In this issue of Blood, Paoluzzi and colleagues describe preclinical results of combining a BH3 mimetic (AT-101) with standard chemotherapy agents in models of B-cell lymphoma, and demonstrate significant synergy in a schedule-dependent manner of administration.
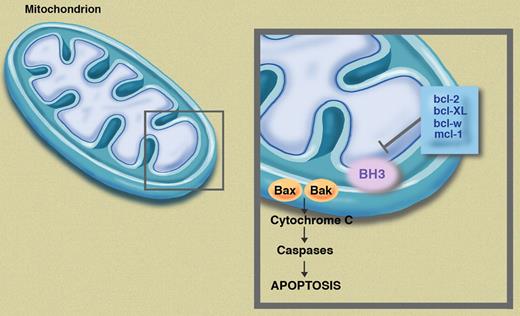
BH3 mimetics, such as AT-101, are designed to interfere with various antiapo-ptotic proteins such as bcl-2, bcl-XL, and mcl-1, thus activating Bax and Bak; these mimetics ultimately promote apoptosis through a complicated pathway (see figure). When a malignant lymphocyte is exposed to chemotherapy, modulation of the cellular apoptotic threshold through this pathway could significantly enhance cytotoxicity.
Antiapoptotic proteins, including bcl-2, bcl-XL, bcl-w, and mcl-1, prevent activation of Bax and Bak, thus inhibiting apoptosis. Pure BH3 mimetics, such as AT-101, allow activation of Bax and Bak, enhancing apoptosis. Illustration by Marie Dauenheimer.
Antiapoptotic proteins, including bcl-2, bcl-XL, bcl-w, and mcl-1, prevent activation of Bax and Bak, thus inhibiting apoptosis. Pure BH3 mimetics, such as AT-101, allow activation of Bax and Bak, enhancing apoptosis. Illustration by Marie Dauenheimer.
Therefore, the targeting of antiapoptotic proteins represents an exciting paradigm to treat lymphoma. Bcl-2, in particular, plays a critical role in normal lymphocyte development, and, through the t(14;18) translocation, in the pathogenesis of follicular lymphoma. A subset of diffuse large B-cell lymphoma cases also overexpresses bcl-2 through amplification; this molecular subtype responds less well to conventional chemotherapy.1 In addition to antisense therapy, several small molecules targeting bcl-2 are currently in clinical development for lymphoma, and very early results of these clinical trials suggest modest single-agent activity.2 However, given the success of monoclonal antibody therapy, developing novel targeted agents for lymphoma in the modern era almost always must include combinations with standard therapies, including chemotherapy, antibody therapy, and other novel agents.3
The article by Paoluzzi and colleagues in this issue of Blood details an elegant series of experiments designed to explore appropriate dose and schedule of AT-101 alone and in combination with a variety of other agents. They demonstrated that duration of exposure was not a critical determinant of cytotoxicity, but dose was critical. In mantle-cell lymphoma cell lines, significant synergy was observed with AT-101 in combination with the proteasome inhibitor carfilzomib, but not bor-tezomib. Pretreatment with AT-101, particularly at a high dose, improved activity of rituximab and cyclophosphamide.
There are several limitations to these studies. The human in vivo mechanisms of therapeutic agents, particularly rituximab, may not be recapitulated well in these artificial model systems. Moreover, perhaps the major disease of interest, follicular lymphoma, lacks predictive cell lines and murine models, and it is unclear if any of the findings would extend to that histology. The chosen agent, AT-101, a pure negative enantiomer of gossypol, may be inferior in efficacy and more toxic than other BH3 mimetics, including a modified version, apo-gossypol.4 Finally, these interesting results with AT-101 may not extend to other members of the BH3 mimetic class of drugs, as specificity of these small molecules for the individual BH3 family members varies significantly.
Despite these considerable limitations, the studies provide an important framework in developing rational clinical trials that combine small-molecule inhibitors with standard agents for lymphoma, and suggest key features of the biology of lymphoma and cellular apoptotic pathways. Given the small numbers of patients available for clinical trials, and the substantial expense of conducting such trials, taking hints about trial design from carefully conducted cell line and murine experiments is now a crucial first step. As the phase 1 and 2 trials are developed with this exciting class of agents, it is hoped that appropriate correlative studies will be performed to both validate the model systems and determine the degree to which the putative target is affected by the agent under evaluation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Acknowledgment:
J.W.F. is a Scholar in Clinical Research of the Leukemia & Lym-phoma Society.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal