Abstract
We previously demonstrated that CD4+CD25+ T regulatory cells (Tregs), important for the maintenance of immune tolerance and prevention of autoimmune disease, from patients with human T lymphotropic virus type I (HTLV-I)–associated myelopathy/tropical spastic paraparesis (HAM/TSP) exhibit reduced Foxp3 expression and Treg suppressor function compared with healthy donors. Since TGF-β signaling has been previously reported to be critical for both Foxp3 expression and Treg function, we examined whether this signaling pathway was dysregulated in patients with HAM/TSP. Levels of TGF-β receptor II (TGF-βRII) as well as Smad7 (a TGF-β–inducible gene) were significantly reduced in CD4+ T cells in patients with HAM/TSP compared with healthy donors, and the expression of TGF-βRII inversely correlated with the HTLV-I tax proviral load. Importantly, both CD4+CD25+ and CD4+CD25− T cells from HAM/TSP patients exhibited reduced TGF-βRII expression compared with healthy donors, which was associated with functional deficits in vitro, including a block in TGF-β–inducible Foxp3 expression that inversely correlated with the HTLV-I tax proviral load, loss of Treg suppressor function, and escape of effector T cells from Treg-mediated control. This evidence suggests that a virus-induced breakdown of immune tolerance affecting both regulatory and effector T cells contributes to the pathogenesis of HAM/TSP.
Introduction
Human T lymphotropic virus type I (HTLV-I) is an exogenous retrovirus that has been estimated to infect between 15 and 20 million people worldwide.1 While most infected individuals remain life-long asymptomatic carriers of the virus, a small percentage progress and develop either an aggressive T-cell leukemia known as adult T-cell leukemia (ATL) or a chronic inflammatory disorder of the central nervous system (CNS) known as HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP).2-5 HAM/TSP is characterized as a chronic progressive myelopathy with involvement principally in the spinal cord.6 CNS white matter lesions of the spinal cord have been reported to harbor activated CD4+ and CD8+ T cells during early stages of disease, with a predominance of CD8+ T cells later in disease.7 HTLV-I viral RNA has been found associated with CD4+ T cells and astrocytes in CNS lesions,8-12 suggesting that virus-infected cells can migrate through the blood-brain barrier and infect CNS resident cells.13 Elevated levels of proinflammatory cytokines IL-6, IFN-γ, and IL-12 have been detected in the serum and CSF of patients with HAM/TSP compared with healthy controls,14,15 and virus-specific CD8+ T cells from HAM/TSP patients exhibit a predominantly effector/memory phenotype.16 IL-1β, TNF-α, and IFN-γ have also been detected in inflammatory lesions during early stages of disease.17 While the mechanisms underlying disease pathogenesis remain unresolved, this evidence strongly suggests that both viral gene expression (in particular the expression of the viral transactivating gene tax) and the host immune response are involved in the pathogenesis of this disease. With respect to viral gene expression, the HTLV-I tax proviral load has been found to directly correlate with both Tax mRNA levels and the frequency of Tax-specific CD8+ T cells in peripheral blood mononuclear cells (PBMCs) from HAM/TSP patients.16,18 It has therefore been suggested that the HTLV-I tax proviral load determines (along with cellular signaling) the level of viral gene expression, which in turn triggers a strong virus-specific CD8+ (as well as CD4+) T-cell response, leading to CNS inflammation, myelin loss, and axonal damage through direct killing of infected cells in the CNS, bystander-mediated cytotoxicity, and/or molecular mimicry.
While HTLV-I has been reported to infect a number of cell types in vitro and in vivo,12,19,20 we have previously demonstrated using molecular and immunologic techniques that the predominant viral reservoir in the peripheral blood is CD4+CD25+ T cells.21 In healthy individuals, CD4+CD25+ T cells that are hyporesponsive to antigenic stimulation and are capable of suppressing the proliferation and cytokine production of CD4+CD25− and CD8+ effector T cells (Teffs) in vitro are defined as regulatory T cells (Tregs; reviewed in Shevach22 ). Pandiyan et al have recently described that CD4+CD25+ T cells may also function to control inflammation through cytokine deprivation-induced apoptosis of effector T cells (Teffs).23 Although phenotypically similar to activated T cells, Tregs can be identified ex vivo by the intracellular expression of the transcriptional regulator Foxp3.24 Foxp3 is critical for the development and function of Tregs in both mice and humans, as genetic mutation of the Foxp3 gene has been found to be responsible for the severe multifocal lymphoproliferative autoimmune phenotype of the scurfy mouse and immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome (IPEX) in humans.25 Previous studies have observed significant reductions in Foxp3 expression and/or Treg function in several human autoimmune diseases, including multiple sclerosis (MS), myasthenia gravis, and type I diabetes,26-28 suggesting that defects in Foxp3 expression and/or Treg function may precipitate loss of immunologic tolerance. Recently, we have reported that CD4+CD25+ T cells from patients with HAM/TSP had reduced levels of Foxp3 mRNA and protein compared with healthy individuals, and that this reduction was associated with a loss of suppressor function.29 This observation suggested that a defective Treg compartment may account for, at least in part, the persistent virus-specific cellular and humoral immune response observed in HAM/TSP patients. Although overexpression of a single HTLV-I gene (tax) was capable of reducing Foxp3 expression and dampening the suppressive function of healthy donor CD4+CD25+ T cells, the mechanisms underlying these events remain unresolved.
TGF-β is a potent anti-inflammatory cytokine capable of suppressing T-cell proliferation and modulating T-cell differentiation.30 A role for TGF-β signaling has been associated with the induction and maintenance of Foxp3 expression and Treg differentiation from the following evidence: (1) stimulation in the presence of TGF-β in vitro has been reported to convert CD4+CD25−Foxp3− T cells into CD4+CD25+Foxp3+ T cells with suppressive function; and (2) the number of CD4+CD25+ T cells as well as the level of Foxp3 expression are severely compromised in the periphery of TGF-β−/− and CD4-promoter-dnTGF-β receptor II (TGF-β-βRII) transgenic mice.31-33 Signaling through the TGF-β receptor has also been reported to confer susceptibility of Teffs to Treg-mediated suppression in vivo, as CD4+CD45RBhigh or CD8+ T cells expressing a dominant-negative TGF-βRII were refractory to suppression mediated by CD4+CD25+ T cells in adoptive transfer models of colitis or diabetes.34,35 These data indicate that TGF-β signaling tightly controls the differentiation and activation of both Tregs and Teffs.
Previous studies have established that CD4+ T cells from patients with HAM/TSP are resistant to the antiproliferative effects of TGF-β in vitro, despite the slightly elevated levels of TGF-β observed in HAM/TSP patients compared with healthy donors.36,37 The reason underlying this discrepancy remains unknown, although a virus-triggered defect in the TGF-β signaling pathway remains a possibility. In the present study, we identify a defect in the TGF-β signaling pathway at the level of TGF-βRII expression as well as expression of the TGF-β–inducible gene smad7 in CD4+ T cells isolated from HAM/TSP patients. Aberrant type II TGF-β receptor expression was observed in both CD4+CD25+ and CD4+CD25− T cells and was associated with disruption of TGF-β–induced Foxp3 expression, Treg suppressive activity, and susceptibility of Teffs to Treg-mediated suppression. Collectively, this study demonstrates that an infectious agent can disrupt both Treg and Teff arms of the cellular immune response as a potential contribution to the pathogenesis of CNS disease in humans.
Methods
Cell culture
HepG2 cells were cultured in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA). Primary human T cells were cultured in RPMI-1640 medium (Invitrogen). Media were supplemented with 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Cambrex, Walkersville, MD), and 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) or 5% human AB serum (Gemini Bio-Products, Woodland, CA). For Foxp3 induction experiments, cells were cultured in X-VIVO 15 (Cambrex).
Subjects and cell preparation
PBMCs were prepared by centrifugation over Ficoll-Hypaque gradients (Cambrex) from HAM/TSP patients, and the cells were viably cryopreserved in liquid nitrogen until tested. HAM/TSP was diagnosed according to WHO guidelines. HTLV-I seropositivity was determined by enzyme-linked immunosorbent assay (ELISA; Abbott Laboratories, Abbott Park, IL), with confirmation by Western blot analysis (Genelabs Technologies, Redwood City, CA). Blood samples were collected after informed consent was obtained in accordance with the Declaration of Helsinki as part of a clinical protocol reviewed and approved by the NIH institutional review panel. A total of 12 HAM/TSP patients, 4 HTLV-I–infected asymptomatic carriers, and 18 healthy donors were used in this study.
Plasmids and siRNA
Expression vectors encoding HTLV-I Tax and empty control vector were a generous gift from Dr Warner Greene (University of California San Francisco). A synthetic TGF-β–responsive reporter vector p3TP-luc and human TGF-βRII (−1240/+36) promoter construct were kindly provided by Dr Seong-Jin Kim (National Cancer Institute [NCI]/NIH, Bethesda, MD). A human Smad7 (−250/+32) promoter construct was provided by Dr Fang Liu (Rutgers University, New Brunswick, NJ). ON-TARGETplus SMARTpool siRNA reagents targeting GAPDH and Smad4 were purchased from Dharmacon (Chicago, IL).
Isolation of primary human CD4+, CD4+CD25−, and CD4+CD25+ T cells
CD4+ cells were isolated from cryopreserved PBMCs using the Human CD4+ T cell Isolation Kit II (Miltenyi Biotech, Auburn, CA) according to the manufacturer's guidelines. Purity of negatively selected CD4+ T cells was routinely more than 97%. CD4+CD25+ (Treg-enriched) and CD4+CD25− (Treg-depleted) cells were isolated using the Human Regulatory T cell Kit (Miltenyi Biotech) where the purity of CD4+CD25+ T cells was routinely more than 94% as determined by flow cytometry.
Transient expression and luciferase assays
HepG2 (3.75 × 105 cells/well) cells were plated into 6-well culture plates (BD Biosciences, San Diego, CA) 1 day prior to transfection with the appropriate plasmid DNA using FuGene 6 transfection reagent (Roche, Indianapolis, IN). Primary human CD4+CD25+ T cells (106) were nucleofected with the specified plasmid DNA or siRNA using the Human T Cell Nucleofection Kit (Amaxa, Gaithersburg, MD). Recombinant human TGF-β (10 ng/mL; R&D Systems, Minneapolis, MN) was added in some experiments 4 hours after transfection. Twenty-four hours after transfection, cells were harvested and luciferase activity was analyzed using the Dual-luciferase Reporter Assay System (Promega, Madison, WI) and a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA) according to the manufacturer's guidelines. pGL4-TKhRluc2 was used as an internal control to normalize for transfection efficiency. Nucleofected T cells were also monitored for transfection efficiency and cell viability 24 hours after transfection as follows. Transfection efficiency was routinely approximately 30% as determined by flow cytometric analysis of EGFP expression. Cell viability, determined by staining with 7-amino-actinomycin D (7-AAD; BD Biosciences), was routinely approximately 70%.
Real-time PCR and RT-PCR (TaqMan)
The HTLV-I proviral DNA load was measured as previously described.16 Real-time reverse-transcription–polymerase chain reaction (RT-PCR) analysis of Foxp3, Smad2, Smad3, Smad4, Smad7, TGF-βRII, GAPDH, and HPRT expression was performed using primer and probe sets purchased from Applied Biosystems (Foster City, CA). Briefly, total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's guidelines, and cDNA was synthesized by reverse transcription using TaqMan Gold RT-PCR Kit reagents and random hexamer primers (Applied Biosystems). Relative quantification of mRNA was performed using the comparative threshold cycle method using HPRT as an endogenous control. Relative target gene expression was quantified using an Applied Biosystems 7900HT FAST Real-Time PCR System. Where indicated, FAST TaqMan settings were used. For each sample, target gene expression was normalized to the expression of HPRT. To determine relative expression levels, the following formula was used: target gene expression = 2−(Ct[target]−Ct[HPRT]).
Flow cytometry
Cells (106) were washed once and resuspended in 100 μL fluorescence-activated cell sorting (FACS) buffer (1× PBS, 0.05% NaN3, 1% FBS). Cells were stained for surface CD4 (RPA-T4; BD Biosciences) and CD25 (M-A251; BD Biosciences) or the appropriate isotype controls for 20 minutes at 4°C. In some experiments, cells were processed further for intracellular Foxp3 (236A/E7; eBioscience, San Diego, CA) staining according to the manufacturer's instructions (eBioscience). Cells were then acquired on a FACSCalibur (BD Biosciences) using CellQuest software (BD Biosciences). Data analysis was performed using FlowJo software (TreeStar, San Diego, CA).
Proliferation assays
CD4+CD25+ and CD4+CD25− T cells (2 × 104 each) from healthy donors and/or HAM/TSP patients were plated alone or in coculture into 96-well round-bottom plates in the presence of soluble anti-CD3 (0.5 μg/mL, HIT3a; BD Biosciences) and irradiated accessory cells (5 × 104). Accessory cells were autologous to the responding cell population. On day 5 of culture, proliferation was analyzed by [3H]-thymidine incorporation during the final 16 hours.
Foxp3 induction assays
CD4+CD25− T cells (106) from healthy donors or HAM/TSP patients were plated in 24-well plates containing IL-2 (100 U/mL), and a combination of the following: plate-bound anti-CD3 (2 μg/mL; UCHT1; BD Biosciences), soluble anti-CD28 (2 μg/mL; 28.2; BD Biosciences), and rhTGF-β1 (5 ng/mL; Peprotech, Rocky Hill, NJ). On day 5, cells were analyzed for CD25 and Foxp3 expression by flow cytometry.
Statistical analyses
Analysis of variance and the Mann-Whitney test was used to compare the data between patients with HAM/TSP, asymptomatic carriers, and healthy donors. Analysis of variance and the unpaired t test were used to compare changes 3TP-luc, Smad7-luc, and TGF-βRII-luc activity caused by overexpression of HTLV-I Tax. Linear regression analysis was used to test the correlation between HTLV-I tax proviral load in PBMCs, TGF-βRII mRNA in CD4+ T cells, and percentage CD25+Foxp3+ T-cell induction. Statistical analyses were performed using Prism (GraphPad Software, San Diego, CA).
Results
Blockade of TGF-β signaling by HTLV-I Tax and in patients with HAM/TSP
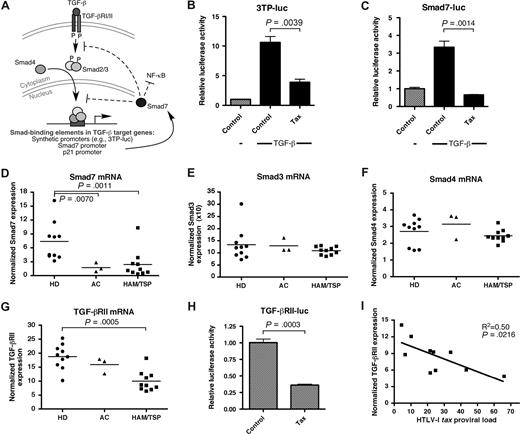
HTLV-I Tax has been implicated in the pathogenesis of HAM/TSP through stimulation of T-cell activation and cytokine production, altering cell signaling pathways, and acting as an antigen for virus-specific CD4+ and CD8+ T cells.38 Previous studies have shown that the HTLV-I Tax protein inhibits cell signaling through the TGF-β pathway, although the precise mechanism remains controversial.39-41 A schematic diagram of the TGF-β signaling pathway is shown in Figure 1A. In this study, we confirmed the ability of Tax to inhibit TGF-β–dependent gene expression by analyzing the activation of a synthetic reporter (3TP-luc) or a Smad7 promoter luciferase reporter construct (Smad7-luc), each containing Smad3/4-binding sites and each responsive to TGF-β. The smad7 gene is a target of TGF-β that functions in a dominant-negative manner to block subsequent TGF-β signaling, and to suppress NF-κB activation and inflammatory cytokine production.42-45 Transcription from both 3TP-luc (Figure 1B) and Smad7-luc (Figure 1C) reporters were stimulated by addition of TGF-β to HepG2 cells. Overexpression of HTLV-I Tax, however, dramatically suppressed the effects of TGF-β. Together, this evidence suggests that expression of TGF-β–inducible genes (eg, smad7) is inhibited by Tax. Moreover, previous studies have demonstrated that Foxp3 is a TGF-β–inducible gene itself.31
Suppression of TGF-β signaling in vitro by HTLV-I Tax and in HTLV-I–infected patients. (A) Schematic diagram of the TGF-β signaling pathway. HepG2 cells were transfected with either a (B) synthetic TGF-β/Smad reporter vector (3TP-luc), (C) Smad7 promoter construct, or (H) TGF-βRII promoter construct in combination with the indicated expression vectors and/or rhTGF-β1. Error bars represent SEM. After 24 hours, cell lysates were assayed for luciferase activity. Total RNA from CD4+ T cells from 10 healthy donors (HD), 3 HTLV-I–infected asymptomatic carriers (AC), and 10 patients with HAM/TSP were assayed for endogenous (D) Smad7, (E) Smad3, (F) Smad4, or (G) TGF-βRII mRNA expression by real-time RT-PCR (TaqMan). The horizontal bar represents the median. (I) A statistically significant negative correlation (P = .022, R2 = 0.50) between the HTLV-I tax proviral load in PBMCs and the TGF-βRII mRNA expression in CD4+ T cells was observed in HTLV-I–infected patients.
Suppression of TGF-β signaling in vitro by HTLV-I Tax and in HTLV-I–infected patients. (A) Schematic diagram of the TGF-β signaling pathway. HepG2 cells were transfected with either a (B) synthetic TGF-β/Smad reporter vector (3TP-luc), (C) Smad7 promoter construct, or (H) TGF-βRII promoter construct in combination with the indicated expression vectors and/or rhTGF-β1. Error bars represent SEM. After 24 hours, cell lysates were assayed for luciferase activity. Total RNA from CD4+ T cells from 10 healthy donors (HD), 3 HTLV-I–infected asymptomatic carriers (AC), and 10 patients with HAM/TSP were assayed for endogenous (D) Smad7, (E) Smad3, (F) Smad4, or (G) TGF-βRII mRNA expression by real-time RT-PCR (TaqMan). The horizontal bar represents the median. (I) A statistically significant negative correlation (P = .022, R2 = 0.50) between the HTLV-I tax proviral load in PBMCs and the TGF-βRII mRNA expression in CD4+ T cells was observed in HTLV-I–infected patients.
Since Tax can disrupt TGF-β signaling in vitro, we next asked whether the TGF-β signaling pathway was affected in patients with HAM/TSP. To address this question, we examined purified CD4+ T cells (which represent a primary target of HTLV-I) from 10 healthy donors, 3 HTLV-I–infected asymptomatic carriers, and 10 HAM/TSP patients using magnetic bead separation and analyzed the expression of an endogenous TGF-β–responsive gene (smad7) by real-time RT-PCR. These studies revealed that Smad7 expression was significantly reduced in both asymptomatic carriers and HAM/TSP patients compared with healthy donors (Figure 1D). This reduction was not the result of aberrant expression of Smad3 (Figure 1E) or Smad4 (Figure 1F), key effector molecules in the TGF-β signaling pathway whose expression has been reported to be unaffected by Tax.39 These data suggest that the expression of TGF-β–responsive genes is inhibited during HTLV-I infection, most likely through the effects of Tax expressed in infected CD4+ T cells. To determine whether other steps in the TGF-β signaling pathway were altered in HTLV-I–infected individuals, the expression of TGF-βRII in CD4+ T cells was examined. Our analysis revealed that TGF-βRII mRNA levels were significantly reduced in HAM/TSP patients, compared with healthy donors (Figure 1G). A similar deficiency in TGF-βRII expression in HTLV-I–infected lymphocytes compared with activated healthy donor lymphocytes has previously been reported by Brady and colleagues (Pise-Masison et al46 ). The reduction of TGF-βRII expression in HAM/TSP patients was likely the direct result of Tax expression within infected CD4+ T cells, since overexpression of Tax down-regulated the transcriptional activation of the TGF-βRII promoter (Figure 1H). Collectively, this evidence suggests the presence of a TGF-β signaling defect in patients with HAM/TSP caused by the HTLV-I transactivating gene tax.
We have previously demonstrated an inverse correlation between Foxp3 expression and the HTLV-I tax proviral load.47 Since TGF-β signaling has been reported to play a critical role in stimulating Foxp3 expression,31,48 we hypothesized that if reduced TGF-βRII levels were causing the loss of Foxp3 expression in HTLV-I–infected individuals, that TGF-βRII expression would also inversely correlate with the HTLV-I tax proviral load. To test this hypothesis, we plotted the levels of TGF-βRII expression in CD4+ T cells against the HTLV-I tax proviral load in PBMCs of HTLV-I–infected individuals. Indeed, a significant inverse correlation was found between the levels of TGF-βRII expression and the HTLV-I tax proviral load from 10 HTLV-I–infected individuals (P = .022, R2 = 0.50, linear regression analysis, Figure 1I), suggesting that dysregulation of TGF-β signaling is involved in the pathogenesis of HAM/TSP, likely through silencing of downstream TGF-β–responsive genes, including foxp3.
Smad3/4 play a role in regulating Foxp3 expression
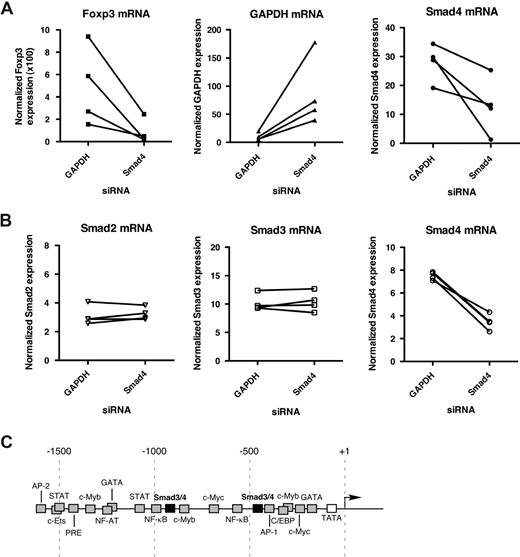
Fantini et al31 and Tran et al48 have previously reported that Foxp3 expression can be induced in CD4+CD25− T cells by stimulating in the presence of TGF-β.31,48 Additional studies have demonstrated that TGF-β−/− mice or CD4-promoter dnTGF-βRII transgenic mice develop lethal autoimmunity and exhibit reduced peripheral CD4+CD25+Foxp3+ T cells and loss of suppressor function.32,33 These reports suggest that TGF-β signaling is critical for the induction and maintenance of Foxp3 expression in the periphery and that a defect in TGF-β signaling could precipitate a decrease in Foxp3 expression and loss of suppressor function in Tregs. Therefore, if TGF-β/Smad signaling is important for Foxp3 expression in human CD4+CD25+ T cells, then a defect in this pathway could be the mechanism for the reduction in Foxp3 expression observed in patients with HAM/TSP. To examine whether TGF-β signaling plays a role in directing Foxp3 expression in human CD4+CD25+ T cells, we introduced siRNA molecules (a predesigned pool of 4 siRNA species) specific for either Smad4 or, as an irrelevant control, GAPDH and assayed endogenous Foxp3 expression after 24 hours by FAST real-time RT-PCR (FAST TaqMan). GAPDH and Smad4 expression was examined in parallel for siRNA knockdown efficiency. The results from 4 independent experiments using different healthy donors are shown in Figure 2A and demonstrate that Smad4 siRNA caused a reduction in Foxp3 mRNA in CD4+CD25+ T cells compared with CD4+CD25+ T cells transfected with control siRNA. To demonstrate the specificity of the Smad4 siRNA, CD4+ T cells from 4 healthy donors were transfected with siRNA to Smad4 or GAPDH and assayed for Smad2, Smad3, and Smad4 expression after 24 hours. The results in Figure 2B indicated that Smad4 siRNA affected only the expression of Smad4 and not Smad transcription factor family members Smad2 or Smad3. These results point to Smad4 playing a potentially important role in regulating Foxp3 expression. Analysis of the human Foxp3 promoter for transcription factor binding sites using Genomatrix MatInspector database49 yielded a number of putative Smad3/4-binding sites (Figure 2C). Future studies will be able to examine in detail the potential interactions between Smad3/4 and the Foxp3 promoter region and the functional consequences of such interactions.
A role for Smad3/4 in the expression of Foxp3 in CD4+CD25+ T cells. (A) CD4+CD25+ T cells from healthy donors were transfected with the indicated siRNA and assayed for Foxp3 mRNA expression after 24 hours by FAST real-time RT-PCR (FAST TaqMan). GAPDH and Smad4 mRNA expression was also analyzed in parallel to monitor siRNA-mediated knockdown efficiency. Shown are results from 4 independent experiments using a different donor for each experiment. (B) To demonstrate Smad4 siRNA specificity, CD4+ T cells from 4 healthy donors were transfected with Smad4 siRNA or GAPDH siRNA (irrelevant control) and assayed for Smad2, Smad3, and Smad4 expression by FAST real-time RT-PCR (FAST TaqMan). (C) Schematic representation of the human Foxp3 promoter regions with putative transcription factor–binding sites.
A role for Smad3/4 in the expression of Foxp3 in CD4+CD25+ T cells. (A) CD4+CD25+ T cells from healthy donors were transfected with the indicated siRNA and assayed for Foxp3 mRNA expression after 24 hours by FAST real-time RT-PCR (FAST TaqMan). GAPDH and Smad4 mRNA expression was also analyzed in parallel to monitor siRNA-mediated knockdown efficiency. Shown are results from 4 independent experiments using a different donor for each experiment. (B) To demonstrate Smad4 siRNA specificity, CD4+ T cells from 4 healthy donors were transfected with Smad4 siRNA or GAPDH siRNA (irrelevant control) and assayed for Smad2, Smad3, and Smad4 expression by FAST real-time RT-PCR (FAST TaqMan). (C) Schematic representation of the human Foxp3 promoter regions with putative transcription factor–binding sites.
Reduced TGF-βRII expression in both CD4+CD25+ and CD4+CD25− T cells is associated with a functional defect in regulatory and effector T-cell populations in patients with HAM/TSP
Although TGF-β–mediated signaling has been recognized as playing a role in inducing and maintaining Foxp3 expression and Treg suppressor function, previous reports also suggest that a TGF-β signaling defect in the Teff population may render these cells unresponsive to Treg-mediated suppression.34,35 To investigate whether the TGF-βRII defect observed in CD4+ T cells in HAM/TSP patients translated into a defect in Treg and/or Teff populations, we isolated CD4+CD25+ and CD4+CD25− T cells from healthy donors and HAM/TSP patients by magnetic bead separation and assessed the expression of TGF-βRII in both populations. As shown in Figure 3A, the expression of TGF-βRII was consistently greater in CD4+CD25− T cells compared with CD4+CD25+ T cells in both healthy donors and HAM/TSP patients. In addition, the expression of TGF-βRII was lower in both CD4+CD25+ and CD4+CD25− T-cell populations from HAM/TSP patients compared with the same populations in healthy donors.
Dysregulation of TGF-βRII expression and loss of regulatory and effector T-cell function in patients with HAM/TSP. (A) CD4+CD25+ and CD4+CD25− T cells from 2 healthy donors and 2 patients with HAM/TSP were analyzed for TGF-βRII mRNA expression by real-time RT-PCR (TaqMan). (B) CD4+CD25−Foxp3− T cells from HAM/TSP patients (n = 5) were cultured for 5 days in the presence of a combination of the following: plate-bound anti-CD3 monoclonal antibody, soluble anti-CD28 monoclonal antibody, rhTGF-β1, and rhIL-2. On day 5, cells were analyzed for conversion into CD4+CD25+Foxp3+ T cells by flow cytometry. A statistically significant inverse correlation between the percentage CD25+Foxp3+ T-cell induction and the HTLV-I tax proviral load in PBMCs was observed in patients with HAM/TSP. (C) CD4+CD25+ and CD4+CD25− T cells from healthy donors or patients with HAM/TSP were cultured separately or in coculture in the presence of anti-CD3 monoclonal antibody and irradiated accessory cells for 5 days. Proliferation was analyzed by [3H]-thymidine incorporation during the final 16 hours. Shown are the results of 2 independent experiments pairing a different healthy donor and HAM/TSP patient in each experiment. Error bars represent SEM.
Dysregulation of TGF-βRII expression and loss of regulatory and effector T-cell function in patients with HAM/TSP. (A) CD4+CD25+ and CD4+CD25− T cells from 2 healthy donors and 2 patients with HAM/TSP were analyzed for TGF-βRII mRNA expression by real-time RT-PCR (TaqMan). (B) CD4+CD25−Foxp3− T cells from HAM/TSP patients (n = 5) were cultured for 5 days in the presence of a combination of the following: plate-bound anti-CD3 monoclonal antibody, soluble anti-CD28 monoclonal antibody, rhTGF-β1, and rhIL-2. On day 5, cells were analyzed for conversion into CD4+CD25+Foxp3+ T cells by flow cytometry. A statistically significant inverse correlation between the percentage CD25+Foxp3+ T-cell induction and the HTLV-I tax proviral load in PBMCs was observed in patients with HAM/TSP. (C) CD4+CD25+ and CD4+CD25− T cells from healthy donors or patients with HAM/TSP were cultured separately or in coculture in the presence of anti-CD3 monoclonal antibody and irradiated accessory cells for 5 days. Proliferation was analyzed by [3H]-thymidine incorporation during the final 16 hours. Shown are the results of 2 independent experiments pairing a different healthy donor and HAM/TSP patient in each experiment. Error bars represent SEM.
Since the data in Figure 1I suggest that loss of TGF-βRII expression was associated with the pathogenesis of HAM/TSP, we investigated whether reduced TGF-βRII expression in CD4+CD25+ and CD4+CD25− T cells from HAM/TSP patients would adversely impact TGF-β–induced Foxp3 expression and/or Treg and Teff function. We first examined the ability of CD4+CD25−Foxp3− T cells from HAM/TSP patients to convert to CD4+CD25+Foxp3+ T cells in the presence of TGF-β. CD4+CD25−Foxp3− T cells from 5 patients with HAM/TSP were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β in serum-free media. CD25 and Foxp3 expression was analyzed 5 days later by flow cytometry. The results from these experiments were plotted against the HTLV-I tax proviral load of each HAM/TSP patient (Figure 3B). Conversion of CD4+CD25−Foxp3− T cells into CD4+CD25+Foxp3+ T cells exhibited a significant inverse correlation with the HTLV-I tax proviral load as determined by real-time PCR (P = .033, R2 = 0.82, linear regression analysis). These results indicate that the ability of TGF-β to induce Foxp3 expression in HAM/TSP patients was repressed as a function of disease progression.
Next we preformed coculture proliferation assays using CD4+CD25+ and CD4+CD25− T cells from healthy donors and HAM/TSP patients to specifically test the ability of Tregs to suppress Teff proliferation as well as the susceptibility of Teffs to Treg-mediated control. We hypothesized that, as previously reported in murine systems,33,34 inhibition of TGF-βRII expression (as a result of HTLV-I infection) would lead to loss of Treg suppression of Teff proliferation as well as resistance of Teffs to Treg-mediated control. The results from this set of experiments are shown in Figure 3C (2 of 3 independent experiments are shown using a different healthy donor and HAM/TSP patient in each experiment). CD4+CD25+ T cells from healthy donors were hyporesponsive to stimulation with anti-CD3, whereas CD4+CD25− T cells from healthy donors proliferated robustly under identical conditions. When cocultured at a 1:1 ratio, healthy donor CD4+CD25+ T cells suppressed the proliferation of healthy donor CD4+CD25− T cells. HAM/TSP CD4+CD25+ T cells were less hyporesponsive to stimulation with anti-CD3 in comparison with healthy donor CD4+CD25+ T cells. HAM/TSP CD4+CD25+ T cells failed to suppress the proliferation of healthy donor CD4+CD25− T cells when cocultured together in a 1:1 ratio, indicating a defect in the ability of HAM/TSP CD4+CD25+ T cells to suppress the proliferation of healthy donor CD4+CD25− T cells (as previously reported29 ). Finally, CD4+CD25+ T cells from healthy donors failed to suppress the proliferation HAM/TSP CD4+CD25− T cells when cocultured together in a 1:1 ratio. These data suggest HAM/TSP CD4+CD25− T cells remain refractory to Treg-mediated suppression. In conclusion, both Treg and Teff populations exhibit a profound functional deficit in patients with HAM/TSP due to virus-induced down-regulation of TGF-βRII. Future studies are required to determine the full implications that dysregulation of TGF-β signaling has on Treg and Teff function observed in HTLV-I–induced neuroinflammatory disease.
Discussion
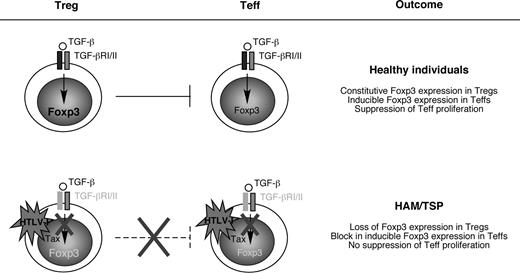
TGF-β signaling plays a critical role in immune tolerance, not only by maintaining Foxp3 expression in peripheral Tregs, but also by promoting Teff susceptibility to Treg-mediated suppression. Evidence presented herein points to a defect in TGF-β signaling as a result of infection with a human retrovirus. As observed in mice with engineered blocks in TGF-β signaling, patients with HAM/TSP exhibit a chronic inflammatory disorder characterized by accumulation of activated CD4+ and CD8+ T cells.16,32,33,50 CD4+CD25+ T cells from HAM/TSP patients also exhibit an activated phenotype marked by decreased expression of CD45RA together with increased expression of IL-2 and IFN-γ compared with CD4+CD25+ T cells from healthy individuals.29 CD4+CD25+ T cells represent the predominant viral reservoir in the peripheral blood in patients with HAM/TSP.21 Therefore persistent T-cell activation in patients with HAM/TSP triggered by viral antigens such as Tax together with down-regulation of Foxp3 may result in decline of the CD4+CD25+Foxp3+ Treg population and accumulation of CD4+CD25+Foxp3− T cells that lack suppressive function, but are capable of exacerbating the disease process (Figure 4).
Loss of immune tolerance caused by HTLV-I Tax. In healthy individuals, CD4+CD25+Foxp3+ Tregs suppress the proliferation of Teffs through (1) constitutive expression of Foxp3, and (2) induction of Foxp3 expression in Teffs (also known as infectious tolerance). However, in patients with HAM/TSP, HTLV-I Tax down-regulates TGF-βRII, which silences TGF-β–responsive genes such as foxp3. The resulting block in Foxp3 expression causes Tregs to lose suppressor function and causes Teffs to become resistant to TGF-β–inducible Foxp3 expression. The result is a breakdown in immune tolerance in patients with HAM/TSP characterized by expansion of potentially pathogenic CD4+ and CD8+ T cells and production of proinflammatory cytokines.
Loss of immune tolerance caused by HTLV-I Tax. In healthy individuals, CD4+CD25+Foxp3+ Tregs suppress the proliferation of Teffs through (1) constitutive expression of Foxp3, and (2) induction of Foxp3 expression in Teffs (also known as infectious tolerance). However, in patients with HAM/TSP, HTLV-I Tax down-regulates TGF-βRII, which silences TGF-β–responsive genes such as foxp3. The resulting block in Foxp3 expression causes Tregs to lose suppressor function and causes Teffs to become resistant to TGF-β–inducible Foxp3 expression. The result is a breakdown in immune tolerance in patients with HAM/TSP characterized by expansion of potentially pathogenic CD4+ and CD8+ T cells and production of proinflammatory cytokines.
HTLV-I Tax is a multifunctional protein known for its ability to activate or silence the transcription of cellular genes in trans (reviewed in Buckle et al51 ). We have demonstrated that Tax inhibited expression of both endogenous Foxp3 (previously reported29 ) and transcriptional activation of the Smad7 and TGF-βRII promoters. The expression of these genes was significantly reduced in HAM/TSP patients, and TGF-βRII expression as well as induction of Foxp3 negatively correlated with the HTLV-I tax proviral load. This evidence suggests that virus-induced dysregulation of TGF-β signaling prevents the expression of TGF-β target genes (eg, Foxp3). Although TGF-β signaling has been implicated in this study as playing an important role in regulating Foxp3 expression, it is possible that HTLV-I Tax may also impact Foxp3 transcription by interacting with and disrupting additional signaling pathways. For example, IL-2 and STAT5b have also been reported to play an essential role for inducing Foxp3 expression.52,53 Therefore, disruption of either of these signaling pathways could also lead to inhibition of Foxp3 expression and loss of Treg function during HTLV-I pathogenesis. However, much evidence exists to argue against this possibility. Both IL-2 and the IL-2Rα (CD25) are up-regulated in patients with HAM/TSP.54 In addition, the transactivating HTLV-I tax gene induces STAT5 mRNA and protein expression, while another viral protein, HTLV-I p12I, enhances STAT5b DNA-binding activity and STAT5b-mediated transcriptional activation.55,56 A recent study has reported that patients who harbor a loss-of-function mutation in STAT5b were reported to exhibit reduced CD25 expression compared with controls, while CD25 is up-regulated in patients with HAM/TSP compared with healthy controls.53,54 STAT5b-mutant CD4+CD25+ T cells exhibited a loss of suppressor function, similar to what we reported herein for CD4+CD25+ T cells from patients with HAM/TSP. However, proliferation of STAT5b-mutant CD4+CD25− T cells was able to be suppressed by Tregs from control patients,53 whereas CD4+CD25− T cells from HAM/TSP patients remained refractory to suppression mediated by CD4+CD25+ T cells from healthy donors. Collectively, the situation in HAM/TSP patients does not resemble that of patients harboring a loss-of-function mutation in STAT5b, but rather the situation reported in murine systems with engineered blocks in TGF-β signaling. In addition to the evidence presented herein indicating that TGF-β signaling is important for Foxp3 expression in Tregs as well as inducible Foxp3 expression in Teffs, a recent study has reported that Tregs block the polarization of antigen-specific T cells toward antigen-presenting cells in a TGF-β–dependent manner.57
Defects in Foxp3 expression and/or Treg function have been reported in a number of human autoimmune diseases.26-28 HAM/TSP is a virus-induced neuroinflammatory disorder in which the expression of Foxp3 and the function of Tregs have been found deficient.29 Hafler and colleagues demonstrated in coculture proliferation assays that Tregs isolated from MS patients were unable to suppress Teffs derived from either MS patients or healthy donors. However, Teffs from MS patients remained susceptible to suppression mediated by Tregs isolated from healthy individuals (Viglietta et al26 ). This evidence suggests that the defect in Treg function in patients with MS reflects an inability of Tregs to suppress proliferation of Teffs rather than the Teff population being refractory to Treg-mediated control. Although Tregs from HAM/TSP patients were shown in this study to also lack suppressive function, unlike MS, Tregs derived from healthy donors could not control the proliferation of Teffs from HAM/TSP patients. This evidence points to a 2-fold defect in immunologic tolerance in patients with HAM/TSP: (1) loss of suppressive function in CD4+CD25+ T cells; and (2) escape of CD4+CD25− T cells from Treg-mediated control. In this study, both TGF-βRII expression and CD4+CD25+Foxp3+ T-cell induction inversely correlated with the HTLV-I tax proviral load in HTLV-I–infected individuals, suggesting that disruption of TGF-β signaling may be a cofactor in the progression of HAM/TSP. Evidence in support of this hypothesis has been reported by Powrie and colleagues, who demonstrated using an adoptive transfer model of autoimmunity that TGF-βRII expression on both regulatory and effector T-cell populations is critical for maintaining a check on the expansion of potentially pathogenic T cells (Fahlen et al34 ). A role for dysregulated TGF-β signaling in the pathogenesis of inflammatory bowel disease and Alzheimer disease has also been recently reported,58,59 reiterating the importance of this pathway in protecting against chronic inflammatory disease.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Kathy Jones (NIH/NCI-Frederick) for expert review of this paper. We also thank J. Weissman and D. Singer (NIH/NCI) for use of their luminometer and the NINDS DNA Sequencing Core Facility.
This research was supported by the Intramural Research Program of the NIH (NINDS).
The content of this publication does not reflect the views orpolicies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: C.G. designed and performed research, analyzed data, and wrote the paper; U.O. designed research and contributed vital new reagents and analytical tools; K.Y. performed research; Y.Y. designed research and contributed vital new reagents; S.J. analyzed the data and wrote the paper.
Y.Y. is presently at the Department of Genome Science, Institute of Medical Science, St Marianna University School of Medicine, Kawasaki, Japan.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Jacobson, National Institutes of Health, National Institute of Neurological Disorders and Stroke, 10 Center Drive, Bldg 10, Rm 5B-16, Bethesda, MD 20892; e-mail: jacobsons@ninds.nih.gov.

![Figure 3. Dysregulation of TGF-βRII expression and loss of regulatory and effector T-cell function in patients with HAM/TSP. (A) CD4+CD25+ and CD4+CD25− T cells from 2 healthy donors and 2 patients with HAM/TSP were analyzed for TGF-βRII mRNA expression by real-time RT-PCR (TaqMan). (B) CD4+CD25−Foxp3− T cells from HAM/TSP patients (n = 5) were cultured for 5 days in the presence of a combination of the following: plate-bound anti-CD3 monoclonal antibody, soluble anti-CD28 monoclonal antibody, rhTGF-β1, and rhIL-2. On day 5, cells were analyzed for conversion into CD4+CD25+Foxp3+ T cells by flow cytometry. A statistically significant inverse correlation between the percentage CD25+Foxp3+ T-cell induction and the HTLV-I tax proviral load in PBMCs was observed in patients with HAM/TSP. (C) CD4+CD25+ and CD4+CD25− T cells from healthy donors or patients with HAM/TSP were cultured separately or in coculture in the presence of anti-CD3 monoclonal antibody and irradiated accessory cells for 5 days. Proliferation was analyzed by [3H]-thymidine incorporation during the final 16 hours. Shown are the results of 2 independent experiments pairing a different healthy donor and HAM/TSP patient in each experiment. Error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/12/10.1182_blood-2007-11-123430/6/m_zh80100819450003.jpeg?Expires=1765887410&Signature=H-zTEagCN20zuKIPKg7VIDnzXIZFAHvCrlah2N6NU6Mc3PChQMgprVr-fJGoqlduPzbtaP6Q4QOKlC83rmVojc~m2LYTLlu8O2jU6rITA9Fyzor4XWATAhiAIn3FKHHdr~WKGaFUCghYstlREXBKhehCVfBrWHjE0J6uaAlrTsVEu0f8TssgB0U1KOmDA1QZJMQtntFoRxMqQll0LiGWSU78lKTQVNxqDTlF14Y7pSLjBm8MWduTGwo-i1SFE7glZ-j5kjddfYpxQjRDxYomYOgIyyNC8vd4bBA2rAMh2A~Lc78uAVJzkHgZHxlLUyjFrjrhqMiDhyuZ4OTAV46hhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)