Abstract
Untoward events involving radioactive material, either accidental or intentional, are potentially devastating. Hematologists and oncologists are uniquely suited to help manage radiation victims, as myelosuppression is a frequent complication of radiation exposure. In the aftermath of a large event, such as a nuclear detonation, there may be a national call for surge capacity that involves hematologists/oncologists across the country in the disaster response. In preparation, the National Marrow Donor Program and American Society for Blood and Marrow Transplantation have established the Radiation Injury Treatment Network (RITN), a voluntary consortium of transplant centers, donor centers, and umbilical cord blood banks. RITN is partnered with the Office of the Assistant Secretary for Preparedness and Response in the United States Department of Health and Human Services to develop treatment guidelines, educate healthcare professionals, coordinate situation response, and provide comprehensive evaluation and care for radiation injury victims. We outline the current plans for event response and describe scenarios, including catastrophic events that would require extensive support from hematologists/oncologists across the country. In addition, we highlight important reference resources and discuss current efforts to develop medical countermeasures against radiation toxicity. Practitioners and institutions across the country are encouraged to become involved and participate in the planning.
Introduction
Events involving radioactive material, either intended or not, are an undeniable possibility and potentially catastrophic.1 Reports of nuclear proliferation in nations unfriendly to the United States and the recent poisoning of a Russian dissident with polonium-2102 are stark reminders of the threat. In addition, more than 400 radiologic accidents have occurred since 1944, resulting in more than 3000 significant exposures.3 Approximately 10 million “sealed sources” of radioactive material (eg, cesium-137, cobalt-60) are used for medical, industrial, agricultural, and research purposes worldwide.4 More than 600 of these were lost or stolen since 1995 and less than half were eventually recovered
Many victims exposed to significant doses of radiation will develop bone marrow suppression. Thus, hematologists and oncologists are uniquely suited to help evaluate and manage radiation exposure victims.5 Depending on the scale of the event, there may be a national call for surge capacity. Hematologists, oncologists and hematopoietic stem cell transplantation (HSCT) specialists across the country could be asked to balance the needs of their local patient populations with requests to accept patient transfers or even travel to other institutions. Therefore, it behooves us to collectively prepare for this contingency.
Radiation Injury Treatment Network
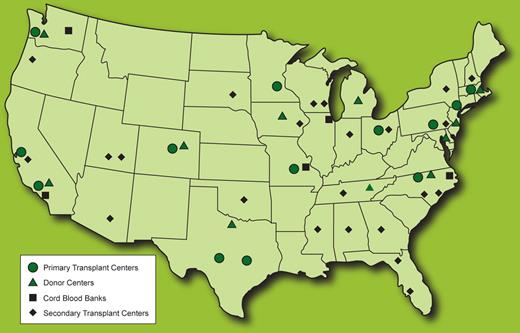
Beginning in 2001, the National Marrow Donor Program (NMDP) and American Society for Blood and Marrow Transplantation established the Radiation Injury Treatment Network (RITN), a voluntary consortium of 52 HSCT centers, donor centers, and umbilical cord blood banks (Figure 1), made possible through partnerships with the Office of Naval Research and the Center for International Blood and Marrow Transplant Research. The goals of RITN (www.ritn.net) are as follows:
To develop treatment guidelines for managing hematologic toxicity among victims of radiation exposure
To educate healthcare professionals about pertinent aspects of radiation exposure management
To coordinate situation response after a radiation event
To provide comprehensive evaluation and treatment for victims at participating HSCT centers.
The Radiation Injury Treatment Network (RITN) consists of 52 transplant centers, donor centers, and umbilical cord blood banks. Primary transplant centers act as the lead institutions within their region during event response.
The Radiation Injury Treatment Network (RITN) consists of 52 transplant centers, donor centers, and umbilical cord blood banks. Primary transplant centers act as the lead institutions within their region during event response.
The European Group for Blood and Marrow Transplantation is establishing a similar network to offer training courses and improve cooperation between institutions.6
The number of patients who will require care after a large-scale event, such as an improvised nuclear device, exceeds the capacity of RITN centers by several orders of magnitude. The number of casualties would depend on many factors, such as the size of the device, the time of day, weather conditions, and the precise location of the detonation. Models suggest that, if a device similar to the bomb detonated over Hiroshima struck a city such as Washington, DC, up to 175 000 victims would require intensive medical care and 30 000 would require management for myelosuppression.7
The logistical complexity of evaluating, transporting, and treating so many victims, considering the expected destruction to local infrastructure, is daunting enough to induce nihilism. Despite the obstacles, experience from radiation accidents indicates that many victims can be salvaged with appropriate care. The LD50 (lethal dose to 50% of persons exposed) for total body irradiation in humans is approximately 3.5 to 4 Gy without supportive care, but the use of antibiotics and transfusions may increase the LD50 to 4.5 to 7 Gy, and survival at doses greater than 10 Gy may be possible with HSCT.8 If a significant frac-tion of victims with acute injuries could be saved with supportive care alone, appropriate planning and response after a large-scale event could salvage tens of thousands or even hundreds of thousands of people. Thus, a large-scale event will offer extraordinary opportunities for qualified practitioners at large and small centers nationwide.
In September 2007, RITN convened a 1-day meeting in Bethesda, MD, to address both progress and outstanding issues in the governmental and nongovernmental responses to radiologic and nuclear events. Speakers reviewed the current threat scenarios and provided updates on radiation biology, the diagnosis and management of radiation injury, efforts to organize a national response, and novel radiation medical countermeasures. The evolving role of hematologists/oncologists within these plans is outlined below. For more extensive descriptions of the acute and chronic effects of radiation, treatment approaches, organizational preparedness for HSCT centers, and radiation biology, the reader is referred to the websites listed in Table 1.5-12
Websites containing additional information on approaches to medical triage, assessment, and management after radiation exposure
| Source . | Website . |
|---|---|
| American Academy of Pediatrics | www.aap.org/policy/radiation.htm |
| American Medical Association Center for Public Health Preparedness and Disaster Response | www.ama-assn.org/ama/pub/category/6206.html |
| Armed Forces Radiobiology Research Institute | www.afrri.usuhs.mil |
| Centers for Disease Control and Prevention | www.bt.cdc.gov/radiation/ |
| Federal Emergency Management Agency | www.fema.gov/hazard/index.shtm |
| Health Physics Society | www.hps.org |
| National Institute of Allergy and Infectious Diseases Radiation Countermeasures Program | http://www3.niaid.nih.gov/research/topics/radnuc/ |
| Radiation Emergency Assistance Center/Training Site | www.orau.gov/reacts |
| Radiation Event Medical Management | www.remm.nlm.gov |
| Radiation Injury Treatment Network | www.ritn.net |
| Uniformed Services University of the Health Sciences Center for Disaster and Humanitarian Assistance Medicine | www.usuhs.mil/mem/cdham.html |
| US Department of Veteran Affairs Emergency Management Strategic Healthcare Group | www1.va.gov/emshg |
| US Food and Drug Administration Office of Crisis Management | www.fda.gov/oc/ocm |
| US Nuclear Regulatory Commission | www.nrc.gov |
| Source . | Website . |
|---|---|
| American Academy of Pediatrics | www.aap.org/policy/radiation.htm |
| American Medical Association Center for Public Health Preparedness and Disaster Response | www.ama-assn.org/ama/pub/category/6206.html |
| Armed Forces Radiobiology Research Institute | www.afrri.usuhs.mil |
| Centers for Disease Control and Prevention | www.bt.cdc.gov/radiation/ |
| Federal Emergency Management Agency | www.fema.gov/hazard/index.shtm |
| Health Physics Society | www.hps.org |
| National Institute of Allergy and Infectious Diseases Radiation Countermeasures Program | http://www3.niaid.nih.gov/research/topics/radnuc/ |
| Radiation Emergency Assistance Center/Training Site | www.orau.gov/reacts |
| Radiation Event Medical Management | www.remm.nlm.gov |
| Radiation Injury Treatment Network | www.ritn.net |
| Uniformed Services University of the Health Sciences Center for Disaster and Humanitarian Assistance Medicine | www.usuhs.mil/mem/cdham.html |
| US Department of Veteran Affairs Emergency Management Strategic Healthcare Group | www1.va.gov/emshg |
| US Food and Drug Administration Office of Crisis Management | www.fda.gov/oc/ocm |
| US Nuclear Regulatory Commission | www.nrc.gov |
Modified from Waselenko et al.7
Triage after a radiation event
Several types of events could result in radiation exposure (Table 2). Intentional events can involve radiologic exposure devices (REDs), radiologic dispersion devices (RDDs), and improvised nuclear devices (INDs). An RED is a radioactive source placed surreptitiously in a public space or other location. An RDD spreads radioactive material over a wide area, either using a conventional explosive (ie, a so-called dirty bomb) or via other means, such as by tainting the food or air supply. Although these devices would probably produce comparatively few fatalities, large numbers of people could be exposed, engendering widespread public confusion and anxiety. Victims of events involving RDDs or REDs would most probably be treated at centers near the incident, except in specific cases that require highly specialized care for burns or marrow suppression. It is improbable that individual hematologists in other regions of the country would be involved.
The spectrum of potential events involving radioactive material
| Category . | Description . | No. of deaths (rough estimates) . |
|---|---|---|
| Radioactive source accident | Loss or theft of a radiologic source (eg, Goiania) | 0-10s |
| Nuclear reactor accident | Release of radioactive gas or material (eg, Chernobyl) | 0-100s |
| Radiologic dispersal device | Device or scheme for dispersing radioactive isotope (eg, dirty bomb* or radioactive material in the food supply) | 0-1000s |
| Radiologic exposure device | Radioactive material intended to expose people in the vicinity (eg, cesium source placed on a train) | 0-100s |
| Improvised nuclear device | Incorporates radioactive material intended to produce a nuclear explosion | 1000s to >1 000 000 |
| Category . | Description . | No. of deaths (rough estimates) . |
|---|---|---|
| Radioactive source accident | Loss or theft of a radiologic source (eg, Goiania) | 0-10s |
| Nuclear reactor accident | Release of radioactive gas or material (eg, Chernobyl) | 0-100s |
| Radiologic dispersal device | Device or scheme for dispersing radioactive isotope (eg, dirty bomb* or radioactive material in the food supply) | 0-1000s |
| Radiologic exposure device | Radioactive material intended to expose people in the vicinity (eg, cesium source placed on a train) | 0-100s |
| Improvised nuclear device | Incorporates radioactive material intended to produce a nuclear explosion | 1000s to >1 000 000 |
Only a small fraction of deaths would be expected to result directly from radiation exposure.
In contrast, an IND using fissionable material, such as plutonium or uranium, would have devastating consequences and require the utilization of all recruitable resources. Approximately 40 000 hospital beds are available at any given time nationwide,13 a capacity far lower than the projected number of potentially salvageable casualties. Thus, medical centers around the country may be asked to accept patients from the disaster area, both victims of the event and local residents who require care for chronic or intercurrent medical conditions. As observed in the aftermath of Hurricane Katrina, healthcare needs for displaced populations can easily overwhelm the infrastructure in regions immediately surrounding a disaster area. For patients with cancer or hematologic disease, this disruption may be particularly dangerous.
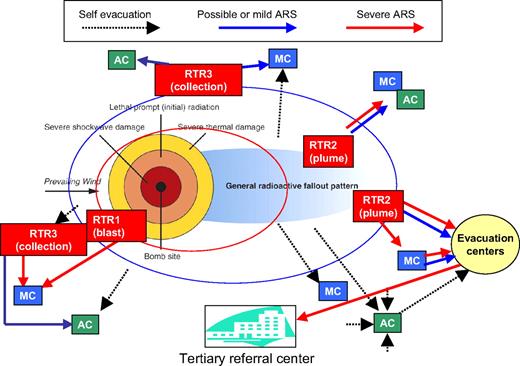
Planners in the military, local and state governments,14 and other healthcare organizations are preparing their responses to radiation events (Table 1). A conceptual model (Figure 2) for triage, transportation, and treatment of victims after an IND, the RTR system (Radiation TRiage, TReatment and TRansportation), is being developed by the Office of the Assistant Secretary for Preparedness and Response in the US Department of Health and Human Services. The complete details will be outlined in a separate publication, but briefly, the initial triage and patient decontamination will occur at RTR sites, whose location and resources will be determined by incident commanders. RTR1 are near the blast and RTR2 are near the plume, both within areas of residual radiation. In contrast, RTR3 will be located outside the region with significant residual radiation.
Schematic for triage and response to a large-scale radiation event developed by the Office of the Assistant Secretary for Preparedness and Response. Triage centers are located in concentric rings around the affected area, providing initial stabilization and decontamination (RTR1-RTR3), more extensive Medical Care (MC), and rapid screening of unexposed or minimally exposed individuals at Assembly Centers (AC). Patients who require further care are evacuated to referral centers in unaffected regions. Complete details will be outlined in a separate publication.
Schematic for triage and response to a large-scale radiation event developed by the Office of the Assistant Secretary for Preparedness and Response. Triage centers are located in concentric rings around the affected area, providing initial stabilization and decontamination (RTR1-RTR3), more extensive Medical Care (MC), and rapid screening of unexposed or minimally exposed individuals at Assembly Centers (AC). Patients who require further care are evacuated to referral centers in unaffected regions. Complete details will be outlined in a separate publication.
Based on previous radiation accidents, the number of unirradiated persons who seek medical attention after an event could dwarf the number exposed. For example, when scavengers in Goiania, Brazil, procured an improperly secured cesium source resulting in 28 cases of radiation sickness, more than 112 000 people presented for screening at the nearby Olympic stadium.15 Unexposed or minimally exposed individuals outside of the disaster zone will be rapidly triaged at Assembly Centers, where victims will be registered but very limited medical care will be available.
Victims requiring further care may be transferred to Medical Care (MC) sites located outside of the disaster zone and potentially across the nation.16 The types of injuries managed at MC centers will depend on the nature of the facilities and their available resources. Only on transfer would hematologists, oncologists, and HSCT specialists outside the disaster zone become involved. Healthcare and other infrastructure at these centers will presumably be intact, affording practitioners the opportunity to use carefully measured interventions, in consultation with RITN, radiation oncologists, and other experts in radiation exposure.
Essential role of biodosimetry
Appropriate triage and care after radiation exposure depends on accurate and timely estimates of radiation dose. Dose information will be important for classifying victims into groups that (1) will not require medical intervention, (2) could benefit from supportive care (eg, granulocyte colony-stimulating factor) to facilitate autologous marrow recovery, (3) require evaluation for HSCT to treat marrow aplasia, and (4) cannot be salvaged.
A variety of information can be used to estimate an individual's radiation exposure. Unlike the homogeneous dosing associated with therapeutic total body irradiation, shielding from nearby structures (eg, buildings) during accidents or terrorist attacks will result in heterogeneous exposures. Therefore, a careful history of the victim's location and subsequent symptoms will be essential. Initial clinical assessment will include the time from event to first emesis and peripheral blood counts, with subsequent lymphocyte depletion kinetics.
Approaches that use only clinical and routine laboratory findings to stratify victims into risk groups6,17-19 are valuable for a small-sized accident, but their utility during large events is not clear. Biodosimetry, the use of biologic markers to assess dose, can enhance the predictive value of clinical findings after radiologic or nuclear events. The “gold standard” for biodosimetry is the quantification of dicentric chromosomes using metaphase cytogenetics in peripheral blood lymphocytes. Dicentric quantification requires multiple days to perform and is only available in select centers, although plans have been formulated to develop major radiation laboratory networks to perform dicentric quantification on a mass scale.20,21 Newer methods for biologic dosimetry, including rapid gene expression analysis, serum proteomics, and measurements of DNA damage, are also under development.
Treating hematologists will need to calculate radiation doses using the information they have available. Online algorithms for estimating dose based on clinical and biologic data are available from the Radiation Event Medical Management website22 at http://www.remm.nlm.gov/ars_wbd.htm or from the Armed Forces Radiobiologic Research Institute at http://www.afrri.usuhs.mil/www/outreach/biodostools.htm#software.
Resources for assisting hematologists with clinical management
Acute radiation syndrome (ARS) can affect virtually any organ but primarily manifests as injury to the hematologic, dermatologic, gastrointestinal, and central nervous systems. The severity of ARS increases proportionally with the radiation dose from mild (< 1-2 Gy) to invariably lethal (> 10-20 Gy). The clinical course of ARS generally includes a prodromal phase, followed by a period of apparent clinical remission, manifest illness, and ultimately recovery or death. Importantly, the latency between exposure and severe manifestations of ARS may provide time to transport victims to RITN sites.
A large fraction of patients with radiation exposure significant enough to induce cytopenias will have multiorgan system damage. Thus, persons exposed to radioactive material may present unique and exceedingly complex management challenges. Few physicians are familiar with the basic manifestations of acute radiation injury or have training in the management of patients with nontherapeutic radiation exposure. Esoteric aspects of care, such as decorporation therapy for internal radiation contamination, are extremely important, but very few healthcare practitioners have experience in this realm. Several resources are available to assist with the evaluation and care of radiation exposure victims (Table 1). Among these, the Radiation Event Medical Management website22 (www.remm.nlm.gov) was developed in a collaboration involving the National Library of Medicine, Office of the Assistant Secretary for Preparedness and Response, and medical experts from around the world. With assistance from RITN members, Radiation Event Medical Management includes admission and treatment order templates directed toward victims of radiologic or nuclear events.
To ensure optimal care and enhance preparedness for subsequent events, it is essential that data on exposure and clinical complications be collected prospectively from victims and compiled centrally. The NMDP has developed a data collection protocol23 for use at RITN centers across the United States. After a large-scale event, centers that accept patients with radiation injury may be asked to contribute patient data to central repositories, either through RITN or governmental agencies.
Role for stem cell transplantation
Some victims of a large-scale event may receive sufficient doses of radiation to cause irreversible myeloablation. As discussed in “Resources for assisting hematologists with clinical management,” these patients will commonly have multiorgan damage. What remains unclear is whether allogeneic HSCT can be a life-sustaining measure in this setting.
To date, 31 patients have undergone allogeneic HSCT after accidental radiation exposure. Median survival after transplantation for these patients is approximately 1 month.3 All 4 patients who survived 1 year reconstituted autologous hematopoiesis, raising the question whether the HSCT provided any benefit. Particularly troubling was the contribution of graft-versus-host disease to mortality in more than 20% of patients.3
In many regards, patients with myeloablation from radiation exposure are similar to those with aplastic anemia. A reduced-intensity conditioning regimen for severe aplastic anemia (where immunosuppression but not myeloablation is required) is being tested in the Blood and Marrow Transplant Clinical Trials Network (BMT CTN Protocol 0301).23 This regimen, modified for radiation victims, is outlined in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Of note, NMDP has plans in place to conduct large numbers of urgent searches for victims following an event, recognizing that only a few searches would probably lead to transplants.
Current efforts to develop medical radiation countermeasures
The US government, particularly the Department of Defense, has a long-standing interest in the development of medical countermeasures against radiation. Beginning in 2005, the Radiation Countermeasures Program at the US National Institute of Allergy and Infectious Diseases has supported the development of medical countermeasures for civilian populations exposed to radiologic or nuclear hazards as a result of accidents or terrorist attacks.24 Current programs and funding opportunities are available at http://www3.niaid.nih.gov/research/topics/radnuc/default.htm.
Several candidate medical countermeasures are listed in Table S1. The complex nature of radiation injury is such that no single drug provides benefit in all circumstances and against all aspects of radiation injury. Antioxidants and radioprotectants are presumably most effective if present at the time of irradiation, whereas therapeutics such as growth factors may target one or more but not all affected organ systems. For this reason, many experts think that combination therapy will be required to produce substantial improvements in outcomes.
The Radiation Countermeasures Program25 funds grants and contracts (1) to study immune reconstitution after radiation injury; (2) to develop orally bioavailable formulations of DTPA, improved decorporating agents, and medical countermeasures against gastrointestinal ARS; and (3) to provide product development support services to corporate and academic partners with promising medical countermeasures. The National Institute of Allergy and Infectious Diseases also funds 8 Centers for Medical Countermeasures against Radiation to support basic research and development activities in this area.
In conclusion, the widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable. Hematologists, oncologists, and HSCT physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.
Many governmental and nongovernmental agencies are involved in the planning. Although the logistical difficulties inherent to any large-scale response are enormous, the potential for life-saving measures is equally grand. Standardized approaches to biodosimetry, evaluation, and treatment are now available for review, comment, and further development. Future efforts will focus on streamlining these processes, providing training to medical practitioners around the country, and validating medical countermeasures to reduce the morbidity and mortality of radiation exposure. Practitioners and institutions across the country are encouraged to become involved and participate in the planning.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Carl Hornfeldt for his assistance with the manuscript and Dr Nicholas Dainiak for his thoughtful review and comments.
The RITN Symposium was supported by National Institutes of Health U19 AI 067798 (N.J.C.).
This project has been supported by funding from the National Marrow Donor Program and the Department of the Navy, Office of Naval Research grant #N00014-06-1-0704 to the National Marrow Donor Program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Naval Research or the National Marrow Donor Program.
National Institutes of Health
Authorship
Contribution: D.M.W., C.C., N.J.C., R.J.H., D.J.W., and D.L.C. are members of the RITN Steering Committee and contributed to planning the RITN Symposium. All authors contributed to writing the paper.
The speakers at the RITN symposium were Marc Benderitter, Institute for Radioprotection and Nuclear Safety; Brooke Buddemeier, Lawrence Livermore National Laboratory; Cullen Case Jr, National Marrow Donor Program; Nelson Chao, Duke University; C. Norman Coleman, Office of Preparedness and Emergency Operations; Carl Curling, Institute for Defense Analysis; Theodor M. Fliedner, Radiation Medicine Research Group and World Health Organization (WHO) Liaison Institute for Radiation Accident Management, Faculty of Medicine, University of Ulm; Martin Hauer-Jensen, University of Arkansas for Medical Sciences, Central Arkansas Veterans Healthcare System, Little Rock, AR; Viktor Meineke, Bundeswehr Institute of Radiobiology; David Rutstein, US Public Health Service; Alla Shapiro, US Food and Drug Administration Center for Drug Evaluation and Research and Office of Counter-Terrorism and Emergency Coordination; Michael Robbins, Wake Forest University School of Medicine; Nelson J. Valverde, Laboratory of Radiological Sciences, State University of Rio de Janeiro, Brazil; Albert J. Wiley, WHO Collaborating Center at Oka Ridge and Radiation Emergency Assistance Center/Train Site.
Information in this manuscript does not represent US Government Policy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David M. Weinstock, Dana-Farber Cancer Institute, D510B, Boston, MA 02115; e-mail: DavidM_Weinstock@dfci.harvard.edu.