Cell traffic from the BM to remote body sites, and the local differentiation of these cells into often-unexpected cell types, have been independently reported by several laboratories. Toth and colleagues now show that, following stroke, a significant fraction of brain endothelial cells are of BM origin.
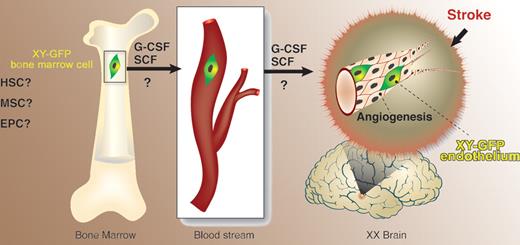
Toth and colleagues report in this issue of Blood on an experimental model that was chosen so as to ensure feasibility of tracking the origin of cells found in the brain. First, bone marrow (BM) from green fluorescence protein (GFP) transgenic male mice was transplanted into female C57Bl recipients. Cerebral artery occlusion in these radiation chimeras caused frontal and parietal cortical brain injury. Upon combined cytokine treatment with G-CSF and SCF, an overall improvement and partial correction of brain damage was observed in the cytokine-treated group compared with controls, and was associated with angiogenesis. Some of the blood vessels contained GFP-positive endothelial cells harboring the Y chromosome, substantiating their BM origin. The study therefore suggests that a cell population within the BM is capable of responding, either directly or indirectly, to G-CSF/SCF. This population consequently leaves the BM microenvironment and migrates, via an unknown route, to the brain (see figure). Presumably, the cells must then cross the blood-brain barrier and settle in the brain, where they participate in formation of new vessels.
Cells of bone marrow origin give rise to endothelial cells in the brain following ischemia-induced damage. HSC indicates hematopoietic stem cell; MSC, multipotent stromal cell; EPC, endothelial progenitor cell; ?, uncertainly as to the nature of the bone marrow cell that gives rise to brain endothelium and as to the exact target for the action of granulocyte colony-stimulating factor (G-CSF) and stem-cell factor (SCF).
Cells of bone marrow origin give rise to endothelial cells in the brain following ischemia-induced damage. HSC indicates hematopoietic stem cell; MSC, multipotent stromal cell; EPC, endothelial progenitor cell; ?, uncertainly as to the nature of the bone marrow cell that gives rise to brain endothelium and as to the exact target for the action of granulocyte colony-stimulating factor (G-CSF) and stem-cell factor (SCF).
These findings are of value as regards therapeutic brain regeneration following ischemic insult. Further analysis is called for regarding the cytokines involved in brain angiogenesis. In addition, and not less important, is the impression that the BM serves as a reservoir of cells that are recruited to various body sites upon need. Such sites include tumors,1 gastric ulcers,2 and wounds,3 to mention just a few examples. One major question raised by these studies is the nature of the cell(s) within the complex BM population endowed with migratory and tissue-repair capacities. In view of previous reports, precursors of brain endothelial cells could be hematopoietic, mesenchymal, or endothelial progenitors, or still-unidentified cells. This issue is not resolved by the present report.
Along with these main findings, the study by Toth and colleagues highlights a major technical limitation in the use of GFP-labeled cells. About 10% of the cells containing the Y chromosome were GFP negative. GFP analysis therefore may underestimate cell frequency, and this problem could account for some of the discrepancies found between studies using this and other methodologies. Related to this point is an issue mentioned by the authors in passing: GFP-positive cells bearing neuronal or glial markers were detected in the ischemic brain at 2 months after treatment. Past reports on the transformation of BM-derived cells into brain neuronal cells4,5 evoked controversy. This issue must, however, be revisited, because the incidence of such cells might be higher than estimated given the limitations of GFP analysis. It should further be examined whether the use of various cytokine combinations, as one option, might shift the balance toward increased production of nerve cells from BM-derived precursors.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal