Abstract
Survivors of childhood acute lymphoblastic leukemia (ALL) are at risk for late effects of cancer therapy. Five-year ALL survivors (< 21 years at diagnosis; n = 5760 eligible, 4151 participants), diagnosed from 1970 to 1986 were compared with the general population and a sibling cohort (n = 3899). Cumulative mortality of 5760 5-year survivors was 13% at 25 years from diagnosis. Recurrent ALL (n = 483) and second neoplasms (SNs; n = 89) were the major causes of death. Among 185 survivors, 199 SNs occurred, 53% in the CNS. Survivors reported more multiple chronic medical conditions (CMCs; odds ratio [OR], 2.8; 95% CI, 2.4-3.2) and severe or life-threatening CMCs (OR, 3.6; 95% CI, 3.0-4.5) than siblings. Cumulative incidence of severe CMCs, including death, 25 years from diagnosis was 21.3% (95% CI, 18.2-24.4; 23.3% [95% CI, 19.4-27.2] and 13.4% [95% CI, 8.4-18.4] for irradiated and nonirradiated survivors, respectively). Survivors reported more adverse general and mental health, functional impairment, and activity limitations compared with siblings (P < .001). Rates of marriage, college graduation, employment, and health insurance were all lower compared with sibling controls (P < .001). Long-term survivors of childhood ALL exhibit excess mortality and morbidity. Survivors who received radiation therapy as part of their treatment or had a leukemia relapse are at greatest risk for adverse outcomes.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy of childhood, accounting for approximately 25% of all childhood cancers.1 Each year, approximately 3000 new cases are diagnosed in the United States.2,3 Since the 1960s, the cure rate of ALL in children has improved dramatically from less than 30% to a current estimated 5-year overall survival of 80% to 86%.4,5 Advances in both laboratory and clinical sciences have contributed to this success.6 While ALL therapies are highly effective, studies have shown excesses of both mortality and morbidity in ALL survivors. These morbidities include second neoplasms, cardiac toxicity, infertility, neurologic toxicity, growth failure, and neurocognitive dysfunction.7-19
At the present time, many of the earliest survivors of childhood ALL are well into adulthood and, in many instances, are having all of their care provided in settings other than their original treatment centers.20 These patients have had considerable exposure to chemotherapy and radiotherapy as children, and it is clear that many of the late effects of those therapies will not be manifest until many years later. It is imperative that a comprehensive study addressing long-term health outcomes is carried out to better understand the true impact of cancer and its therapy on these individuals.
The study population is part of the Childhood Cancer Survivor Study (CCSS) cohort,21 the largest and most comprehensively characterized research cohort of long-term childhood cancer survivors. While some of the previous reports from the CCSS have focused exclusively on the childhood ALL survivors,11,22 many included ALL cases along with survivors of other pediatric malignancies.18,20,23-36,49,50,57-59,61-66 Even though the late mortality, second neoplasms, chronic health conditions, and health status in survivors of childhood cancers, including ALL, have been reported by CCSS previously,20,23-25 the current report provides results of a more in-depth and detailed analyses of ALL survivors, which in some instances used updated data, to provide a more comprehensive assessment of outcomes in this important subgroup of childhood cancer survivors. We also provide unique data on the status of survivors of childhood ALL based on their relapse and irradiation status.
Methods
Study population and surveys
CCSS cohort members were diagnosed before age 21 years with leukemia, brain tumor, Hodgkin disease, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone malignancy at one of 26 participating institutions in the United States and Canada (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) between January 1, 1970, and December 31, 1986, and survived at least 5 years following diagnosis.21 Of the 20 626 survivors of childhood cancer in the cohort, 3058 (14.8%) were lost to follow-up after extensive efforts failed to locate them. Of the remaining 17 568 subjects, 14 363 (81.8%) completed the baseline questionnaire. Of 5778 ALL survivors in the cohort, 4151 (85.8%) completed the questionnaire. To determine the potential for bias, we previously compared demographic and cancer-related characteristics of participants, nonparticipants, and those who were lost to follow-up. We found that these 3 groups were similar with regard to sex, diagnosis type, age at diagnosis, type of cancer treatment, and age at which they were asked to participate in the study (or, for those lost to follow-up, their age at the time the cohort was assembled).21,37
Medical records of participating survivors who signed a release were abstracted in a systematic manner at each institution. The baseline survey, initiated in 1994, assessed a wide range of health outcomes including subsequent neoplasms; leukemia recurrences; chronic health conditions and health status; and demographic and socioeconomic status. Follow-up surveys were conducted in 2000, 2002, and 2004. Mortality prior to January 1, 2002, was ascertained by a search of the National Death Index and from information reported on the surveys. At baseline, a random sample of survivors (4790) was asked to contact a nearest aged sibling for participation in a comparison group. Of these, 3899 (81.4%) completed the baseline questionnaire; these siblings also received the follow-up surveys. The Human Subjects Committee at each participating institution reviewed and approved the CCSS protocol. Informed consent was obtained in accordance with the Declaration of Helsinki.
Statistical analysis
Descriptive statistics of demographic and treatment characteristics were calculated for the 4151 ALL survivors, and compared with those of the 3899 siblings, taking into account the potential intrafamily correlation between a survivor and his/her sibling by bootstrapping families.38 Comparisons were also made between irradiated and nonirradiated ALL survivors as well as between ALL survivors with and without a recurrence in the first 5 years after the original diagnosis. As most patients with ALL treated on contemporary protocols do not receive radiation therapy (RT), survivors who were both nonirradiated and nonrelapsed were analyzed separately and compared with siblings to approximate current therapeutic standards.
Survival analysis was conducted for 5760 ALL survivors; 18 Canadians, whose vital status could not be determined by the National Death Index and who did not complete the baseline survey, were excluded from the 5778 eligible ALL survivors of the CCSS cohort. Overall survival probabilities of the 5760 ALL survivors at 10 and 20 years after entry into the CCSS cohort (15 and 25 years after diagnosis) were estimated by the Kaplan-Meier method and the survival curves were compared between groups of interest (radiation exposure and treatment era) by log-rank test. Assessment of mortality by radiation exposure was limited to the survivors who had signed a medical-record release form. US survivors were censored on December 31, 2002, the cutoff date of the National Death Index search, while Canadian survivors were censored at the earlier of December 31, 2002, and the last survey date. Information on cause of death was obtained from death certificates and from review of the study questionnaires. For the calculation of standardized mortality ratios (SMRs) and for overall and cause-specific mortality, person-years at risk were computed from the time of cohort entry (5 years from diagnosis) to the date of death or censoring date, stratified by age, sex, and calendar year and multiplied by year-, age-, and sex-specific US mortality rates for a specific cause of death. A 95% confidence interval for each SMR was calculated based on Poisson probability models.
Cumulative incidence probabilities of ALL recurrence and of the occurrence of a second neoplasm (SN) were estimated taking death as a competing risk.39 Recurrences and SNs that occurred before cohort entry were included as prevalent cases at the beginning of follow-up of the cohort. Standardized incidence ratios (SIRs) of overall and specific types of subsequent malignancies (excluding meningiomas and other neoplasms with an ICDO behavior code other than malignant code 3) were calculated in the same manner as SMRs, using the US Surveillance, Epidemiology, and End Results cancer incidence rates.4 Health status and chronic medical conditions were assessed for ALL survivors 18 years or older at the time of the completion of the baseline questionnaire.20,23 They were cross-sectionally compared between survivors and siblings, using logistic regression adjusting for sex, race, and age (using a natural cubic spline), taking into account for intrafamily correlation by bootstrapping families.38
The severity of chronic medical conditions was scored using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.40 There are 5 grades: 1, mild; 2, moderate; 3, severe; 4, life threatening or disabling; and 5, death. Details of the health status measurements in survivors and siblings have been reported earlier.23
We calculated age-standardized rates of marriage, educational attainment, employment, and health insurance coverage, using the survivor's age as the standard and compared with siblings. We also compared nonrelapsed versus relapsed survivors, and irradiated versus nonirradiated survivors with respect to these psychosocial outcomes. The comparisons between survivors and siblings were carried out using polytomous or binary logistic regression, adjusting for sex, race, and age (using a natural cubic spline), with bootstrapping of families to account for potential within-family correlation. The comparisons between nonrelapsed survivors and relapsed survivors and between nonirradiated survivors and irradiated survivors were adjusted for sex, race, age (using a natural cubic spline), cumulative anthracycline dose, and alkylating agent score. For each alkylating agent, the total dose received per body surface area (mg/m2) is summed for each study subject. Dose distributions for all subjects in the overall study are made for each agent and were divided into thirds. Each study subject is assigned a score of 0, 1, 2, or 3 for each separate agent, depending on whether he/she received none or fell into the lower, middle, or upper third of the distribution, respectively. The scores of the individual alkylating agents are then summed for each study subject and the summed scores were divided into tertiles, and each subject was assigned a score of 0, 1, 2, or 3 total alkylator score depending on whether he/she received none or fell into the lower, middle, or upper third of the distribution, respectively.41
Analysis of the socioeconomic outcomes was restricted to those survivors and siblings aged 25 to 49 years at baseline interview and for whom the outcome-specific data were available. All statistical analyses were carried out using SAS version 9.1 (SAS Institute, Cary, NC).
Results
Patient characteristics
Table 1 shows characteristics of the 4151 ALL survivors and 3899 siblings included in this study. Fifty-three percent of survivors and 48% of siblings were male. Median age at diagnosis for survivor was 4 years (range, 0-21 years). Survivors were younger than siblings, with median age of 26 years at follow-up compared with 31 years for siblings. Mean follow-up time was 21.2 years (range, 5-35 years) for survivors. Most of the survivors and siblings were non-Hispanic white; however, a greater proportion of survivors was of ethnic and racial minority backgrounds and had a household income of less than $20 000 per year. Among survivors, 2573 (62.0%) had received radiation therapy and 735 (17.7%) had experienced at least one relapse of their ALL, with 475 (64.6%) of these relapsing before entering the cohort and 722 (98.2%) relapsing before completion of the baseline questionnaire. Among the survivors who never relapsed and never received irradiation, 268 received both alkylator agents as well as anthracyclines, while 253 received only alkylators and 39 received only anthracyclines. Among the same group, 448 survivors did not receive either the alkylators or anthracyclines.
Characteristics of the study population
| Characteristic . | All ALL survivors, no. (%) . | Siblings, n (%) . | P* . | Nonrelapsed survivors, n (%) . | Relapsed survivors, n (%) . | P . | Nonirradiated survivors, n (%) . | Radiated survivors, n (%) . | P . |
|---|---|---|---|---|---|---|---|---|---|
| Number in each group | 4151 | 3899 | 3416 | 735 | 1069 | 2573 | |||
| Sex | <.001 | <.001 | <.001 | ||||||
| Male | 2212 (53.3) | 1878 (48.2) | 1738 (50.9) | 474 (64.5) | 494 (46.2) | 1410 (54.8) | |||
| Female | 1939 (46.7) | 2021 (51.8) | 1678 (49.1) | 261 (35.5) | 575 (53.8) | 1163 (45.2) | |||
| Race | <.001 | .89 | .22 | ||||||
| White, non-Hispanic | 3383 (81.5) | 3414 (87.6) | 2790 (81.7) | 593 (80.7) | 876 (90.0) | 2143 (83.3) | |||
| Black, non-Hispanic | 167 (4.0) | 103 (2.6) | 137 (4.0) | 30 (4.1) | 33 (3.1) | 85 (3.3) | |||
| Hispanic, Latino | 269 (6.5) | 138 (3.5) | 221 (6.5) | 48 (6.5) | 74 (6.9) | 133 (5.2) | |||
| Other, unknown | 332 (8.0) | 244 (6.3) | 268 (7.8) | 64 (8.7) | 86 (8.0) | 212 (8.2) | |||
| Household income | <.001 | .77 | <.001 | ||||||
| Less than $20 000 | 633 (18.3) | 352 (10.2) | 526 (18.4) | 107 (17.9) | 126 (13.6) | 415 (19.4) | |||
| $20 000 and over | 2818 (81.7) | 3108 (89.8) | 2328 (81.6) | 490 (82.1) | 802 (86.4) | 1723 (80.6) | |||
| Age at questionnaire, y | <.001 | <.001 | <.001 | ||||||
| 0 to 9 | 58 (1.4) | 12 (0.3) | 12 (0.4) | 46 (2.3) | 5 (0.5) | 44 (1.7) | |||
| 10 to 19 | 628 (15.1) | 379 (9.7) | 411 (12.0) | 217 (29.5) | 187 (17.4) | 355 (13.8) | |||
| 20 to 29 | 2084 (50.2) | 1353 (34.7) | 1780 (52.1) | 304 (41.4) | 658 (61.6) | 1176 (45.7) | |||
| 30 to 39 | 1215 (29.3) | 1332 (34.2) | 1059 (31.0) | 156 (21.2) | 190 (17.8) | 877 (34.1) | |||
| 40 to 49 | 165 (4.0) | 728 (18.7) | 153 (4.5) | 12 (1.6) | 29 (2.7) | 120 (4.7) | |||
| 50 and over | 1 (0.0) | 95 (2.4) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) | |||
| Age at diagnosis, y | .78 | <.001 | |||||||
| 0 to 4 | 2254 (54.3) | NA | NA | 1844 (54.0) | 410 (55.8) | 658 (61.5) | 1336 (51.9) | ||
| 5 to 9 | 1140 (27.5) | NA | NA | 949 (27.8) | 191 (26.0) | 282 (26.4) | 709 (27.6) | ||
| 10 to 14 | 532 (12.8) | NA | NA | 437 (12.8) | 95 (12.9) | 92 (8.6) | 371 (14.4) | ||
| 15 to 21 | 225 (5.4) | NA | NA | 186 (5.4) | 39 (5.3) | 37 (3.5) | 157 (6.1) | ||
| Survival time since diagnosis, y | <.001 | <.001 | |||||||
| 5 to 14 | 337 (8.1) | NA | NA | 98 (2.9) | 239 (32.5) | 33 (3.1) | 257 (10.0) | ||
| 15 to 24 | 2633 (63.4) | NA | NA | 2273 (66.5) | 360 (49.0) | 868 (81.2) | 1457 (56.6) | ||
| 25 to 35 | 1181 (28.5) | NA | NA | 1045 (30.6) | 136 (18.5) | 168 (15.7) | 859 (33.4) | ||
| Treatment era | .06 | <.001 | |||||||
| 1970 to 1974 | 650 (15.7) | NA | NA | 514 (15.0) | 136 (18.5) | 103 (9.6) | 466 (18.1) | ||
| 1975 to 1979 | 1089 (26.2) | NA | NA | 901 (26.4) | 188 (25.6) | 109 (10.2) | 835 (32.5) | ||
| 1980 to 1986 | 2412 (58.1) | NA | NA | 2001 (58.6) | 411 (55.9) | 857 (80.2) | 1272 (49.4) | ||
| Chemotherapy (CT) | |||||||||
| Anthracyclines | 1782 (48.7) | NA | NA | 1291 (42.8) | 491 (77.1) | <.001 | 347 (32.5) | 1430 (55.6) | <.001 |
| Alkylating agents | 2006 (54.9) | NA | NA | 1493 (49.4) | 513 (80.5) | <.001 | 566 (53.0) | 1433 (55.7) | .13 |
| Both anthracyclines plus alkylating agents | 1444 (39.5) | NA | NA | 994 (32.9) | 450 (70.6) | <.001 | 305 (28.5) | 1135 (44.1) | <.001 |
| Radiation therapy (RT) | <.001 | NA | |||||||
| Cranial/craniospinal RT | 2266 (92.2) | NA | NA | 1877 (97.3) | 384 (72.7) | NA | 2261 (92.0) | ||
| Total body irradiation (TBI) | 134 (5.5) | NA | NA | 31 (1.6) | 103 (19.5) | NA | 134 (5.5) | ||
| Other sites | 62 (2.3) | NA | NA | 21 (1.1) | 41 (7.8) | NA | 62 (2.5) | ||
| Bone marrow transplant (BMT) | 205 (4.9) | NA | NA | 50 (1.5) | 155 (21.1) | <.001† | 13 (1.2) | 167 (6.5) | NA |
| Characteristic . | All ALL survivors, no. (%) . | Siblings, n (%) . | P* . | Nonrelapsed survivors, n (%) . | Relapsed survivors, n (%) . | P . | Nonirradiated survivors, n (%) . | Radiated survivors, n (%) . | P . |
|---|---|---|---|---|---|---|---|---|---|
| Number in each group | 4151 | 3899 | 3416 | 735 | 1069 | 2573 | |||
| Sex | <.001 | <.001 | <.001 | ||||||
| Male | 2212 (53.3) | 1878 (48.2) | 1738 (50.9) | 474 (64.5) | 494 (46.2) | 1410 (54.8) | |||
| Female | 1939 (46.7) | 2021 (51.8) | 1678 (49.1) | 261 (35.5) | 575 (53.8) | 1163 (45.2) | |||
| Race | <.001 | .89 | .22 | ||||||
| White, non-Hispanic | 3383 (81.5) | 3414 (87.6) | 2790 (81.7) | 593 (80.7) | 876 (90.0) | 2143 (83.3) | |||
| Black, non-Hispanic | 167 (4.0) | 103 (2.6) | 137 (4.0) | 30 (4.1) | 33 (3.1) | 85 (3.3) | |||
| Hispanic, Latino | 269 (6.5) | 138 (3.5) | 221 (6.5) | 48 (6.5) | 74 (6.9) | 133 (5.2) | |||
| Other, unknown | 332 (8.0) | 244 (6.3) | 268 (7.8) | 64 (8.7) | 86 (8.0) | 212 (8.2) | |||
| Household income | <.001 | .77 | <.001 | ||||||
| Less than $20 000 | 633 (18.3) | 352 (10.2) | 526 (18.4) | 107 (17.9) | 126 (13.6) | 415 (19.4) | |||
| $20 000 and over | 2818 (81.7) | 3108 (89.8) | 2328 (81.6) | 490 (82.1) | 802 (86.4) | 1723 (80.6) | |||
| Age at questionnaire, y | <.001 | <.001 | <.001 | ||||||
| 0 to 9 | 58 (1.4) | 12 (0.3) | 12 (0.4) | 46 (2.3) | 5 (0.5) | 44 (1.7) | |||
| 10 to 19 | 628 (15.1) | 379 (9.7) | 411 (12.0) | 217 (29.5) | 187 (17.4) | 355 (13.8) | |||
| 20 to 29 | 2084 (50.2) | 1353 (34.7) | 1780 (52.1) | 304 (41.4) | 658 (61.6) | 1176 (45.7) | |||
| 30 to 39 | 1215 (29.3) | 1332 (34.2) | 1059 (31.0) | 156 (21.2) | 190 (17.8) | 877 (34.1) | |||
| 40 to 49 | 165 (4.0) | 728 (18.7) | 153 (4.5) | 12 (1.6) | 29 (2.7) | 120 (4.7) | |||
| 50 and over | 1 (0.0) | 95 (2.4) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) | |||
| Age at diagnosis, y | .78 | <.001 | |||||||
| 0 to 4 | 2254 (54.3) | NA | NA | 1844 (54.0) | 410 (55.8) | 658 (61.5) | 1336 (51.9) | ||
| 5 to 9 | 1140 (27.5) | NA | NA | 949 (27.8) | 191 (26.0) | 282 (26.4) | 709 (27.6) | ||
| 10 to 14 | 532 (12.8) | NA | NA | 437 (12.8) | 95 (12.9) | 92 (8.6) | 371 (14.4) | ||
| 15 to 21 | 225 (5.4) | NA | NA | 186 (5.4) | 39 (5.3) | 37 (3.5) | 157 (6.1) | ||
| Survival time since diagnosis, y | <.001 | <.001 | |||||||
| 5 to 14 | 337 (8.1) | NA | NA | 98 (2.9) | 239 (32.5) | 33 (3.1) | 257 (10.0) | ||
| 15 to 24 | 2633 (63.4) | NA | NA | 2273 (66.5) | 360 (49.0) | 868 (81.2) | 1457 (56.6) | ||
| 25 to 35 | 1181 (28.5) | NA | NA | 1045 (30.6) | 136 (18.5) | 168 (15.7) | 859 (33.4) | ||
| Treatment era | .06 | <.001 | |||||||
| 1970 to 1974 | 650 (15.7) | NA | NA | 514 (15.0) | 136 (18.5) | 103 (9.6) | 466 (18.1) | ||
| 1975 to 1979 | 1089 (26.2) | NA | NA | 901 (26.4) | 188 (25.6) | 109 (10.2) | 835 (32.5) | ||
| 1980 to 1986 | 2412 (58.1) | NA | NA | 2001 (58.6) | 411 (55.9) | 857 (80.2) | 1272 (49.4) | ||
| Chemotherapy (CT) | |||||||||
| Anthracyclines | 1782 (48.7) | NA | NA | 1291 (42.8) | 491 (77.1) | <.001 | 347 (32.5) | 1430 (55.6) | <.001 |
| Alkylating agents | 2006 (54.9) | NA | NA | 1493 (49.4) | 513 (80.5) | <.001 | 566 (53.0) | 1433 (55.7) | .13 |
| Both anthracyclines plus alkylating agents | 1444 (39.5) | NA | NA | 994 (32.9) | 450 (70.6) | <.001 | 305 (28.5) | 1135 (44.1) | <.001 |
| Radiation therapy (RT) | <.001 | NA | |||||||
| Cranial/craniospinal RT | 2266 (92.2) | NA | NA | 1877 (97.3) | 384 (72.7) | NA | 2261 (92.0) | ||
| Total body irradiation (TBI) | 134 (5.5) | NA | NA | 31 (1.6) | 103 (19.5) | NA | 134 (5.5) | ||
| Other sites | 62 (2.3) | NA | NA | 21 (1.1) | 41 (7.8) | NA | 62 (2.5) | ||
| Bone marrow transplant (BMT) | 205 (4.9) | NA | NA | 50 (1.5) | 155 (21.1) | <.001† | 13 (1.2) | 167 (6.5) | NA |
Numbers of subjects may not add up exactly to the total number of subjects due to missing values.
NA indicates not applicable.
P values indicate statistical significance levels of group differences with respect to the distribution of each characteristic. For CT and BMT characteristics, however, a P value corresponds to the therapeutic exposure listed in each row and indicates the statistical significance level of group differences with respect to the exposure.
Survival
Overall survival (OS) at 25 years from diagnosis was 86.8% for all ALL survivors (95% confidence interval [CI], 85.9-97.8; Figure 1A). OS was 96.1% (95% CI, 94.7-97.1) for survivors treated without RT and 87.3% (95% CI, 85.8-88.6) for survivors treated with RT (P < .001; Figure 1B). For patients treated in 1970-1974, 1975-1979, and 1980-1986, 20-year survival from diagnosis improved from 82% to 87% to 90% (P < .001; Figure 1C). Among those who did not have a relapse within 5 years of their original diagnosis, OS was 92.6% (95% CI, 91.5-93.5), compared with 63.3% (95% CI, 58.8-67.6) for those who did relapse within 5 years (P < .001; figure not shown). OS for nonirradiated, nonrelapsed survivors (survivors without history of RT and no relapse within 5 years from diagnosis) was 97.8% (95% CI, 96.7-98.6; figure not shown).
Overall survival of ALL survivors. (A) Overall survival of ALL survivors in comparison with US population. For panels A and C, assessment of late mortality included all 5760 patients of the 5778 eligible 5-year ALL survivors, excluding 18 Canadians who were not covered by NDI search and did not fill out any questionnaire. (B) Overall survival in ALL survivors with and without history of irradiation. (C) Overall survival of ALL survivors in different treatment era.
Overall survival of ALL survivors. (A) Overall survival of ALL survivors in comparison with US population. For panels A and C, assessment of late mortality included all 5760 patients of the 5778 eligible 5-year ALL survivors, excluding 18 Canadians who were not covered by NDI search and did not fill out any questionnaire. (B) Overall survival in ALL survivors with and without history of irradiation. (C) Overall survival of ALL survivors in different treatment era.
Among the 5760 5-year ALL survivors, 730 deaths occurred; cumulative mortality was 13% at 25 years from diagnosis. Recurrent ALL accounted for 483 (66.2%) deaths, while 89 individuals (12.2%) died of SN and 15 (2.1%) died of cardiac causes. Ten survivors died of infectious complications; 7, of pulmonary toxicity; 7, of other sequelae of their ALL therapy; and 64, of other causes. In 55 cases, the cause of death could not be verified.
Second neoplasms
Excluding the 254 cases of nonmelanoma skin cancer, but including meningiomas, 185 survivors reported 199 SNs. Of these, 190 (95%) occurred in 177 survivors after entry into the cohort. RT patients reported 81% (n = 162) of the SNs, with 155 occurring after entering the cohort. A total of 106 SNs (53.3%) occurred in the CNS, including meningioma (66), astrocytoma (22), other glial tumors (5), medulloblastoma (3), and nonspecified CNS tumors (10). Other reported SNs included breast cancer (11), thyroid cancer (16), acute myelogenous leukemia (AML; 4), sarcoma (13), non-Hodgkin lymphoma (7), skin cancer (9), and others (26). SNs reported in patients without history of RT included AML (1), CNS (4), breast cancer (4), lymphoma (3), skin cancer (2), sarcoma (1), and others (4). The cumulative incidence (Figure 2) of SN at 25 years was 5.2% (95% CI, 4.3-6.1), with irradiated and nonirradiated survivors having an incidence of 6.2% (95% CI, 5.0-7.3) and 3.1% (95% CI, 1.1-5.0), respectively. The SIR for malignant neoplasms was 5.0 (95% CI, 4.1-6.0).
Cumulative incidence of SNs in ALL survivors with and without history of irradiation.
Cumulative incidence of SNs in ALL survivors with and without history of irradiation.
Chronic medical conditions
One or more chronic medical conditions were reported by 50.0% of survivors, compared with 37.8% of siblings (P < .001; Table 2). Survivors were found to be 3.7 times more likely (95% CI, 3.0-4.5) than siblings to report a severe or life-threatening chronic medical condition and 2.8 times more likely (95% CI, 2.4-3.2) to report multiple chronic medical conditions, after adjusting for age at interview, sex, and ethnicity (Table 3). Highest risks were seen for musculoskeletal (OR, 7.7), cardiac (OR, 6.9), and neurologic (OR, 5.3) conditions (Table 3). Most of the grade 3 to 4 musculoskeletal morbidity reported was related to major joint replacement (14), cardiac conditions including congestive heart failure (CHF) on medication (15), coronary artery disease on medications (18), myocardial infarction (1), cardiac arrest (2), and cerebrovascular accidents (47), while the pulmonary conditions includes pulmonary fibrosis on O2 (1), and thromboembolic disease (76). The grade 3 to 4 neurologic conditions included cranial nerve paralysis (6), severe cognitive deficits (23), and monoquadriplegia and other paralysis syndromes (36).
Number and percentage of ALL survivors and siblings with a chronic physical health condition, with CTCAE v3 severity score
| CTCAE v3 severity score* . | ALL survivors,† n (%) . | Siblings,†n (%) . | P‡ . |
|---|---|---|---|
| Number in each group | 2599 | 3083 | |
| No condition | 1299 (50.0) | 1919 (62.2) | |
| Grade 1, mild | 535 (20.6) | 627 (20.4) | |
| Grade 2, moderate | 376 (14.5) | 358 (11.6) | <.001‖ |
| Grade 3, severe | 250 (9.6) | 144 (4.7) | |
| Grade 4, life threatening | 127 (4.9) | 35 (1.1) | |
| Grade 5, death | 12 (0.4) | NA | |
| Any grade 1 to health condition§ | 1298 (49.9) | 1164 (37.8) | <.001 |
| Any grade 3 to 4 health condition§ | 382 (14.7) | 179 (5.8) | <.001 |
| Multiple health conditions | |||
| 2 or more health conditions | 667 (25.7) | 433 (14.0) | <.001 |
| 3 or more health conditions | 381 (14.7) | 180 (5.8) | <.001 |
| CTCAE v3 severity score* . | ALL survivors,† n (%) . | Siblings,†n (%) . | P‡ . |
|---|---|---|---|
| Number in each group | 2599 | 3083 | |
| No condition | 1299 (50.0) | 1919 (62.2) | |
| Grade 1, mild | 535 (20.6) | 627 (20.4) | |
| Grade 2, moderate | 376 (14.5) | 358 (11.6) | <.001‖ |
| Grade 3, severe | 250 (9.6) | 144 (4.7) | |
| Grade 4, life threatening | 127 (4.9) | 35 (1.1) | |
| Grade 5, death | 12 (0.4) | NA | |
| Any grade 1 to health condition§ | 1298 (49.9) | 1164 (37.8) | <.001 |
| Any grade 3 to 4 health condition§ | 382 (14.7) | 179 (5.8) | <.001 |
| Multiple health conditions | |||
| 2 or more health conditions | 667 (25.7) | 433 (14.0) | <.001 |
| 3 or more health conditions | 381 (14.7) | 180 (5.8) | <.001 |
Survivors' health conditions do not include any problems prior to the cancer diagnosis or acute problems prior to 5 years after cancer diagnosis.
NA indicates not applicable.
Common Terminology Criteria for Adverse Events version 3.
Restricted to those age 18 years and older. To be eligible, siblings had to be alive at time of enrollment. Survivors may have died in the interval between 5 years after cancer diagnosis and time of study. The composite percentage of survivors with grade 3 or 4 conditions includes conditions that were reported before the time of death in the 12 survivors who died.
Adjusted for race, sex, and age at interview.
The number may not reflect the sum of grade 1 to grade 4. Grade 1 to grade 5 was calculated by taking the maximum grade per subject. A subject with grade 5 may have other lower grades.
Comparison between no condition versus any condition.
Frequencies and odds ratios of chronic medical conditions and adverse health status in siblings, all ALL survivors, and nonrelapsed, nonirradiated survivors (restricted to those age 18 years and older)
| Outcome . | Siblings, n (%) . | All ALL survivors, n (%) . | OR for all ALL survivors vs siblings (95% CI) † . | P . | Nonrelapsed, nonirradiated survivors, n (%) . | OR for nonrelapsed, nonirradiated survivors vs siblings (95% CI)† . | P . |
|---|---|---|---|---|---|---|---|
| Number in each group | 3083 | 2599 | 426 | ||||
| Chronic medical conditions | |||||||
| Hearing | 12 (0.4) | 27 (1.0) | 3.0 (1.5-6.8) | <.001 | 4 (0.9) | 2.7 (0.5-7.8) | .17 |
| Vision | 21 (0.7) | 30 (1.2) | 1.6 (0.9-3.1) | .19 | 2 (0.5) | 0.5 (0.1-1.6) | .26 |
| Endocrine | 56 (1.8) | 114 (4.4) | 3.1 (2.3-4.5) | <.001 | 5 (1.2) | 1.0 (0.2-2.2) | .96 |
| Pulmonary | 37 (1.2) | 78 (3.0) | 4.2 (2.8-6.6) | <.001 | 8 (1.9) | 2.7 (1.0-5.1) | .05 |
| Cardiac | 21 (0.7) | 82 (3.2) | 6.9 (4.212.9) | <.001 | 6 (1.4) | 3.8 (0.9-10.0) | .06 |
| Gastrointestinal | 14 (0.5) | 18 (0.7) | 2.2 (1.0-5.0) | .04 | 1 (0.2) | 1.0 (0.2-5.7) | .96 |
| Renal | 5 (0.2) | 21 (0.8) | 4.8 (2.118.9) | <.001 | 2 (0.5) | 4.1 (0.5-37.0) | .29 |
| Musculoskeletal | 3 (0.1) | 14 (0.5) | 7.7 (2.821.3) | <.001 | 4 (0.9) | 22.8 (4.0-128.1) | .03 |
| Neurologic | 13 (0.4) | 62 (2.4) | 5.3 (3.111.4) | <.001 | 3 (0.7) | 1.6 (0.5-5.4) | .49 |
| Grade 3 to 4 | 179 (5.8) | 382 (14.7) | 3.7 (3.0-4.5) | <.001 | 34 (8.0) | 2.0 (1.3-3.0) | .01 |
| 2 or more in grades 1 to 4 | 433 (14.0) | 667 (25.7) | 2.8 (2.4-3.2) | <.001 | 73 (17.1) | 1.9 (1.4-2.5) | <.001 |
| Adverse health status | |||||||
| General health | 157 (5.1) | 230 (8.9) | 2.1 (1.6-2.7) | <.001 | 24 (5.6) | 1.2 (0.7-1.9) | .45 |
| Mental health | 302 (9.8) | 389 (15.0) | 1.7 (1.4-2.0) | <.001 | 61 (14.3) | 1.6 (1.1-2.1) | .01 |
| Activity limitation | 178 (5.8) | 230 (8.9) | 1.8 (1.5-2.3) | <.001 | 28 (6.6) | 1.3 (0.8-2.0) | .25 |
| Functional impairment | 79 (2.6) | 227 (8.7) | 4.1 (3.1-5.6) | <.001 | 18 (4.2) | 2.0 (1.0-3.3) | .05 |
| Outcome . | Siblings, n (%) . | All ALL survivors, n (%) . | OR for all ALL survivors vs siblings (95% CI) † . | P . | Nonrelapsed, nonirradiated survivors, n (%) . | OR for nonrelapsed, nonirradiated survivors vs siblings (95% CI)† . | P . |
|---|---|---|---|---|---|---|---|
| Number in each group | 3083 | 2599 | 426 | ||||
| Chronic medical conditions | |||||||
| Hearing | 12 (0.4) | 27 (1.0) | 3.0 (1.5-6.8) | <.001 | 4 (0.9) | 2.7 (0.5-7.8) | .17 |
| Vision | 21 (0.7) | 30 (1.2) | 1.6 (0.9-3.1) | .19 | 2 (0.5) | 0.5 (0.1-1.6) | .26 |
| Endocrine | 56 (1.8) | 114 (4.4) | 3.1 (2.3-4.5) | <.001 | 5 (1.2) | 1.0 (0.2-2.2) | .96 |
| Pulmonary | 37 (1.2) | 78 (3.0) | 4.2 (2.8-6.6) | <.001 | 8 (1.9) | 2.7 (1.0-5.1) | .05 |
| Cardiac | 21 (0.7) | 82 (3.2) | 6.9 (4.212.9) | <.001 | 6 (1.4) | 3.8 (0.9-10.0) | .06 |
| Gastrointestinal | 14 (0.5) | 18 (0.7) | 2.2 (1.0-5.0) | .04 | 1 (0.2) | 1.0 (0.2-5.7) | .96 |
| Renal | 5 (0.2) | 21 (0.8) | 4.8 (2.118.9) | <.001 | 2 (0.5) | 4.1 (0.5-37.0) | .29 |
| Musculoskeletal | 3 (0.1) | 14 (0.5) | 7.7 (2.821.3) | <.001 | 4 (0.9) | 22.8 (4.0-128.1) | .03 |
| Neurologic | 13 (0.4) | 62 (2.4) | 5.3 (3.111.4) | <.001 | 3 (0.7) | 1.6 (0.5-5.4) | .49 |
| Grade 3 to 4 | 179 (5.8) | 382 (14.7) | 3.7 (3.0-4.5) | <.001 | 34 (8.0) | 2.0 (1.3-3.0) | .01 |
| 2 or more in grades 1 to 4 | 433 (14.0) | 667 (25.7) | 2.8 (2.4-3.2) | <.001 | 73 (17.1) | 1.9 (1.4-2.5) | <.001 |
| Adverse health status | |||||||
| General health | 157 (5.1) | 230 (8.9) | 2.1 (1.6-2.7) | <.001 | 24 (5.6) | 1.2 (0.7-1.9) | .45 |
| Mental health | 302 (9.8) | 389 (15.0) | 1.7 (1.4-2.0) | <.001 | 61 (14.3) | 1.6 (1.1-2.1) | .01 |
| Activity limitation | 178 (5.8) | 230 (8.9) | 1.8 (1.5-2.3) | <.001 | 28 (6.6) | 1.3 (0.8-2.0) | .25 |
| Functional impairment | 79 (2.6) | 227 (8.7) | 4.1 (3.1-5.6) | <.001 | 18 (4.2) | 2.0 (1.0-3.3) | .05 |
CI indicates confidence interval.
* Adjusted for age at interview, sex, and ethnicity.
Adjusted for cumulative anthracycline dose, alkylator score, age at interview, age at diagnosis, sex, and ethnicity.
After adjusting for age at interview, sex, and ethnicity, nonirradiated, nonrelapsed survivors were 2.0 times as likely to report a severe or life-threatening condition and 1.9 times as likely to report 2 or more chronic medical conditions, compared with siblings (Table 3). Nonirradiated, nonrelapsed survivors were 2.7 times and 22.8 times as likely to report pulmonary and musculoskeletal conditions, respectively, compared with siblings, but the likelihood of their reporting other chronic conditions was similar to that of siblings (Table 3). The musculoskeletal condition reported was major joint replacement (4), and pulmonary morbidities included thromboembolic disease (7) and respiratory arrest (1). Relapsed survivors were more likely to report a severe, life-threatening chronic condition, multiple chronic medical conditions, and endocrine, cardiac, visual, renal, and neurologic chronic medical conditions than nonrelapsed survivors (Table 4). Irradiated survivors were more likely to report multiple chronic medical conditions, severe, life-threatening chronic conditions, and endocrine conditions (Table 4).
Frequencies and odds ratios of chronic medical conditions and adverse health status in nonrelapsed, relapsed, nonirradiated and irradiated survivors (restricted to those age 18 years and older)
| Outcome . | Nonrelapsed survivors, n (%) . | Relapsed survivors, n (%) . | OR for relapsed versus nonrelapsed survivors (95% CI)* . | P . | Nonirradiated survivors, n (%) . | Irradiated survivors, n (%) . | OR for irradiated versus nonirradiated survivors† (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| No. in each group | 2222 | 377 | 451 | 1770 | ||||
| Chronic medical conditions | ||||||||
| Hearing | 20 (0.9) | 7 (1.9) | 2.3 (0.9-5.7) | .09 | 4 (0.9) | 22 (1.2) | 1.1 (0.4-3.4) | .88 |
| Vision | 21 (1.0) | 9 (2.4) | 3.3 (1.4-7.9) | .006 | 4 (0.9) | 23 (1.3) | 1.4 (0.4-4.2) | .61 |
| Endocrine | 71 (3.2) | 43 (11.4) | 5.4 (3.4-8.6) | <.001 | 6 (1.3) | 90 (5.1) | 4.1 (1.6-10.4) | .003 |
| Pulmonary | 59 (2.7) | 19 (5.0) | 1.8 (1.0-3.4) | .06 | 13 (2.9) | 59 (3.3) | 1.2 (0.6-2.5) | .53 |
| Cardiac | 54 (2.4) | 28 (7.4) | 2.8 (1.5-4.9) | <.001 | 10 (2.2) | 59 (3.3) | 1.6 (0.8-3.3) | .22 |
| Gastrointestinal | 14 (0.6) | 4 (1.1) | 1.5 (0.4-5.3) | .53 | 2 (0.4) | 16 (0.9) | 1.9 (0.4-8.5) | .42 |
| Renal | 11 (0.5) | 10 (2.7) | 5.2 (2.0-13.8) | <.001 | 3 (0.7) | 16 (0.9) | 1.4 (0.4-5.0) | .59 |
| Musculoskeletal | 11 (0.5) | 3 (0.8) | 1.3 (0.4-5.0) | .68 | 4 (0.9) | 10 (0.6) | 0.6 (0.2-2.2) | .46 |
| Neurologic | 40 (1.8) | 22 (5.8) | 3.2 (1.7-6.2) | <.001 | 6 (1.3) | 46 (2.6) | 1.6 (0.6-3.8) | .34 |
| Grade 3 to 4 | 268 (12.1) | 114 (30.2) | 3.4 (2.5-4.5) | <.001 | 46 (10.2) | 291 (16.4) | 1.7 (1.2-2.4) | .005 |
| 2 or more in grades 1 to 4 | 492 (22.1) | 175 (46.4) | 3.0 (2.3-3.9) | <.001 | 86 (19.1) | 479 (27.1) | 1.5 (1.1-2.0) | .005 |
| Adverse health status | ||||||||
| General health | 190 (8.6) | 40 (10.6) | 1.7 (1.1-2.6) | .01 | 30 (6.7) | 162 (9.2) | 1.4 (0.9-2.2) | .12 |
| Mental health | 334 (15.0) | 55 (14.6) | 1.1 (0.8-1.6) | .62 | 66 (14.6) | 276 (15.6) | 1.0 (0.7-1.3) | .86 |
| Activity limitation | 169 (7.6) | 61 (16.2) | 2.5 (1.7-3.7) | <.001 | 32 (7.1) | 164 (9.3) | 1.5 (1.0-2.3) | .07 |
| Functional impairment | 186 (8.4) | 41 (10.9) | 1.7 (1.1-2.5) | .02 | 23 (5.1) | 173 (9.8) | 2.2 (1.4-3.5) | .001 |
| Outcome . | Nonrelapsed survivors, n (%) . | Relapsed survivors, n (%) . | OR for relapsed versus nonrelapsed survivors (95% CI)* . | P . | Nonirradiated survivors, n (%) . | Irradiated survivors, n (%) . | OR for irradiated versus nonirradiated survivors† (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| No. in each group | 2222 | 377 | 451 | 1770 | ||||
| Chronic medical conditions | ||||||||
| Hearing | 20 (0.9) | 7 (1.9) | 2.3 (0.9-5.7) | .09 | 4 (0.9) | 22 (1.2) | 1.1 (0.4-3.4) | .88 |
| Vision | 21 (1.0) | 9 (2.4) | 3.3 (1.4-7.9) | .006 | 4 (0.9) | 23 (1.3) | 1.4 (0.4-4.2) | .61 |
| Endocrine | 71 (3.2) | 43 (11.4) | 5.4 (3.4-8.6) | <.001 | 6 (1.3) | 90 (5.1) | 4.1 (1.6-10.4) | .003 |
| Pulmonary | 59 (2.7) | 19 (5.0) | 1.8 (1.0-3.4) | .06 | 13 (2.9) | 59 (3.3) | 1.2 (0.6-2.5) | .53 |
| Cardiac | 54 (2.4) | 28 (7.4) | 2.8 (1.5-4.9) | <.001 | 10 (2.2) | 59 (3.3) | 1.6 (0.8-3.3) | .22 |
| Gastrointestinal | 14 (0.6) | 4 (1.1) | 1.5 (0.4-5.3) | .53 | 2 (0.4) | 16 (0.9) | 1.9 (0.4-8.5) | .42 |
| Renal | 11 (0.5) | 10 (2.7) | 5.2 (2.0-13.8) | <.001 | 3 (0.7) | 16 (0.9) | 1.4 (0.4-5.0) | .59 |
| Musculoskeletal | 11 (0.5) | 3 (0.8) | 1.3 (0.4-5.0) | .68 | 4 (0.9) | 10 (0.6) | 0.6 (0.2-2.2) | .46 |
| Neurologic | 40 (1.8) | 22 (5.8) | 3.2 (1.7-6.2) | <.001 | 6 (1.3) | 46 (2.6) | 1.6 (0.6-3.8) | .34 |
| Grade 3 to 4 | 268 (12.1) | 114 (30.2) | 3.4 (2.5-4.5) | <.001 | 46 (10.2) | 291 (16.4) | 1.7 (1.2-2.4) | .005 |
| 2 or more in grades 1 to 4 | 492 (22.1) | 175 (46.4) | 3.0 (2.3-3.9) | <.001 | 86 (19.1) | 479 (27.1) | 1.5 (1.1-2.0) | .005 |
| Adverse health status | ||||||||
| General health | 190 (8.6) | 40 (10.6) | 1.7 (1.1-2.6) | .01 | 30 (6.7) | 162 (9.2) | 1.4 (0.9-2.2) | .12 |
| Mental health | 334 (15.0) | 55 (14.6) | 1.1 (0.8-1.6) | .62 | 66 (14.6) | 276 (15.6) | 1.0 (0.7-1.3) | .86 |
| Activity limitation | 169 (7.6) | 61 (16.2) | 2.5 (1.7-3.7) | <.001 | 32 (7.1) | 164 (9.3) | 1.5 (1.0-2.3) | .07 |
| Functional impairment | 186 (8.4) | 41 (10.9) | 1.7 (1.1-2.5) | .02 | 23 (5.1) | 173 (9.8) | 2.2 (1.4-3.5) | .001 |
CI indicates confidence interval.
Adjusted for age at interview, age at diagnosis, sex, and ethnicity.
Adjusted for cumulative anthracycline dose, alkylator score, age at interview, age at diagnosis, sex, and ethnicity.
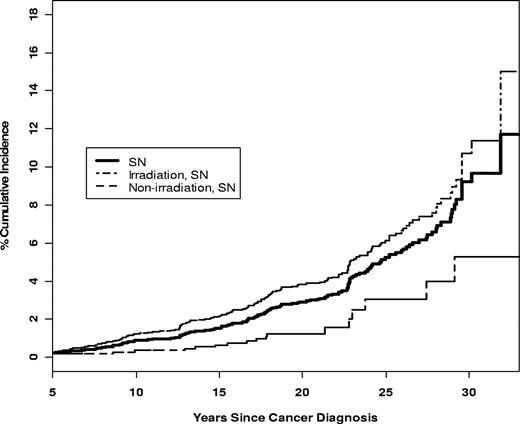
Among all ALL survivors, the cumulative incidence 25 years from diagnosis of any chronic medical condition (grades 1-5) was 64.9% (95% CI, 61.0-68.9), while that of a severe, life-threatening condition was 21.3% (95% CI, 18.2-24.4). Among irradiated survivors, the cumulative incidence at 25 years from diagnosis of any chronic medical condition was 68.5% (95% CI, 63.5-73.4), compared with 53.3% (95% CI, 46.2-60.5) for nonirradiated survivors. Similarly, the cumulative incidence at 25 years from diagnosis of a severe, life-threatening chronic condition including death (grades 3-5) was 23.3% (95% CI, 19.4-27.2) for irradiated survivors, compared with 13.4% (95% CI, 8.4-18.4) for nonirradiated survivors (Figure 3). Ninety-two percent of nonirradiated, nonrelapsed survivors reported no grade 3 to 4 CMCs.
Cumulative incidence of severe chronic medical conditions in ALL survivors with and without history of irradiation.
Cumulative incidence of severe chronic medical conditions in ALL survivors with and without history of irradiation.
Health status
Among ALL survivors and siblings, 18 years or older at the time of the baseline questionnaire, significantly more survivors reported poor general health, mental health problems, activity limitations, and functional impairment compared with siblings (Table 3). When adjusted for age at interview, sex, and ethnicity, nonirradiated, nonrelapsed survivors reported adverse mental status and functional impairment 1.6 and 2.0 times as often, respectively, as did siblings, but neither general health status nor reported activity limitations differed significantly between the 2 groups (Table 3).
When adjusted for age at interview, sex, and ethnicity, relapsed survivors were 1.6, 1.7, and 2.6 times as likely to report poor general health, functional impairment, and activity limitations, respectively, compared with nonrelapsed survivors (Table 4). When adjusted for age at interview, sex, ethnicity, cumulative anthracycline dose, and alkylator score, irradiated survivors were 2.2 times more likely to report functional impairment than were nonirradiated survivors (Table 4).
Marriage, employment, education, and health insurance
We compared ALL survivors and siblings between 25 to 49 years of age with respect to marriage, educational attainments, employment status, and health insurance coverage (Table 5). In analyses stratified by sex, survivors' rates of marriage, college graduation, and of health insurance coverage were all significantly lower than those of siblings when adjusted for age (Table 5). With respect to employment, both female and male survivors were more likely to be unemployed than their sibling counterparts, but for males, the difference did not show statistical significance (P = .07).
Frequencies of socioeconomic outcomes in siblings, all ALL survivors, nonrelapsed, nonirradiated survivors, relapsed, nonrelapsed, nonirradiated, and irradiated survivors*
| Outcomes . | Siblings† . | ALL survivors . | P for survivors versus siblings . | Nonrelapsed, nonirradiated survivors . | P for nonrelapsed, nonirradiated survivors versus siblings‡ . | Relapsed survivors . | P for relapsed survivors versus nonrelapsed survivors . | Irradiated survivors . | P for irradiated survivors versus nonirradiated survivors . |
|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||
| Marital status, no. | 1302 | 1206 | <.001 | 183 | .56 | 180 | .04 | 836 | .11 |
| Never married, % | 31.3 | 44.6 | 39.3 | 48.9 | 45.9 | ||||
| Married, % | 59.9 | 48.3 | 53.6 | 40.6 | 47.5 | ||||
| No longer married, % | 8.9 | 7.1 | 7.1 | 10.5 | 6.6 | ||||
| Education, no. | 1299 | 1207 | <.001 | 184 | .99 | 180 | .30 | 835 | .03 |
| Not high school graduate, % | 3.0 | 6.4 | 3.3 | 3.9 | 6.1 | ||||
| High school graduate, % | 48.5 | 55.7 | 50.0 | 59.4 | 55.0 | ||||
| College graduate, % | 48.5 | 37.9 | 46.7 | 36.7 | 38.9 | ||||
| Employment, no. | 979 | 888 | .07 | 143 | .96 | 128 | .87 | 631 | .80 |
| Full time, % | 93.7 | 90.4 | 91.6 | 89.1 | 90.0 | ||||
| Part time, % | 3.9 | 4.8 | 4.9 | 5.5 | 5.2 | ||||
| Unemployed, % | 2.4 | 4.7 | 3.5 | 5.5 | 4.8 | ||||
| Health insurance‡, no. | 1201 | 1136 | .02 | 181 | .21 | 162 | .69 | 774 | .27 |
| Public health insurance, % | 3.0 | 5.5 | 5.0 | 6.2 | 5.6 | ||||
| Private health insurance, % | 83.4 | 78.3 | 84.5 | 75.3 | 78.8 | ||||
| Uninsured, % | 13.6 | 16.2 | 10.5 | 18.5 | 15.6 | ||||
| Female | |||||||||
| Marital status, no. | 1442 | 1139 | <.001 | 231 | .21 | 117 | .23 | 780 | <.001 |
| Never married, % | 23.0 | 42.1 | 32.0 | 48.7 | 45.9 | ||||
| Married, % | 67.4 | 48.4 | 56.7 | 44.4 | 45.1 | ||||
| No longer married, % | 9.7 | 9.6 | 11.3 | 6.8 | 9.0 | ||||
| Education, no. | 1442 | 1135 | <.001 | 230 | .64 | 118 | .54 | 778 | .04 |
| Not high school graduate, % | 2.6 | 5.0 | 2.6 | 5.9 | 4.9 | ||||
| High school graduate, % | 41.5 | 51.6 | 46.5 | 46.6 | 50.5 | ||||
| College graduate, % | 55.9 | 43.3 | 50.9 | 47.5 | 44.6 | ||||
| Employment, no. | 962 | 757 | .01 | 170 | .55 | 68 | .04 | 517 | .41 |
| Full time, % | 81.0 | 78.2 | 77.6 | 70.6 | 78.5 | ||||
| Part time, % | 16.7 | 15.3 | 18.2 | 14.7 | 14.7 | ||||
| Unemployed, % | 2.3 | 6.5 | 4.1 | 14.7 | 6.8 | ||||
| Health insurance, no. | 1339 | 1081 | <.001 | 228 | .69 | 106 | .76 | 731 | <.001 |
| Public health insurance, % | 5.1 | 11.8 | 6.1 | 13.2 | 12.6 | ||||
| Private health insurance, % | 84.9 | 73.8 | 82.9 | 74.5 | 72.0 | ||||
| Uninsured, % | 10.0 | 14.3 | 11.0 | 12.3 | 15.5 | ||||
| Outcomes . | Siblings† . | ALL survivors . | P for survivors versus siblings . | Nonrelapsed, nonirradiated survivors . | P for nonrelapsed, nonirradiated survivors versus siblings‡ . | Relapsed survivors . | P for relapsed survivors versus nonrelapsed survivors . | Irradiated survivors . | P for irradiated survivors versus nonirradiated survivors . |
|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||
| Marital status, no. | 1302 | 1206 | <.001 | 183 | .56 | 180 | .04 | 836 | .11 |
| Never married, % | 31.3 | 44.6 | 39.3 | 48.9 | 45.9 | ||||
| Married, % | 59.9 | 48.3 | 53.6 | 40.6 | 47.5 | ||||
| No longer married, % | 8.9 | 7.1 | 7.1 | 10.5 | 6.6 | ||||
| Education, no. | 1299 | 1207 | <.001 | 184 | .99 | 180 | .30 | 835 | .03 |
| Not high school graduate, % | 3.0 | 6.4 | 3.3 | 3.9 | 6.1 | ||||
| High school graduate, % | 48.5 | 55.7 | 50.0 | 59.4 | 55.0 | ||||
| College graduate, % | 48.5 | 37.9 | 46.7 | 36.7 | 38.9 | ||||
| Employment, no. | 979 | 888 | .07 | 143 | .96 | 128 | .87 | 631 | .80 |
| Full time, % | 93.7 | 90.4 | 91.6 | 89.1 | 90.0 | ||||
| Part time, % | 3.9 | 4.8 | 4.9 | 5.5 | 5.2 | ||||
| Unemployed, % | 2.4 | 4.7 | 3.5 | 5.5 | 4.8 | ||||
| Health insurance‡, no. | 1201 | 1136 | .02 | 181 | .21 | 162 | .69 | 774 | .27 |
| Public health insurance, % | 3.0 | 5.5 | 5.0 | 6.2 | 5.6 | ||||
| Private health insurance, % | 83.4 | 78.3 | 84.5 | 75.3 | 78.8 | ||||
| Uninsured, % | 13.6 | 16.2 | 10.5 | 18.5 | 15.6 | ||||
| Female | |||||||||
| Marital status, no. | 1442 | 1139 | <.001 | 231 | .21 | 117 | .23 | 780 | <.001 |
| Never married, % | 23.0 | 42.1 | 32.0 | 48.7 | 45.9 | ||||
| Married, % | 67.4 | 48.4 | 56.7 | 44.4 | 45.1 | ||||
| No longer married, % | 9.7 | 9.6 | 11.3 | 6.8 | 9.0 | ||||
| Education, no. | 1442 | 1135 | <.001 | 230 | .64 | 118 | .54 | 778 | .04 |
| Not high school graduate, % | 2.6 | 5.0 | 2.6 | 5.9 | 4.9 | ||||
| High school graduate, % | 41.5 | 51.6 | 46.5 | 46.6 | 50.5 | ||||
| College graduate, % | 55.9 | 43.3 | 50.9 | 47.5 | 44.6 | ||||
| Employment, no. | 962 | 757 | .01 | 170 | .55 | 68 | .04 | 517 | .41 |
| Full time, % | 81.0 | 78.2 | 77.6 | 70.6 | 78.5 | ||||
| Part time, % | 16.7 | 15.3 | 18.2 | 14.7 | 14.7 | ||||
| Unemployed, % | 2.3 | 6.5 | 4.1 | 14.7 | 6.8 | ||||
| Health insurance, no. | 1339 | 1081 | <.001 | 228 | .69 | 106 | .76 | 731 | <.001 |
| Public health insurance, % | 5.1 | 11.8 | 6.1 | 13.2 | 12.6 | ||||
| Private health insurance, % | 84.9 | 73.8 | 82.9 | 74.5 | 72.0 | ||||
| Uninsured, % | 10.0 | 14.3 | 11.0 | 12.3 | 15.5 | ||||
Restricted to those aged 25 to 49 years. Numbers of subjects with nonmissing values are shown in the row of each outcome variable name.
Age standardized to ALL survivors' age distribution in each sex.
Age standardized to nonrelapsed, nonirradiated ALL survivors' age distribution in each sex.
With the exception of employment, relapsed female survivors had similar socioeconomic outcomes to those without a relapse (Table 5). Among male relapsed survivors, the rate of marriage was significantly lower compared with siblings, but no differences were found for education, employment, or health insurance (Table 5).
Female survivors who were irradiated showed significantly lower rates of marriage, of educational attainment, and of health insurance coverage compared with nonirradiated females, but employment rates did not differ (Table 5). Irradiated males showed significantly lower rates of high school and college graduation compared with nonirradiated males.
Discussion
Using the unique resource of the CCSS, this study provides the largest and most comprehensive assessment of the health of childhood ALL survivors to date. It is clear from this analysis that more than 20 years from diagnosis these adults continue to have medical and psychosocial sequelae of their original diagnosis and therapy. All groups, including those nonirradiated, nonrelapsed survivors (whose outcomes we believe most represent the future of children treated with contemporary therapies) reported late effects. The nature and frequency of the chronic medical conditions reported as well as the reported social and mental health status varied across these groups.
Few large studies have addressed global, long-term outcomes in ALL survivors. In one such study by Pui et al,19 the authors reported on 856 ALL patients treated over a 30-year period at the St Jude Children's Research Hospital. All were in continuous first remission for at least 10 years from diagnosis. They reported an excellent overall survival that, although inferior to that of the general US population, was more than 95% at 30 years from original remission. In the St Jude study,19 the nonirradiated group had significantly better survival than irradiated survivors. MacArthur et al15 found that 5-year survivors of all childhood cancers had a 9-fold higher risk of death compared with the general population. ALL survivors within their cohort were one of the highest risk groups for late mortality, findings very similar to ours and to those in the CCSS cohort overall.24 The major causes of excess mortality in ALL survivors were recurrence of ALL and SN,15 which was true for the CCSS population as well. We and others have demonstrated decreasing late mortality with each subsequent treatment era,42,43 clearly the result of more effective therapies improving the likelihood of relapse-free survival.
Second neoplasms (SNs) are one of the most serious and devastating morbidities associated with cancer therapy and have been strongly associated with the use of therapeutic radiation for treatment of the original cancer.25,44 The cumulative incidence of SN in our study was slightly higher than in some of the earlier reports45-49 due to the high proportion of patients within our cohort receiving RT. In our study, the central nervous system was the most common site of these second neoplasms, a finding consistent with that of many other investigators.19,50,51 Regrettably, the cumulative incidence of SNs is continuing to rise, even 25 years after diagnosis. It is important to point out that survivors who did not receive any radiation also developed SNs, at a rate much lower than the irradiated group but still at a higher rate than would be expected in the general population, suggesting a need for a close surveillance of all survivors.
MacArthur et al showed that survivors, whose ALL had recurred within 5 years from diagnosis, had a 61-fold higher likelihood of dying compared with the general population.15 Our study confirmed the excess mortality risk in this group, although we found a lower overall likelihood of death (SMR, 36). We also found substantially more morbidities in the relapsed group compared with the nonrelapsed group. Survivors with a history of relapse reported more chronic medical conditions, both severe and multiple, and poorer health status compared with their nonrelapsed counterparts. Remarkably, their socioeconomic outcomes were similar to those of the sibling cohort, suggesting the tremendous resilience and adaptability of this group relative to the multiple stressful and life-changing experiences associated with cancer and its therapy.
The adverse effects of cancer and its therapy on mental health, general health, and health-related quality of life have been reported before,52 but often in the setting of small, single-institution studies. CCSS has also previously reported on chronic health issues, health status, and psychologic outcomes of the survivor population.20,23,53 We found an excess of chronic medical conditions and poorer self-reported health status in ALL survivors including those who never relapsed and never received RT, although it is very important to emphasize that the overwhelming majority (92%) of survivors who did not relapse or receive RT did not report any severe CMC. We also found that the most-affected domains of health status were related to type and degree of specific treatment exposures. Relapsed and irradiated survivors reported a higher frequency of and more severe chronic medical conditions, activity limitations, and functional impairment. Relapsed survivors reported poorer general health compared with nonrelapsed survivors, but mental health status did not differ between either the irradiated and the nonirradiated groups or the relapsed and nonrelapsed groups.
The overall negative impact of cancer and its therapy on the social well being and educational attainment of survivors has been described in earlier reports, with survivors reporting lower graduation rates, higher use of special education services, lower marriage and higher divorce rates, lower employment rates, and lower health insurance coverage rates.19,26,27,54-56 Female survivors, those who received cranial radiation, and brain tumor survivors have been shown to be at greatest risk for these types of outcomes.27,60-62 Among our adult ALL survivors, both males and females uniformly reported worse socioeconomic outcomes than did siblings in all areas. Irradiated survivors, especially females, reported poorer socioeconomic outcomes. Conversely, survivors who did not receive radiation and had never relapsed did very well, comparable with the siblings in all the outcomes measured.
This study has some limitations. First, with the exception of death and second neoplasm, the medical conditions examined in this study were self-reported and not verified through medical records and thus may be subject to overreporting or underreporting. Second, our analysis of the reported health status and chronic conditions included only survivors and siblings 18 years and older, and the analysis of socioeconomic outcomes was restricted to individuals aged 25 to 49 years. Thus our findings might have limited application for younger survivors. In addition, because of these age limitations, the number of nonirradiated survivors available for these analyses was reduced. Finally, because survivors' siblings are likely to have been exposed to some of the same stressors as survivors, associated with the cancer experience within a family,63-65 health status comparisons of survivors with their siblings may underestimate the true effects of cancer and its therapy.
Tremendous advances have been made in the treatment of children with cancer, and specifically in the treatment of children with ALL over the past 30 years. Nevertheless, all children diagnosed with ALL are exposed to rigorous therapies, often very early in life. Our findings show that ALL survivors continue to experience excess mortality, chronic morbidity, and poor socioeconomic outcomes for many years following the completion of therapy. These late effects are greatest among survivors who received radiation therapy or who have survived a relapse. However, nonirradiated and nonrelapsed survivors also report excessive chronic health issues and poor health status, although their socioeconomic outcomes are similar to those of their siblings. It is apparent that by limiting the use of therapeutic radiation and by developing therapies that reduce the likelihood of relapse, the late effects of therapy will be reduced. This does not, however, diminish our need to understand the outcomes of our patients, currently in adulthood, who were treated with aggressive therapeutic regimens, and often with radiation. With large numbers of leukemia survivors joining the population every year, it is essential that medical professionals, educators, employers, legislators, and society in general are aware of the issues faced by the survivors. Ongoing research and refinements of therapy will, it is hoped, further the goal of enabling our patients to return fully to their premorbid potential.
The online version of this article contains a data supplement.
Presented in abstract form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 10, 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Cancer Institute (NCI) Grant U24 55727 (L.L.R., Principal Investigator), the Intramural Research Program of the National Institutes of Health (NIH) and the National Cancer Institute, funding to the University of Minnesota from the Children's Cancer Research Fund, and funding to St Jude Children's Research Hospital from the American Lebanese Syrian Associated Charities (ALSAC). This research used the CCSS, a resource supported by the National Cancer Institute to promote and facilitate research on long-term survivors of cancer diagnosed in childhood and adolescence. Investigators may apply to use the CCSS by proposing an analysis of existing data or proposing new initiatives that would use the cohort. Interested investigators are encouraged to visit the CCSS website at http://www.stjude.org/ccss to learn more about this unique resource.
The study design, data collection, analysis, interpretation of the results, and the preparation of this paper were the sole responsibility of the authors.
National Institutes of Health
Authorship
Contribution: R.M., Y.Y., L.L.R., and J.P.N. conceived of and designed the study; R.M., S.L., D.C.D., Y.Y., L.L.R., and J.P.N. analyzed and interpreted the data; R.M., Y.Y., S.S., K.C.O., L.L.R., and J.P.N. drafted the paper; R.M., Y.Y., S.S., K.C.O., L.L.R., and J.P.N. critically reviewed the paper; S.L., D.C.D., Y.Y., and W.L. provided statistical expertise; R.M., L.L.R., and J.P.N. provided administrative and technical support; L.L.R. obtained funding; R.M., L.L.R., and J.P.N. supervised the study.
A list of the institutions and investigators in the Childhood Cancer Survivor Study is provided in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph P. Neglia, Department of Pediatrics, University of Minnesota School of Medicine, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: jneglia@umn.edu.