In this issue of Blood, Sprague and colleagues report that platelet-derived membrane vesicles (PDMVs) orchestrate an immune response sufficient to deliver CD154 signals, which stimulate antigen-specific IgG production and modulate germinal-center formation, by cooperating with responses elucidated by CD4+ T cells.
Attention has been focused recently on circular membrane fragments called membrane vesicles (MVs), which for many years have been largely overlooked. MVs are shed from the surface membranes of cells, as well as secreted from the endosomal compartment as circular membrane fragments.1 They contain numerous proteins and lipids similar to those present in the membranes of the cells from which MVs originate. Furthermore, because they engulf some cytoplasm during membrane blebbing, they may also contain proteins and mRNA.2 Thus, MVs may stimulate target cells directly via surface-expressed ligands acting as a kind of “signaling complex.” It has been postulated that this MV-mediated cell-cell communication system emerged very early in evolution and served as a template for the development of cell-cell interaction mechanisms involving soluble bioactive mediators and fine-tuned ligand-receptor interactions. MVs may, in addition, transfer surface receptors from one cell to another; deliver proteins, mRNA, bioactive lipids, and even whole organelles (eg, mitochondria) into target cells; and finally, serve as a vehicle to transfer infectious particles such as HIV or prions between cells (through a “Trojan horse” mechanism).
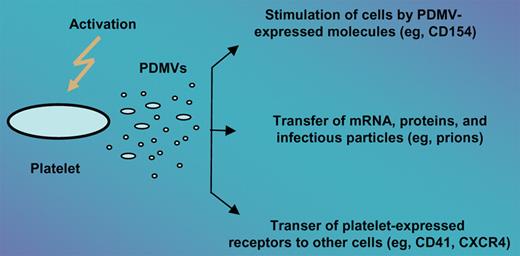
It is well known that activated platelets are a rich source of MVs released both from their surface as well as from endosomal compartments (exosomes). These PDMVs may (1) directly stimulate hematopoietic cells, lymphocytes, and endothelium3 ; (2) transfer platelet-expressed receptors (eg, CD41 or CXCR4)4,5 to the surface of other cells; and even (3) transfer mRNA, proteins, and infectious particles (eg, prions) between cells. Thus, PDMVs may exert several pleiotropic effects that are relevant to hematopoiesis and/or lymphopoiesis (see figure).
Different mechanisms by which PDMVs may interact with target cells. PDMVs may (1) stimulate target cells directly via surface-expressed ligands acting as a kind of signaling complex (eg, CD154), (2) transfer surface receptors from one cell to another (eg, CD41, CXCR4), or (3) deliver proteins, mRNA, and bioactive lipids, and even serve as a vehicle (“Trojan horse” mechanism) to transfer infectious particles between cells (eg, prions).
Different mechanisms by which PDMVs may interact with target cells. PDMVs may (1) stimulate target cells directly via surface-expressed ligands acting as a kind of signaling complex (eg, CD154), (2) transfer surface receptors from one cell to another (eg, CD41, CXCR4), or (3) deliver proteins, mRNA, and bioactive lipids, and even serve as a vehicle (“Trojan horse” mechanism) to transfer infectious particles between cells (eg, prions).
In this issue of Blood, Sprague and colleagues report that PDMVs orchestrate immune responses by being able to deliver a CD154 signal that stimulates antigen-specific IgG production and modulates germinal-center formation through cooperation with CD4+ T cells. CD154 is a ligand for CD40 receptor, which is critical to the initiation and propagation of the adaptive immune response and is expressed on several cell types, including various subsets of T lymphocytes and platelets. Humans and mice lacking functional CD154 fail to isotype-switch from the IgM antibody isotype, which leads to hyper-IgM syndrome, and are also unable to mount the germinal-center response necessary for differentiation of memory B lymphocytes and plasmocytes.
It has been known for many years that platelets modulate inflammatory responses. Because PDMVs released from platelets circulate in peripheral blood, the data published by Sprague et al in this issue of Blood explain why platelets may modulate inflammation and adaptive immunity at sites distant from the location of activation. In a set of elegant experiments, the authors challenged a well-established paradigm: that the CD154 signal is delivered solely by CD4+ cells to B cells.
This paper is an important step toward understanding the complexity of cell-cell communication, in which MVs released from activated cells play an important and still underappreciated role. Not only platelets, but all other cells present in the hematopoietic microenvironment or at sites of tissue injury/inflammation, secrete MVs; thus, the MV-related network seems to modulate several important biological responses. Thus, MVs should not be envisioned any longer as biologically irrelevant cell dust. Accumulating evidence demonstrates that they are important mediators of intercellular communication (eg, by delivering CD154 signal, as shown by Sprague and colleagues). Based on these findings, further studies are required to determine whether, in addition to CD154, other components of PDMVs directly or indirectly affect the immune response.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal