The tachykinins comprise a family of peptides, traditionally classified as neurotransmitters, that includes substance P and neurokinins A and B. It is now clear that these peptides mediate a variety of biological functions, including smooth muscle cell contraction, vasodilation, plasma extravasation, neurogenic inflammation, and hematopoiesis. In this issue of Blood, work by Jones and colleagues extends the functional repertoire of tachykinins by revealing an important role in hemostasis and thrombosis.
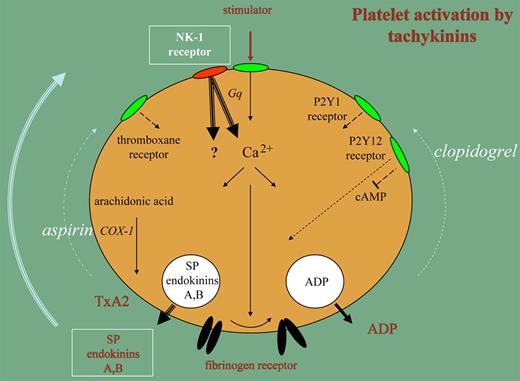
It now appears that platelets contain substance P and endokinins A and B, which are released upon stimulation and cause positive feedback activation. Platelets circulate in a dormant state, but contact with components in the damaged vessel wall and stimulators generated in plasma start a sequence of reactions resulting in aggregation, secretion of granule contents, and generation of a procoagulant surface. There is a stream of activation signals initiated by receptor stimulation that leads to mobilization of Ca2+ ions from internal stores and is facilitated by the GTP-binding protein Gq through signal amplification. Often, this pathway functions far below maximal capacity. The support of 2 feedback mechanisms is essential for rapid execution of platelet aggregation and secretion. One of those mechanisms is the release of ADP, which feeds back to P2Y receptors; the other is the release of thromboxane A2, which activates thromboxane receptors. The success of clopidogrel and aspirin as antithrombotic medication is based on the interference with P2Y12 signaling and thromboxane A2 formation, respectively.
The studies by the Gibbins group make it clear that when platelets are stimulated, a third mechanism for feedback stimulation is activated. The secretion reaction liberates substance P and endokinins A and B, which signal through NK-1 receptors on the platelet surface. NK-1 signaling accelerates the Gq-mediated Ca2+ mobilization, leading to faster and more extensive aggregation and secretion. The prolongation of the bleeding time and reduction of thromboembolism by deficiencies in NK-1 signaling in an animal model are impressive illustrations of the importance of this feedback loop in hemostasis and thrombosis.
Platelet stimulation leads to a rise in Ca2+, which activates the fibrinogen receptor enabling aggregation (solid arrows). This pathway is accelerated by release of ADP and thromboxane A2 (TxA2) through P2Y and thromboxane receptors (dotted arrows). Clopidogrel and aspirin interfere with these pathways. The secretion of Substance P (SP) and endokinins A, B forms a third mechanism for feedback activation of platelet functions (bold arrows).
Platelet stimulation leads to a rise in Ca2+, which activates the fibrinogen receptor enabling aggregation (solid arrows). This pathway is accelerated by release of ADP and thromboxane A2 (TxA2) through P2Y and thromboxane receptors (dotted arrows). Clopidogrel and aspirin interfere with these pathways. The secretion of Substance P (SP) and endokinins A, B forms a third mechanism for feedback activation of platelet functions (bold arrows).
Aspirin and clopidogrel also interfere with tachykinin-induced platelet functions and the result of NK-1 mediated Ca2+ mobilization might well be neutralized by these agents at more downstream steps in the NK-1 signaling pathway. However, in addition to Gq-mediated Ca2+ increases, the NK-1 receptor might trigger activation mechanisms that escape inhibition by aspirin and clopidogrel. The NK-1 receptor has been reported to couple to other GTP-binding proteins in other cells, causing activation of alternative signaling pathways. Furthermore, receptor-independent modes of action for tachykinins in some cells have been reported.1,2
The relevance of alternative signaling pathways in the activation of platelets by tachykinins is uncertain, but the effects of perturbing tachykinin receptors on hemostasis and thrombosis indicate that this new mechanism may be a novel route for the development of antithrombotic treatments.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal