Abstract
Hemojuvelin (HJV) is a coreceptor for bone morphogenetic protein (BMP) signaling that regulates hepcidin expression and iron metabolism. However, the precise combinations of BMP ligands and receptors used by HJV remain unknown. HJV has also been demonstrated to bind to neogenin, but it is not known whether this interaction has a role in regulating hepcidin expression. In the present study, we show that BMP-2, BMP-4, and BMP-6 are endogenous ligands for HJV in hepatoma-derived cell lines, and that all 3 of these ligands are expressed in human liver. We demonstrate in vitro that HJV selectively uses the BMP type II receptors ActRIIA and BMPRII, but not ActRIIB, and HJV enhances utilization of ActRIIA by BMP-2 and BMP-4. Interestingly, ActRIIA is the predominant BMP type II receptor expressed in human liver. While HJV can use all 3 BMP type I receptors (ALK2, ALK3, and ALK6) in vitro, only ALK2 and ALK3 are detected in human liver. Finally, we show that HJV-induced BMP signaling and hepcidin expression are not altered by neogenin overexpression or by inhibition of endogenous neogenin expression. Thus, HJV-mediated BMP signaling and hepcidin regulation occur via a distinct subset of BMP ligands and BMP receptors, independently of neogenin.
Introduction
Hereditary hemochromatosis (HH) is a genetic disorder characterized by high levels of iron absorption from the diet, leading to iron overload. HH is linked to mutations in several genes, namely HFE, TFR2 (encoding transferrin receptor 2), HFE2 (encoding hemojuvelin), or HAMP (encoding hepcidin).1 A small peptide secreted by the liver, hepcidin is an important regulator of body iron stores.1-4 Hepcidin decreases both intestinal iron absorption and macrophage iron release by binding to the iron exporter ferroportin and inducing its internalization and degradation.5 Impaired induction of hepcidin expression in response to iron loading appears to be a common pathogenic mechanism for HH due to mutations in HFE, TFR2, or HFE2, suggesting that these genes are involved in the upstream regulation of hepcidin
Hemojuvelin (HJV; also known as RGMc) is a member of the repulsive guidance molecule (RGM) family, which also includes RGMa and DRAGON (RGMb).6,7 All RGMs enhance cellular responses to bone morphogenetic protein (BMP) signals.8-10 BMPs are members of the transforming growth factor-β (TGF-β) superfamily. BMPs play a crucial role in regulating cell proliferation, cell differentiation, apoptosis, and the development of tissues.11-14 While a physiologic role for the BMP signaling function of RGMa and DRAGON has yet to be determined, HJV-mediated BMP signaling increases hepcidin mRNA expression both in vitro and in vivo.10,15,16 Furthermore, mutations in HJV associated with HH have impaired ability to generate BMP signals and induce hepcidin expression.10 Targeted disruption of the BMP/TGF-β signaling pathway in the mouse liver results in low hepcidin levels and iron overload, similar to the phenotype seen in HH.17 These data suggest that HFE2 mutations trigger iron overload by causing impaired hepatic BMP signaling, decreased hepcidin expression, and consequent ferroportin overactivity.
BMPs transduce their signals by binding to combinations of type I and II serine/threonine kinase receptors. There are 3 known BMP type II receptors (BMPRII, ActRIIA, and ActRIIB) and 3 known BMP type I receptors (ALK3, ALK6, and ALK2).11-14 Upon ligand binding, constitutively active type II receptors phosphorylate type I receptors. Type I receptors then phosphorylate intracellular receptor-activated Smads (R-Smads). Activated R-Smads complex with the common partner Smad4 and translocate to the nucleus to regulate gene transcription.11,12
For many TGF-β superfamily members, membrane-anchored proteins act as accessory receptors or coreceptors to assist with ligand binding to receptor, or to alter receptor specificity. For example, betaglycan mediates TGF-β2 binding to TGF-β type II receptor,18 and also increases the affinity of inhibin for the activin and BMP type II receptors.19-21 We have previously demonstrated that HJV-mediated BMP signaling is dependent on BMP ligands, BMP type I receptors, and BMP R-Smads.10 We have also demonstrated that HJV binds to BMP-2 and BMP-4 ligands and interacts with the BMP type I receptor ALK6.10 These data suggest that HJV acts as a BMP coreceptor to mediate BMP signaling via the classical pathway; however, the precise combinations of BMP ligands and receptors used by HJV remain largely unknown.
RGM family members, including HJV, have also been demonstrated to bind to the receptor neogenin.22,23 HJV-mediated iron accumulation in human embryonic kidney (HEK) 293 cells is enhanced in the presence of neogenin, thus suggesting a link between HJV, neogenin, and intracellular iron homeostasis.24 Interestingly, recent data suggest that HJV shedding can be mediated by neogenin in vitro.25 The ability of HJV to interact with neogenin raises the question of whether neogenin is involved in HJV-mediated BMP signaling and hepcidin regulation.
In the present study, we investigated the molecular mechanisms of HJV-mediated BMP signaling and hepcidin regulation using specific small interfering RNA (siRNA) to inhibit endogenous BMP ligands, BMP receptors, and neogenin in Hep3B and Huh-7 hepatoma cells. We also investigated the cohort of BMP ligands and receptors endogenously expressed in human liver. We found that HJV selectively uses distinct sets of BMP ligands, BMP type II receptors, and BMP type I receptors to induce BMP signaling and hepcidin expression. We provide evidence that HJV mediates BMP signaling and hepcidin regulation independently of neogenin.
Methods
Cell culture and transfection
Liver tissue
Human liver tissue was collected from the histologically normal margins of a resection specimen in a patient with colorectal cancer metastatic to the liver. This tissue was acquired from the institutional Tissue Bank under a Human Studies Committee–approved protocol at Massachusetts General Hospital. Informed consent was obtained in accordance with the Declaration of Helsinki. Additional human liver total RNA from a patient with sudden death was purchased from Clontech (Palo Alto, CA).
RT-PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA). First-strand cDNA synthesis was performed using iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Transcripts of BMP-2, BMP-4, BMP-5, BMP-6, BMP-7, BMP-9, BMPRII, ActRIIA, and ActRIIB were amplified using primers previously described.16,26 Transcripts of GDF-5, GDF-6, ALK2, ALK3, ALK6, and neogenin were amplified using primers summarized in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
siRNA targeting
siRNA duplexes in annealed and purified form were obtained from Ambion (Austin, TX). Sense sequences for ALK2, ALK3, ALK6, and neogenin are summarized in Table S2. BMP-2, BMP-4, BMP-6, BMP-7, BMPRII, ActRIIA, and ActRIIB siRNA sequences were previously described and validated for efficacy and specificity.16,26 siRNA transfections were performed as previously described.26
Measurement of gene expression
Real-time quantification of mRNA transcripts was performed as previously described.26 First-strand cDNA was amplified with the primers as shown in Table S3 (ALK2, ALK3, ALK6, and neogenin) or as previously described (BMP-2, BMP-4, BMP-6, BMP-7, BMPRII, ActRIIA, ActRIIB, hepcidin, and RPL19).16,26,27
Luciferase assays
Hep3B, KGN, or Huh-7 cells were transiently transfected with a BMP-responsive reporter (BRE-Luc; BMP-responsive portion of the Id1 gene promoter),28 an activin-responsive reporter ((CAGA)12MPL-Luc),29 or a hepcidin promoter luciferase reporter (Hep-Luc; 2.7-kb promoter upstream of the hepcidin translation start site)10,30,31 in combination with pTK-RL (Promega, Madison, WI) in a ratio of 10:1 to control for transfection efficiency, with or without cotransfection with siRNAs, in the absence or presence of Flag-tagged human HJV cDNA (Flag-HJV) or anti–BMP-6 antibody (R&D Systems, Minneapolis, MN). Approximately 24 hours after transfection, the medium was replaced with serum-free medium, with or without BMP or activin ligands (R&D Systems). At 16 hours later, the cells were lysed, and luciferase activity was determined with the Dual Reporter Assay kits (Promega). Experiments were performed in triplicate wells. Relative light units were calculated as ratios of firefly and Renilla luciferase values.
Data analysis
Results from luciferase assay experiments are expressed as the means plus or minus SD of triplicates from representative experiments. A total of 3 independent experiments were performed in each experimental setting. Differences were assessed by the Student t test with a P value less than .05 used to indicate significance.
Results
BMP-2, BMP-4, and BMP-6 are endogenous ligands for HJV in liver cells
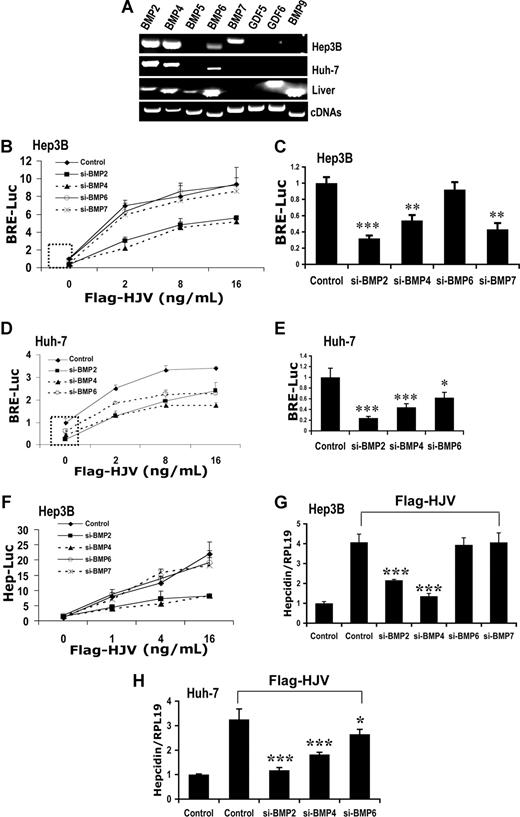
We have previously shown that HJV binds BMP-2 and BMP-4. Our data also suggest that HJV binds BMP-5 and BMP-6, but not BMP-7 or BMP-9.16 However, the endogenous BMP ligands used by HJV to regulate BMP signaling and hepcidin expression in the liver remain unknown. We therefore screened human liver and hepatoma-derived cells for expression of BMP and closely related growth and differentiation factor (GDF) ligands by reverse transcription–polymerase chain reaction (RT-PCR; Figure 1A). In human liver, BMP-2, BMP-4, BMP-5, BMP-6, BMP-9, and GDF-6 mRNA are all endogenously expressed, while BMP-7 and GDF-5 mRNA are not. Only BMP-2, BMP-4, BMP-6, and BMP-7 were detected in Hep3B cells. Huh-7 cells expressed only BMP-2, BMP-4, and BMP-6. Neither cell line expressed BMP-5, BMP-9, GDF-5, or GDF-6. Expression of BMP-5, GDF-6, and BMP-9 was also not detected in HepG2 cells.16
Impact of siRNA-mediated specific inhibition of BMP ligand expression on BMP signaling and hepcidin expression induced by HJV in Hep3B and Huh-7 cells. (A) Total RNA from Hep3B and Huh-7 cells and human liver was extracted for RT-PCR to determine the expression of BMP-2, BMP-4, BMP-5, BMP-6, BMP-7, GDF-5, GDF-6, and BMP-9. Purified plasmid cDNAs containing these ligands were used as positive controls. (B-E) Hep3B cells (B,C) or Huh-7 cells (D,E) were transfected with the BMP-responsive firefly luciferase reporter (BRE-Luc) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or siRNA specific for BMP-2, BMP-4, BMP-6, or BMP-7 (Control, si-BMP-2, siBMP-4, siBMP-6, and si-BMP-7; 60 nM) for 46 hours prior to measurement of luciferase activity. Firefly luciferase values were normalized for transfection efficiency relative to Renilla activity. Values shown are the means of triplicate measurements plus or minus SD. Basal BRE luciferase activities in the absence or presence of BMP-2, BMP-4, BMP-6, and BMP-7 siRNAs (dotted square in panels B and D) were replotted in panels C and E. (F) Hep3B cells were transfected with the hepcidin promoter firefly luciferase reporter (Hep-Luc) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or si-BMP-2, si-BMP-4, si-BMP-6, or si-BMP-7 at 60 nM. After 46 hours, cell lysates were analyzed for luciferase activity as in panels B-E. (G,H) Hep3B cells (G) or Huh-7 cells (H) were transfected with empty vector (pcDNA3) and control siRNA or with Flag-HJV cDNA in combination with control siRNA, si-BMP-2, si-BMP-4, si-BMP-6, or si-BMP-7 at 60 nM. After 46 hours, cells were collected for RNA extraction and real time RT-PCR analyses to quantify hepcidin and RPL19 mRNA levels. Hepcidin expression values were normalized to RPL19 mRNA levels. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
Impact of siRNA-mediated specific inhibition of BMP ligand expression on BMP signaling and hepcidin expression induced by HJV in Hep3B and Huh-7 cells. (A) Total RNA from Hep3B and Huh-7 cells and human liver was extracted for RT-PCR to determine the expression of BMP-2, BMP-4, BMP-5, BMP-6, BMP-7, GDF-5, GDF-6, and BMP-9. Purified plasmid cDNAs containing these ligands were used as positive controls. (B-E) Hep3B cells (B,C) or Huh-7 cells (D,E) were transfected with the BMP-responsive firefly luciferase reporter (BRE-Luc) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or siRNA specific for BMP-2, BMP-4, BMP-6, or BMP-7 (Control, si-BMP-2, siBMP-4, siBMP-6, and si-BMP-7; 60 nM) for 46 hours prior to measurement of luciferase activity. Firefly luciferase values were normalized for transfection efficiency relative to Renilla activity. Values shown are the means of triplicate measurements plus or minus SD. Basal BRE luciferase activities in the absence or presence of BMP-2, BMP-4, BMP-6, and BMP-7 siRNAs (dotted square in panels B and D) were replotted in panels C and E. (F) Hep3B cells were transfected with the hepcidin promoter firefly luciferase reporter (Hep-Luc) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or si-BMP-2, si-BMP-4, si-BMP-6, or si-BMP-7 at 60 nM. After 46 hours, cell lysates were analyzed for luciferase activity as in panels B-E. (G,H) Hep3B cells (G) or Huh-7 cells (H) were transfected with empty vector (pcDNA3) and control siRNA or with Flag-HJV cDNA in combination with control siRNA, si-BMP-2, si-BMP-4, si-BMP-6, or si-BMP-7 at 60 nM. After 46 hours, cells were collected for RNA extraction and real time RT-PCR analyses to quantify hepcidin and RPL19 mRNA levels. Hepcidin expression values were normalized to RPL19 mRNA levels. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
We then tested whether siRNA-mediated specific inhibition of endogenous BMP ligands in Hep3B cells affected HJV-mediated BMP signaling as measured by a BMP-responsive luciferase reporter (BRE-Luc) assay. BMP-2, BMP-4, BMP-6, and BMP-7 mRNA expression was each specifically inhibited 80% to 97% by their respective gene-specific siRNA duplexes (60 nM), with minimal effects on the expression of other ligands as measured by quantitative real-time RT-PCR (Figure S1). Inhibition of endogenous BMP-2 or BMP-4 expression dramatically reduced HJV-induced BRE luciferase activity compared with control siRNA (Figure 1B). In contrast, inhibition of BMP-6 or BMP-7 did not affect HJV-induced BRE luciferase activity compared with control siRNA (Figure 1B). These results suggest BMP-2 and BMP-4 are endogenous ligands for the coreceptor HJV in Hep3B cells.
Although inhibition of BMP-6 did not inhibit HJV-mediated BMP signaling in Hep3B cells, we have previously shown that soluble HJV.Fc inhibited hepcidin promoter activation induced by exogenous BMP-6,16 suggesting that HJV may bind to BMP-6. Of note, siRNA inhibition of BMP-6 did not change basal BRE luciferase activity in Hep3B cells, while inhibition of BMP-2, BMP-4, and BMP-7 significantly reduced basal BRE luciferase activity (Figure 1B,C). Furthermore, basal BRE luciferase activity was not altered by BMP-6 neutralizing antibody (Figure S2A). These results suggest that the expression of BMP-6 protein is too low to contribute to endogenous BMP signaling in Hep3B cells. To explore whether BMP-6 can act as an endogenous ligand for HJV, we used Huh-7 cells, which express higher levels of BMP-6 (Figure 1A). In Huh-7 cells, siRNA inhibition of BMP-2, BMP-4, and BMP-6 each reduced both basal and HJV-induced BRE luciferase activity (Figure 1D,E). Basal and HJV-induced BRE luciferase activity in Huh-7 cells was also reduced by a neutralizing anti–BMP-6 antibody (Figure S2B). Thus, BMP-6 can function as an endogenous ligand for HJV-mediated BMP signaling.
Since BMP signaling positively regulates hepcidin expression, we next investigated whether siRNA-mediated specific inhibition of endogenous BMP ligands also inhibited HJV-induced hepcidin transcription as measured by a hepcidin promoter luciferase reporter10 (Figure 1F) and hepcidin mRNA levels as measured by quantitative real-time RT-PCR (Figure 1G,H). Mirroring our results using the BRE-Luc reporter assay, BMP-2 and BMP-4 siRNA dramatically reduced HJV-induced hepcidin promoter luciferase activity in Hep3B cells, while BMP-6 and BMP-7 siRNA had no significant effect (Figure 1F). Similar results were obtained for hepcidin mRNA levels (Figure 1G). In Huh-7 cells, HJV induction of hepcidin expression was reduced by BMP-2, BMP-4, and BMP-6 siRNA (Figure 1H). These results suggest that HJV-induced hepcidin expression is dependent on BMP ligands, and that HJV can use BMP-2, BMP-4, and BMP-6, but not BMP-7, to up-regulate hepcidin expression.
HJV uses BMP type II receptors BMPRII and ActRIIA, enhances utilization of ActRIIA by BMP-2 and BMP-4, and primarily uses ActRIIA to regulate hepcidin expression
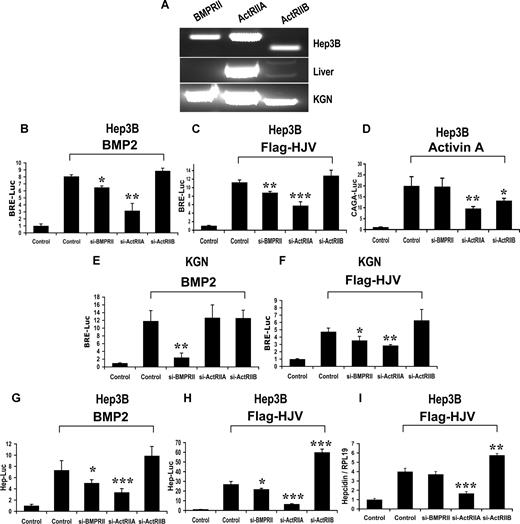
To investigate the utilization of BMP type II receptors by HJV, we first screened human liver and hepatoma cell lines for expression of BMPRII, ActRIIA, and ActRIIB by RT-PCR. In human liver, ActRIIA is the predominant type II receptor expressed, while BMPRII was not detected (Figure 2A). Similar results were seen in 2 separate human liver samples from different sources, including the histologically normal margins of a resection specimen of colorectal cancer metastatic to the liver, and a liver sample from a patient with sudden death. The lack of BMPRII expression in human liver was confirmed using an additional primer pair (Figure S3). ActRIIA was also the most highly expressed BMP type II receptor in Hep3B cells (Figure 2A), Huh-7 cells, and HepG2 cells (Figure S5A), although BMPRII was also detected in both Hep3B and Huh-7 cells.
Impact of siRNA-mediated specific inhibition of BMP type II receptor expression on BMP signaling and hepcidin expression induced by HJV in Hep3B and KGN cells. (A) Expression of BMP type II receptors BMPRII, ActRIIA, and ActRIIB in Hep3B cells and human liver (RT-PCR). KGN cells were used as a positive control. (B-F) Hep3B (B-D) or KGN cells (E,F) were transfected with BRE-Luc (B,C,E,F) or the activin-responsive firefly luciferase reporter (CAGA-Luc; panel D) and pRL-TK Renilla luciferase vector in combination with control siRNA or siRNA specific for BMPRII, ActRIIA, or ActRIIB (60 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (B,E) or activin A (D) or cotransfected with HJV cDNA (8 ng/mL; panels C,F). Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (G,H) Hep3B cells were transfected with the hepcidin promoter reporter construct (Hep-Luc) and pRL-TK, in combination with control siRNA or siRNA specific for BMPRII, ActRIIA, or ActRIIB (40 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (G), or after cotransfection with HJV cDNA (8 ng/mL; panel H) prior to measurement of luciferase activity. (I) Hep3B cells were transfected with empty vector (pcDNA3) and control siRNA, or with Flag-HJV cDNA in combination with control siRNA, si-BMPRII, si-ActRIIA, or si-ActRIIB at 40 nM. At 46 hours after transfection, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
Impact of siRNA-mediated specific inhibition of BMP type II receptor expression on BMP signaling and hepcidin expression induced by HJV in Hep3B and KGN cells. (A) Expression of BMP type II receptors BMPRII, ActRIIA, and ActRIIB in Hep3B cells and human liver (RT-PCR). KGN cells were used as a positive control. (B-F) Hep3B (B-D) or KGN cells (E,F) were transfected with BRE-Luc (B,C,E,F) or the activin-responsive firefly luciferase reporter (CAGA-Luc; panel D) and pRL-TK Renilla luciferase vector in combination with control siRNA or siRNA specific for BMPRII, ActRIIA, or ActRIIB (60 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (B,E) or activin A (D) or cotransfected with HJV cDNA (8 ng/mL; panels C,F). Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (G,H) Hep3B cells were transfected with the hepcidin promoter reporter construct (Hep-Luc) and pRL-TK, in combination with control siRNA or siRNA specific for BMPRII, ActRIIA, or ActRIIB (40 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (G), or after cotransfection with HJV cDNA (8 ng/mL; panel H) prior to measurement of luciferase activity. (I) Hep3B cells were transfected with empty vector (pcDNA3) and control siRNA, or with Flag-HJV cDNA in combination with control siRNA, si-BMPRII, si-ActRIIA, or si-ActRIIB at 40 nM. At 46 hours after transfection, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
We then determined the effect of BMP type II receptor siRNAs on exogenous BMP- or HJV-induced BMP signaling in Hep3B cells. BMP type II receptor siRNAs significantly and specifically reduced endogenous type II receptor mRNA expression by 80% to 90%, but did not affect the expression of other type II receptors (Figure S4). These siRNAs also dramatically reduced the protein expression levels of corresponding transfected type II receptors (Figure S4B). BRE luciferase activity induced by exogenous BMP-2 was slightly, but significantly, reduced by BMPRII siRNA, and was more dramatically reduced by ActRIIA siRNA in Hep3B cells (Figure 2B). In contrast, BMP-2 signaling was not affected by inhibition of ActRIIB expression (Figure 2B). Similar data were observed when cells were treated with exogenous BMP-4 (data not shown), and when cells were transfected with Flag-HJV cDNA in place of exogenous BMP stimulation (Figure 2C). As a control, activin A–induced CAGA-luciferase activity was inhibited by both ActRIIA and ActRIIB inhibition, indicating the ActRIIB siRNA was effective (Figure 2D).
We also tested utilization of BMP type II receptors by HJV in Huh-7 and HepG2 cells, where BMPRII expression was lower or absent, more closely mimicking human liver. Exogenous BMP-2– and HJV-induced BRE luciferase activity was reduced by inhibition of ActRIIA expression, but not by inhibition of BMPRII or ActRIIB expression in both Huh-7 and HepG2 cells (Figure S5C,E). Taken together, these results suggest that HJV can use both ActRIIA and BMPRII, but not ActRIIB to mediate BMP signaling; however, ActRIIA appears to be the primary type II receptor used by HJV in Hep3B, HepG2, and Huh-7 cells.
Although we found that BMP-2 and BMP-4 primarily use ActRIIA in hepatoma-derived cells, BMPRII is the principal type II receptor used by BMP-2 and BMP-4 ligands in most cell lines studied.26,32,33 We have previously demonstrated that RGMa facilitates the use of ActRIIA by endogenous BMP-2 and BMP-4 ligands that normally prefer signaling via BMPRII in KGN cells, a human granulosa cell line.26 We therefore examined whether HJV can also alter utilization of BMP type II receptors by BMP-2 and BMP-4 ligands in KGN cells. We first demonstrated that BMP-2 and BMP-4 are the sole ligands for the HJV coreceptor in KGN cells by showing that inhibition of both ligands reduced HJV-mediated BMP signaling to baseline (Figure S6). Furthermore, although KGN cells express BMP-6 mRNA in addition to BMP-2 and BMP-4,26 inhibition of BMP-6 by BMP-6 siRNA or BMP-6–neutralizing antibody had no effect on basal or HJV-induced BMP signaling in these cells (Figure S6). Next, we examined the effects of siRNA inhibition of BMP type II receptors on exogenous BMP-2– or HJV-mediated BMP signaling in KGN cells. BMP-2 signaling was blocked by siRNA inhibition of BMPRII expression, but not altered by inhibition of ActRIIA or ActRIIB expression (Figure 2E). Similar results were observed when cells were treated with exogenous BMP-4 (data not shown). In contrast, HJV-induced BRE luciferase activity was significantly inhibited by both BMPRII and ActRIIA siRNAs (Figure 2F). The inhibition of HJV-induced BRE luciferase activity by both BMPRII and ActRIIA siRNAs was not changed by incubation with a neutralizing BMP-6 antibody (Figure S7), excluding the possibility that BMP-6 contributed to the increased utilization of ActRIIA by HJV. These results suggest that HJV enhances utilization of ActRIIA by BMP-2 and BMP-4 in KGN cells, similar to our previously reported finding for RGMa.26
We then determined the effects of inhibiting endogenous BMP type II receptors on BMP- and HJV-induced hepcidin expression. Hepcidin promoter luciferase activity induced by exogenous BMP-2 (Figure 2G) and BMP-4 (data not shown) was reduced significantly by ActRIIA siRNA, and to a lesser extent by BMPRII siRNA, but was not altered by ActRIIB siRNA. Similarly, hepcidin promoter luciferase activity induced by Flag-HJV was reduced by ActRIIA siRNA, and to a lesser extent by BMPRII siRNA. Interestingly, hepcidin promoter activity was significantly enhanced by inhibition of ActRIIB expression (Figure 2H). Hepcidin mRNA expression induced by Flag-HJV was significantly reduced by ActRIIA siRNA, but was not significantly altered by inhibition of BMPRII (Figure 2I). As seen with the hepcidin promoter luciferase assay (Figure 2H), inhibition of ActRIIB significantly increased HJV mediated hepcidin mRNA levels (Figure 2I). These results suggest that HJV acts primarily through ActRIIA to regulate hepcidin mRNA expression in Hep3B cells.
HJV uses classical BMP type I receptors to regulate BMP signaling and hepcidin expression
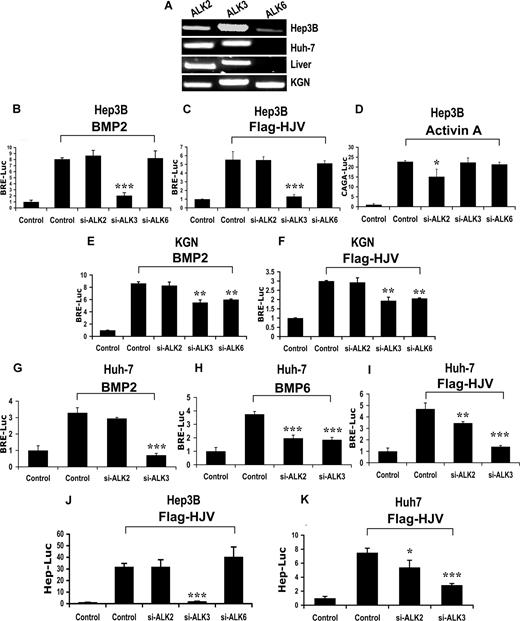
To investigate the utilization of BMP type I receptors by HJV, we screened for the expression of BMP type I receptors ALK2, ALK3, and ALK6 in human liver, Hep3B cells, and Huh-7 cells (Figure 3A). KGN cells were used as a positive control.34 ALK2 and ALK3 were abundantly expressed in human liver and all cell lines. ALK6 was not detected in human liver (Figures 3A, S3) or Huh-7 cells, and was only present at a low levels in Hep3B cells (Figure 3A).
Impact of siRNA-mediated specific inhibition of BMP type I receptor expression on BMP signaling and hepcidin expression induced by HJV in Hep3B, KGN, and Huh-7 cells. (A) Expression of BMP type I receptors ALK2, ALK3, and ALK6 in Hep3B, Huh-7, and KGN cells, and human liver (RT-PCR). (B-I) Hep3B (B-D), KGN (E,F), or Huh-7 cells (G-I) were transfected with BRE-Luc or CAGA-Luc and pRL-TK Renilla luciferase vector, in combination with control siRNA or siRNA specific for ALK2, ALK3, and ALK6 (60 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (B,E,G), 20 ng/mL activin A (D), or 20 ng/mL BMP-6 (H), or after cotransfection with 8 ng/mL HJV cDNA (C,F,I). Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (J,K) Hep3B (J) or Huh-7 cells (K) were transfected with Hep-Luc and pRL-TK, in combination with control siRNA or siRNA specific for ALK2, ALK3, or ALK6 (60 nM), in the absence or presence of HJV cDNA (8 ng/mL) for 46 hours prior to measurement of luciferase activity. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
Impact of siRNA-mediated specific inhibition of BMP type I receptor expression on BMP signaling and hepcidin expression induced by HJV in Hep3B, KGN, and Huh-7 cells. (A) Expression of BMP type I receptors ALK2, ALK3, and ALK6 in Hep3B, Huh-7, and KGN cells, and human liver (RT-PCR). (B-I) Hep3B (B-D), KGN (E,F), or Huh-7 cells (G-I) were transfected with BRE-Luc or CAGA-Luc and pRL-TK Renilla luciferase vector, in combination with control siRNA or siRNA specific for ALK2, ALK3, and ALK6 (60 nM). Cells were incubated in the absence or presence of 20 ng/mL BMP-2 (B,E,G), 20 ng/mL activin A (D), or 20 ng/mL BMP-6 (H), or after cotransfection with 8 ng/mL HJV cDNA (C,F,I). Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (J,K) Hep3B (J) or Huh-7 cells (K) were transfected with Hep-Luc and pRL-TK, in combination with control siRNA or siRNA specific for ALK2, ALK3, or ALK6 (60 nM), in the absence or presence of HJV cDNA (8 ng/mL) for 46 hours prior to measurement of luciferase activity. Values shown are the means of triplicate measurements plus or minus SD. *P < .05; **P < .01; ***P < .001.
We then tested the effect of BMP type I receptor siRNAs on exogenous BMP- and HJV-induced BRE-Luc activity in Hep3B cells. BMP type I receptor siRNAs (60 nM) selectively reduced endogenous mRNA expression of each of the 3 receptors by 75% to 90% with minimal effect on the expression of the other receptors (Figure S8A). These siRNAs also dramatically reduced the protein expression levels of corresponding transfected type I receptors (Figure S8B). BRE luciferase activity induced by exogenous BMP-2 or HJV was reduced by inhibition of ALK3 but was not affected by inhibition of ALK2 or ALK6 expression in Hep3B cells (Figure 3B,C). As a control, activin A–induced CAGA-luciferase activity was significantly inhibited by ALK2 siRNA, indicating the ALK2 siRNA was effective (Figure 3D). These results suggest that HJV uses ALK3, but not ALK2 and ALK6 as a type I receptor to enhance BMP signaling in Hep3B cells.
BMP-2 has been previously shown to use the BMP type I receptor ALK6,14 and we have previously shown that HJV interacts with ALK6.10 We hypothesized that the inability of ALK6 siRNA to inhibit BMP-2– or HJV-mediated BMP signaling in Hep3B cells may be due to low ALK6 protein expression in these cells, even though ALK6 mRNA was detected. We therefore explored the utilization of BMP type I receptors by HJV in KGN cells, a cell line which expresses higher levels of endogenous ALK6 mRNA in addition to ALK3 (Figure 3A). In KGN cells, inhibition of ALK3 or ALK6 significantly reduced BMP-2–induced (Figure 3E) or HJV-mediated (Figure 3F) BRE luciferase activity, indicating HJV can use ALK6 to enhance BMP signaling when it is expressed at sufficient levels.
Previous studies have shown that BMP-6 can signal through the BMP type I receptor ALK2.35 Although we have shown that BMP-6 does not contribute to basal or HJV-induced BMP signaling in Hep3B cells, BMP-6 is an endogenous ligand for HJV in Huh-7 cells. We therefore examined whether HJV uses ALK2 to enhance endogenous BMP-6 signaling in Huh-7 cells. As seen in Hep3B cells, exogenous BMP-2–induced BRE luciferase activity was abolished by inhibition of ALK3 expression, but was not altered by inhibition of ALK2 expression in Huh-7 cells (Figure 3G). In contrast, exogenous BMP-6–induced BRE luciferase activity was reduced to a similar extent by inhibition of ALK2 and ALK3 expression in Huh-7 cells (Figure 3H). HJV-induced BRE luciferase activity was also significantly reduced by inhibition of ALK2 or ALK3 expression in Huh-7 cells. (Figure 3I). To examine whether the reduction in HJV-mediated BMP signaling by si-ALK2 was due to the endogenous BMP-6, we used anti–BMP-6 antibody to specifically neutralize endogenous BMP-6 protein (Figure S9). In the presence of neutralizing anti–BMP-6 antibody, inhibition of ALK2 expression was no longer able to reduce BMP signaling mediated by HJV (Figure S9). These results suggest that HJV can use ALK2 to induce BMP signaling in the presence of high levels of BMP-6.
We then tested the effects of BMP type I receptor siRNAs on HJV-mediated activation of the hepcidin promoter. Consistent with the BRE-Luc assay data (Figure 3C), HJV-induced hepcidin promoter activity was significantly reduced by inhibition of ALK3, but was not significantly altered by inhibition of ALK2 and ALK6 in Hep3B cells (Figure 3J). In Huh-7 cells, HJV-induced hepcidin promoter activity was significantly reduced by inhibition of ALK2 or ALK3 expression (Figure 3K). Thus, ALK3 and ALK2 are important type I receptors for hepcidin regulation in Hep3B and Huh-7 cells.
Neogenin does not affect HJV-mediated BMP signaling and hepcidin expression
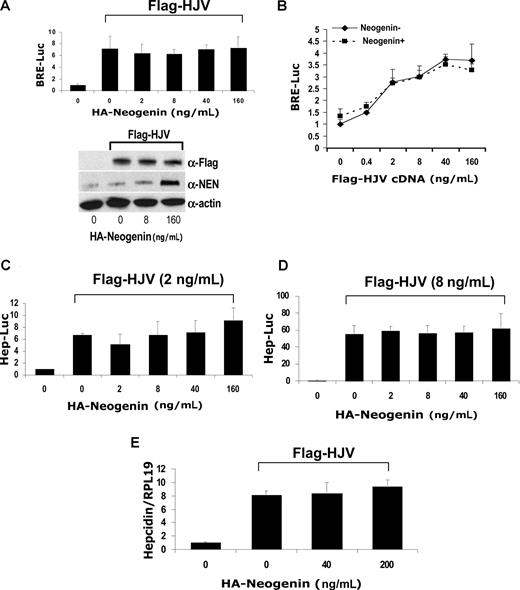
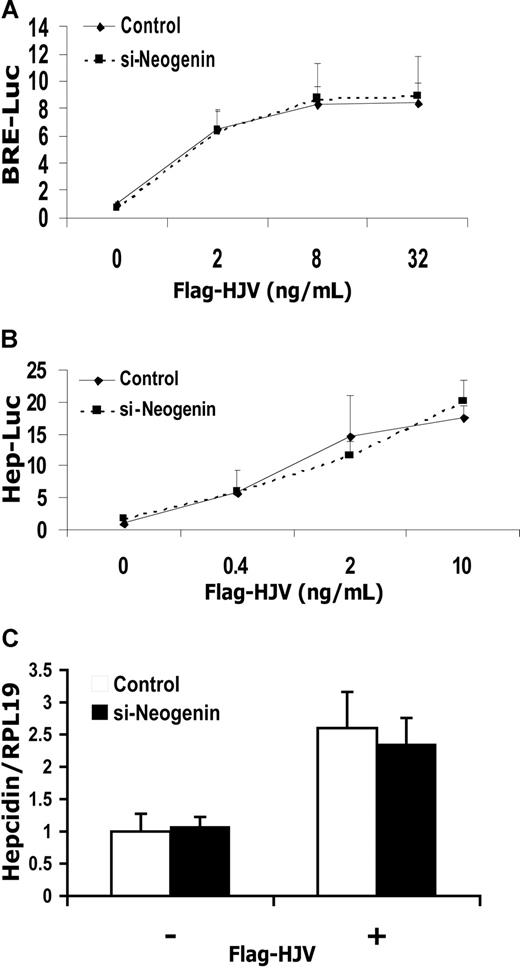
It has been shown previously that HJV interacts with neogenin.24 We therefore investigated whether neogenin influences HJV-mediated BMP signaling and hepcidin regulation using both neogenin overexpression and siRNA inhibition assays. For overexpression assays, we generated a cDNA encoding mouse mature neogenin with an HA tag at the N-terminus (HA-neogenin; Figure S10A). We confirmed that HA-neogenin coimmunoprecipitated with Flag-HJV (Figure S10B), similar to previously described findings.24 Transfection of Hep3B cells with HA-neogenin cDNA did not affect HJV-mediated induction of BRE luciferase activity, even though significant amounts of neogenin protein were produced at the highest concentration of cDNA used (Figure 4A,B). Hepcidin promoter activity was induced by transfection with HJV at 2 ng/mL (Figure 4C) or 8 ng/mL (Figure 4D) as previously described but was not affected by cotransfection with increasing amounts of HA-neogenin cDNA (Figure 4C,D). Similarly, overexpression of HA-neogenin did not alter HJV induced hepcidin mRNA expression in Hep3B cells (Figure 4E). For inhibition assays, we demonstrated that neogenin siRNA significantly inhibited neogenin mRNA and protein expression in Hep3B cells (Figure S11). Inhibition of endogenous neogenin in Hep3B cells had no effect on HJV-induced BMP signaling (Figure 5A) or hepcidin expression (Figure 5B,C). These results suggest that HJV acts independently of neogenin to enhance BMP signaling and hepcidin expression in Hep3B cells.
HJV-mediated BMP signaling and hepcidin expression are not altered by neogenin overexpression in Hep3B cells. (A) Hep3B cells were transfected with BRE-Luc and pRL-TK, either alone or with a fixed amount of Flag-HJV cDNA in combination with increasing amounts of HA-neogenin cDNA for 46 hours. Cell lysates were analyzed for luciferase activity as in Figure 1B-E (top panel) or by Western blot in succession with Flag antibody (M5; α-Flag), neogenin antibody (α-NEN), and actin antibody (α-actin, as a loading control; bottom panel). (B) Hep3B cells were transfected with BRE-Luc and pRL-TK, either alone or with fixed amount of HA-neogenin cDNA (40 ng/mL), in combination with increasing amounts of Flag-HJV cDNA. Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (C,D) Hep3B cells were transfected with Hep-Luc and pRL-TK, either alone or with Flag-HJV at 2 ng/mL (C) or 8 ng/mL (D), in combination with increasing amounts of HA-neogenin cDNA. Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (E) Hep3B cells were transfected with empty vector (pcDNA3) or Flag-HJV cDNA in combination with increasing amount of HA-neogenin cDNA. After 46 hours, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD.
HJV-mediated BMP signaling and hepcidin expression are not altered by neogenin overexpression in Hep3B cells. (A) Hep3B cells were transfected with BRE-Luc and pRL-TK, either alone or with a fixed amount of Flag-HJV cDNA in combination with increasing amounts of HA-neogenin cDNA for 46 hours. Cell lysates were analyzed for luciferase activity as in Figure 1B-E (top panel) or by Western blot in succession with Flag antibody (M5; α-Flag), neogenin antibody (α-NEN), and actin antibody (α-actin, as a loading control; bottom panel). (B) Hep3B cells were transfected with BRE-Luc and pRL-TK, either alone or with fixed amount of HA-neogenin cDNA (40 ng/mL), in combination with increasing amounts of Flag-HJV cDNA. Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (C,D) Hep3B cells were transfected with Hep-Luc and pRL-TK, either alone or with Flag-HJV at 2 ng/mL (C) or 8 ng/mL (D), in combination with increasing amounts of HA-neogenin cDNA. Cell lysates were analyzed for luciferase activity as in Figure 1B-E. (E) Hep3B cells were transfected with empty vector (pcDNA3) or Flag-HJV cDNA in combination with increasing amount of HA-neogenin cDNA. After 46 hours, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD.
HJV-mediated BMP signaling and hepcidin expression are not altered by inhibition of endogenous neogenin expression in Hep3B cells. (A,B) Hep3B cells were transfected with BRE-Luc (A) or Hep-Luc (B) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or neogenin siRNA. After 46 hours, cell lysates were analyzed for luciferase activity as in Figure 1B-F. (C) Hep3B cells were transfected with empty vector (pcDNA3) or Flag-HJV cDNA in combination with control siRNA or si-neogenin. After 46 hours, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD.
HJV-mediated BMP signaling and hepcidin expression are not altered by inhibition of endogenous neogenin expression in Hep3B cells. (A,B) Hep3B cells were transfected with BRE-Luc (A) or Hep-Luc (B) and pRL-TK Renilla luciferase vector, either alone or with increasing amounts of Flag-HJV cDNA, in combination with control siRNA or neogenin siRNA. After 46 hours, cell lysates were analyzed for luciferase activity as in Figure 1B-F. (C) Hep3B cells were transfected with empty vector (pcDNA3) or Flag-HJV cDNA in combination with control siRNA or si-neogenin. After 46 hours, hepcidin relative to RPL19 mRNA levels were quantified by real-time RT-PCR as in Figure 1G,H. Values shown are the means of triplicate measurements plus or minus SD.
Discussion
Juvenile hemochromatosis (JH) is an early-onset hereditary disorder of iron overload which results from mutations in either HAMP (encoding hepcidin) or HFE2 (encoding HJV).1 We reported previously that HJV is a coreceptor for BMP signaling and that HJV-mediated BMP signaling increases hepcidin expression.10 In the present study, we further investigated the precise molecular mechanisms by which HJV mediates BMP signaling and regulates hepcidin expression.
Here, we demonstrated that inhibition of endogenous BMP-2, BMP-4, or BMP-6 expression reduced HJV-induced BMP signaling and hepcidin expression. This result is consistent with our previous findings that BMP-2 and BMP-4 antibody inhibits HJV-mediated BMP signaling,10 that soluble HJV.Fc binds to BMP-2 and BMP-4 ligands,10 and that soluble HJV.Fc inhibits hepcidin promoter activity stimulated by BMP-2, BMP-4, and BMP-6.16 This is also consistent with previous data that soluble mouse hemojuvelin inhibits BMP-2 and BMP-4 induction of hepcidin expression in primary hepatocyte cultures.36 Inhibition of endogenous BMP-7 expression reduced basal BMP signaling, but did not change HJV-induced BMP signaling and hepcidin expression. This is consistent with our previous findings that soluble HJV.Fc binding to BMP-2 was not inhibited by BMP-7,10 and that soluble HJV.Fc did not significantly inhibit BMP-7–induced hepcidin promoter activity.16 In addition, BMP-7 is not expressed in human liver. Collectively, our results demonstrate that BMP-2, BMP-4, and BMP-6 are endogenous ligands for HJV-mediated BMP signaling and hepcidin regulation, but BMP-7 is not a ligand for HJV.
BMP-6 and BMP-7, in conjunction with BMP-5, form a subfamily within the BMP family of ligands with 71% to 80% amino acid identity in the mature region.37 Interest-ingly, despite their sequence similarity, BMP-6 and BMP-7 exhibit a number of biochemical and biological differences. For example, the affinity of follistatin for BMP-6 is 5-fold higher than that for BMP-7.38,39 In addition, BMP-6 and BMP-7 have differential effects on neurite outgrowth of cerebellar granule cell neurons.40 Our results suggest that HJV is able to distinguish between the closely related BMP-6 and BMP-7 ligands. Future studies will be needed to determine whether HJV directly binds BMP-6. A comparative sequence analysis of these ligands may help to identify regions in BMP-6 that are important for interaction with HJV.
In human liver, we show that BMP-2, BMP-4, BMP-5, BMP-6, GDF-6, and BMP-9, but not BMP-7, are endogenously expressed. Taken together with our in vitro data, we hypothesize that BMP-2, BMP-4, and BMP-6 are good candidates for endogenous ligands for HJV-mediated BMP signaling and hepcidin regulation in vivo. Of note, BMP-5 and BMP-9 have also been shown to induce hepcidin expression in vitro.15,16 BMP-5 induction of hepcidin expression can be inhibited by soluble HJV.Fc. However, soluble HJV.Fc does not inhibit BMP-9–induced activation of the hepcidin promoter in Hep3B cells,16 and mouse soluble HJV does not inhibit BMP-9–induced hepcidin expression in primary hepatocyte cultures.36 These data suggest that BMP-5 may also be an endogenous ligand for HJV, but BMP-9 is unlikely a ligand for HJV.
Previous studies have shown that BMP-2 and BMP-4 use both BMP type II receptors BMPRII and ActRIIA; however, BMPRII appears to be the principal type II receptor in most cell lines studied.26,32,33 Here, we show that in Hep3B cells, BMP-2/BMP-4–induced or HJV-mediated BMP signaling and hepcidin promoter activation occurs via both BMPRII and ActRIIA, with a preference for ActRIIA. By quantitative real-time PCR, only ActRIIA siRNA, but not BMPRII siRNA, significantly inhibited HJV induction of hepcidin expression. These data indicate that HJV preferentially uses ActRIIA to regulate hepcidin expression. Interestingly, ActRIIA is the predominant BMP type II receptor expressed in human liver, while BMPRII was not detected. This suggests that the presence of HJV may be important for allowing BMP ligands to transduce signals in human liver via ActRIIA.
Recently, we showed that RGMa enhances utilization of the BMP type II receptor ActRIIA by endogenous BMP-2 and BMP-4 ligands in KGN cells, which do not express endogenous RGM family members.26 Interestingly, HJV also facilitates utilization of ActRIIA by BMP-2 and BMP-4 in KGN cells. Of note, Hep3B cells express endogenous HJV and RGMb/Dragon mRNA (data not shown). The presence of the RGM family members may explain the preferential utilization of ActRIIA by BMP-2 and BMP-4 in Hep3B cells.
In Hep3B cells, siRNA inhibition of ActRIIB significantly increased HJV-stimulated hepcidin luciferase activity and hepcidin expression. This raises the possibility that ActRIIB mediates an inhibitory effect on hepcidin expression. Alternatively, inhibition of ActRIIB expression may liberate additional BMP type I receptors that normally would complex with ActRIIB, thus leading to increased BMP-mediated stimulation of hepcidin expression. Future studies will be needed to further understand this finding. Of note, a small amount of ActRIIB may be expressed in human liver.
The utilization of BMP type I receptors by exogenous BMP ligands in our study is consistent with previous studies showing that BMP-2 and BMP-4 signal through ALK3, ALK6, but not ALK2, while BMP-6 signals through ALK2, ALK3, and ALK6.14,33 In the present study, we found that HJV-mediated BMP signaling occurs via ALK3 and ALK6, but not ALK2, in Hep3B and KGN cells, where BMP-2 and BMP-4 are the endogenous ligands for HJV. In Huh-7 cells, HJV-induced BMP signaling occurs via ALK2 in addition to ALK3 due to the presence of endogenous BMP-6. HJV presumably also uses ALK6 to mediate BMP-6 signaling, but we could not test this directly due to the absence of ALK6 in the Huh-7 cells. Thus, the utilization of type I receptors in the presence of HJV mirrors utilization of type I receptors by BMP ligands in the absence of HJV, suggesting that HJV does not alter utilization of type I receptors by BMP ligands. Our RT-PCR data show that both ALK2 and ALK3 were present in human liver, but ALK6 was not detected. Thus, ALK3 and/or ALK2 appear to be important BMP type I receptors for regulating hepcidin expression in vivo in human liver.
RGMa and HJV (RGMc) have been shown to interact with neogenin,24 and neogenin mRNA is expressed in human liver (data not shown). Consistent with these prior findings, we were able to coimmunoprecipitate HA-neogenin with Flag-HJV. However, we found that neither overexpression of HA-neogenin nor inhibition of endogenous neogenin altered HJV-induced BRE-Luc activity, hepcidin promoter activity, or hepcidin mRNA expression in Hep3B cells. This suggests that HJV acts independently of neogenin to regulate BMP signaling and hepcidin expression. Consistent with these findings, a recent study demonstrated that the RGMa-neogenin signal transduction pathway involved in axonal guidance appears to involve the small GTPase RhoA and its downstream effector Rho kinase, but not the BMP signaling pathway.41
Zhang and colleagues have previously reported that neogenin mediates shedding of HJV from the cell surface of HEK293 cells.25 Both we16 and others27 have shown that soluble HJV inhibits hepcidin expression, presumably by binding to BMP ligands and sequestering them from cell-surface signaling receptors. Thus, one could predict that neogenin might inhibit BMP signaling and hepcidin expression by the generation of shed inhibitory soluble HJV. However, Zhang and colleagues did not test the shed HJV protein for functional ability to inhibit BMP signaling or hepcidin expression. In addition, Zhang and colleagues did not study neogenin-mediated HJV shedding in Hep3B cells or other hepatoma-derived cell lines. Although we did not examine the shedding of HJV in our study, whether or not neogenin mediates shedding of HJV in Hep3B cells, there was clearly no effect of neogenin with respect to BMP signaling by HJV. If neogenin did mediate shedding of HJV in Hep3B cells, the shed HJV may not have been functional or sufficient in quantity to inhibit BMP signaling or hepcidin expression. The significance of the interaction between neogenin and HJV remains to be a topic for future studies.
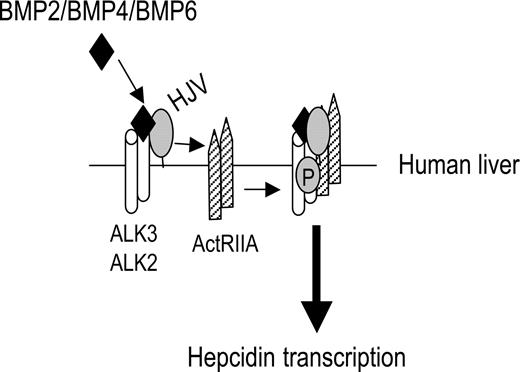
In summary, we found that BMP-2, BMP-4, and BMP-6, but not BMP-7, are endogenous ligands for HJV to induce BMP signaling and hepcidin expression in hepatoma-derived cells. HJV alters utilization of BMP type II receptors by BMP-2 and BMP-4 ligands, acting preferentially through ActRIIA and to a lesser extent BMPRII, but not ActRIIB. HJV can use all 3 BMP type I receptors, ALK2, ALK3, and ALK6, with a selectivity that mirrors the selectivity of the BMP ligand present in the signaling complex. The predominant expression of ActRIIA, ALK2, and ALK3 in human liver supports the potential importance of these BMP receptors for HJV-mediated hepcidin regulation in vivo (Figure 6). Our findings clearly show that HJV uses a classical BMP pathway to induce BMP signaling and hepcidin expression, and that this function is independent of the interaction between HJV and neogenin.
Schematic diagram depicting HJV action in regulating hepcidin expression in human liver. ActRIIA is the predominant BMP type II receptor, and ALK3 and ALK2 are predominant BMP type I receptors expressed in the human liver. HJV facilitates endogenous ligands BMP-2, BMP-4, and BMP-6 to signal through ActRIIA in combination with ALK3 and/or ALK2 to regulate hepcidin expression
Schematic diagram depicting HJV action in regulating hepcidin expression in human liver. ActRIIA is the predominant BMP type II receptor, and ALK3 and ALK2 are predominant BMP type I receptors expressed in the human liver. HJV facilitates endogenous ligands BMP-2, BMP-4, and BMP-6 to signal through ActRIIA in combination with ALK3 and/or ALK2 to regulate hepcidin expression
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Nancy Andrews for helpful discussions and for providing Huh-7 cells, Dr Kohei Miyazono for providing the HA-ALK2 construct, and Drs Hideyuki Beppu and Paul Yu for providing the BMPRII-GFP construct. We also thank Ms Sandra Chen for technical assistance.
H.Y.L. was supported by National Institutes of Health (NIH) grant RO1 DK-071837 and RO1 DK-069533, and a grant from the Roche Foundation for Anemia Research. J.L.B. was supported by NIH grant K08 DK-075846.
National Institutes of Health
Authorship
Contribution: H.Y.L. designed the research and wrote the paper; Y.X. designed the research, performed the research, analyzed data, and wrote the paper; J.L.B. designed the research and wrote the paper; Y.S. designed the research and contributed vital new reagents; and R.T.C. contributed vital new reagents and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Herbert Y. Lin, Program in Membrane Biology, Richard B. Simches Research Center, 185 Cambridge St, CPZN-8216, Boston, MA 02114; e-mail: hlin@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal