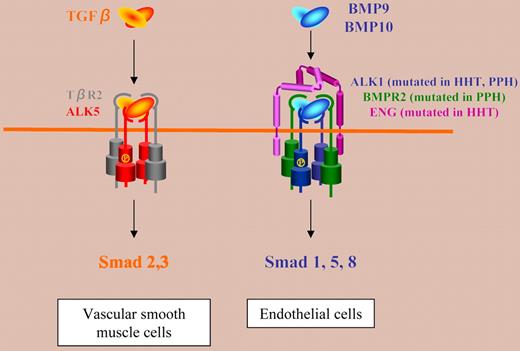
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal-dominant vascular disorder characterized by recurrent nosebleeds, mucocutaneous telangiectases, and arteriovenous malformations (AVMs) in the brain, lung, liver, and gastrointestinal (GI) tract. This disease is caused by heterozygous mutations in either endoglin (ENG) or activin receptor-like kinase 1 (ALK1) genes. The standing assumption is that, in endothelial cells, TGFβ binds to TGFβR2 and ALK1, together with the canonical TGFβ receptor ALK5 and endoglin.1 This model has led many medical textbooks to list HHT as a TGFβ signaling disease.
In this issue of Blood, Park and colleagues report important new results on the role of ALK1 versus ALK5 and TGFβR2 in HHT that cast doubts on this model. They addressed this question in vivo by comparing the phenotypes of mice in which the Alk1, Alk5, or Tgfbr2 gene was conditionally deleted in restricted vascular endothelia using a novel endothelial Cre transgenic line (Tg(Alk1-cre)). They found that Alk1-conditional deletion resulted in fetuses that died by embryonic day 18.5 (E18.5). These mice displayed dilated, tortuous, and irregular blood vessels in the yolk sac and lungs and severe AVM in the yolk sac vasculature, mimicking the main pathological features of HHT. In contrast, Alk5-or Tgfbr2-conditional deletion did not affect murine vessel morphogenesis. These data indicate that neither ALK5 nor TGFβR2 are required for ALK1 signaling pertinent to the pathogenesis of HHT and that HHT may not be a TGFβ subfamily disease. Previous work from the same group has already challenged the TGFβ/ALK1/ALK5 model, since transgenic β-gal reporter mice showed that ALK1 and ALK5 were not expressed in the same cells, Alk1 being expressed in endothelial cells and Alk5 in vascular smooth muscle cells.2
What could be the signaling pathway involved in HHT, if not TGFβ? Very recently, the bone morphogenetic proteins BMP9 and BMP10 have been reported to bind to ALK1.3 Moreover, BMPR2 and endoglin have been shown to increase BMP9 signaling through ALK1. Whether BMP9 and/or BMP10 are in vivo ligands for ALK1 pertinent to HHT is becoming an exciting question. The implication of BMPs in this disease is substantiated by a recent report showing that Bmpr2-specific short hairpin RNA transgenic mice exhibit GI bleeding and HHT-like vascular lesions.4
The data presented by Park and colleagues suggest a new signaling model (see figure), in which BMP9 and/or BMP10 (but not TGFβ) bind to ALK1 and endoglin together with BMPR2 in endothelial cells and contribute to the formation of vascular beds between arteries and veins. This pathway, which is altered in HHT, might also be involved in primary pulmonary hypertension (PPH) linked to mutations of BMPR2 and ALK1.
The transgenic Tg(Alk1-cre) mice that Park and colleagues have developed in this work should also be used as an animal model to study at least one of the phenotypes of HHT. Indeed, although these mice do not reproduce all the pathophysiological features of HHT, since they die by E18.5, they do present consistently with AVM. These animals will be a valuable resource for studying the molecular pathways involved in the progression of AVM found in HHT and also for testing new therapies for HHT.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal