Lack of regulation of the complement system is associated with thrombosis. In this issue of Blood, Ståhl and colleagues report that deficiency of factor H in patients with atypical hemolytic uremic syndrome activates platelets and generates platelet microparticles.
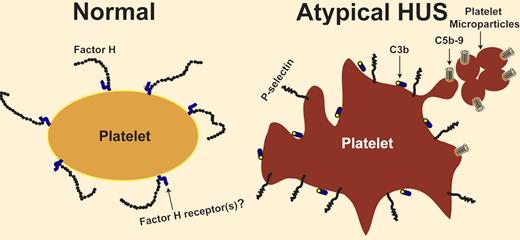
Several membrane and plasma proteins regulate the activity of the complement system. Deficiency of CD55 and CD59 on the cell surface causes paroxysmal nocturnal hemoglobinuria, which is characterized by intravascular hemolysis and recurrent venous and arterial thrombosis. Deficiency of factor H, a complement-regulatory plasma glycoprotein, is associated with a microangiopathic hemolytic anemia indistinguishable from hemolytic uremic syndrome. This condition lacks a diarrheal prodrome and hence is known as atypical hemolytic uremic syndrome (aHUS).1,2 The structure of factor H comprises 20 homologous units known as short consensus repeats or sushi domains. The C-terminal domains of factor H contain the cell-surface binding sites; most of the mutations detected in aHUS patients are located within these domains. The role of factor H deficiency in pathogenesis of aHUS has been confirmed in a mouse model. Mice with an engineered mutant factor H, missing the C-terminal domains, developed spontaneous HUS before 12 weeks of age.3 How is the deficiency in a complement-regulatory protein related to thrombosis? Complement activity on a permissive surface, such as a bacterial surface, results in deposition of C3b (and its degradation products), and in generation of a membrane attack complex (MAC or C5b-9) that perforates the membrane and lyses the target cell. Presence of factor H on a cell surface converts it to a nonpermissive surface and limits complement activation and propagation (see figure). Absence of the regulatory function of factor H (due to an abnormal quantity or quality of factor H) might affect any cell exposed to plasma, including endothelial cells and blood cells. What if the surface attacked by the complement system is a platelet surface? Studies pioneered by Polley et al and Sims et al showed that complement activation results in platelet activation and in release of platelet microparticles with procoagulant activity.4,5 However, the interaction between platelets and complement is not a one-way relationship. After being activated with different agonists, platelets are capable of activating the complement system on their surface.6,7 Therefore, a closed loop of activation can develop between platelets and the complement system. Activated platelets activate the complement, which in turn propagates platelet activation (see figure). Consequently, lack of complement regulation on platelets might cause a vicious cycle of platelet activation resulting in thrombosis. This is the theoretical basis for studying platelets as a possible culprit for thrombosis in disorders characterized by dysregulation of the complement system. Neither the presence of activated platelets in patients with aHUS nor the ability of aHUS plasma to activate normal platelets rule out an important role for complement-induced damage of endothelial cells, white blood cells, and perhaps red blood cells in the pathogenesis of thrombotic microangiopathy. However, it means that complement researchers should pay closer attention to platelets, and investigators with an interest in thrombotic disorders should update their complement knowledge.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal