To complete their maturation and participate in the humoral immune response, immature B cells that leave the bone marrow are targeted to specific areas in the spleen, where they differentiate into mature cells. Previously, we showed that immature B cells actively down-regulate their integrin-mediated migration to lymph nodes or to sites of inflammation, enabling their targeting to the spleen for final maturation. This inhibition is mediated by IFN-γ, which is transcribed and secreted at low levels by these immature B cells; IFN-γ expression is extinguished following B-cell maturation. Stimulation of the MHC class I receptor, Ly49D, triggers a signaling cascade that increases transcription of both IL-12 (p40) and IL-18; these, in turn, induce the secretion of IFN-γ. In the present study, we demonstrate that Ly49D-dependent secretion of IL-12 and IL-18 induces IL-15 expression by immature B cells, and that these 3 factors together regulate IFN-γ production that inhibits their ability to home to the lymph nodes or to sites of inflammation. Thus, IL-15 controls immature B-cell homing, resulting in shaping the B-cell repertoire to enable an efficient immune response.
Introduction
The surveillance of the body for foreign antigens is a critical function of the immune system. Lymphocytes migrate (home) from the blood into tissues and secondary lymphoid organs, and return to the blood via lymph vessels and the thoractic duct. The adhesion of peripheral blood lymphocytes to the endothelium of secondary lymphoid tissues or peripheral sites of inflammation, and their migration through endothelial cells, involve multiple steps. Transient interactions with the vessel wall are followed by chemoattractant-mediated activation signals that promote firm adhesion between integrin molecules on the leukocyte and immunoglobulin superfamily ligands on the endothelium, and spreading of lymphocytes on the apical endothelial surface. Finally, cells migrate through the intercellular borders and transmigrate to sites beneath the epithelial monolayers.1,,,,,,–8
Previous studies focused on the recruitment of leukocytes to the lymph nodes or to sites of inflammation. However, little is known about the molecular mechanisms that negatively control or prevent homing of cells to these sites. Our studies focus on the pathways that restrict the homing of specific subsets of immune cells and thereby fine-tune the immune response within specific lymphoid and peripheral tissues.
Precursor B cells differentiate into immature B-lymphocytes after successful expression of a surface immunoglobulin receptor (IgM).9,10 Newly generated immature B cells are released from bone marrow and migrate to the spleen, where they differentiate into long-lived mature B cells, before antigen encounter. Like other naive lymphocytes, before their arrival in the spleen, immature B cells might recirculate to nonsplenic secondary lymphoid organs, which are specialized tissues for collecting antigens,11 or to sites of infection and inflammation. At these secondary lymphoid organs, which do not support final B-cell maturation, premature antigen encounter would lead to the death of the immature B cells and elimination of effective clones due to the negative selection process. Therefore, before final maturation in the spleen, immature B cells are excluded from these sites.
Previously, we demonstrated that immature B cells can down-regulate their own migration into nonsplenic sites. This inhibition is mediated by 2 independent pathways: secretion of low-dose IFN-γ,12 and expression of the chemokine receptor CCR2.13
Immature B cells regulate their homing in an autocrine manner by secreting low levels of IFN-γ, which engage the IFN-γ receptor, leading to inhibition of cytoskeletal rearrangement; such rearrangement is required to promote integrin-mediated adhesion and migration of B cells.14,15 IFN-γ secretion by immature B cells is regulated by the MHC class I receptor Ly49D. This activating receptor is expressed on peripheral immature B cells, and recognizes MHC class I on peripheral tissues. The engagement of Ly49D by MHC class I molecules induces secretion of IFN-γ by immature B cells, thereby down-regulating their ability to home to the lymph nodes or to sites of inflammation.16 Ly49D activity is tightly regulated by the inhibitory receptor Ly49G2. Low levels of the inhibitory receptor Ly49G2, coexpressed with high levels of the activating receptor Ly49D on the circulating immature B cells, enable the secretion of precisely regulated low levels of IFN-γ. This expression pattern ensures the control of the migration of peripheral immature B cells, preventing their premature encounter with an antigen while enabling entry to the lymph nodes once they mature.17
The cytokines IL-12 and IL-18 have been shown to stimulate IFN-γ production.18,–20 Recently, we showed that Ly49D stimulation leads to the elevated transcription and secretion of IL-18 and IL-12B (p40), resulting in augmented IFN-γ expression in immature B cells, and autocrine control of their cytoskeletal rearrangement and migration.21
IL-15 is a 14- to 15-kDa cytokine of the 4 α-helix bundle family that shares structural and functional similarities with IL-2 and IL-21.22,–24 It was previously shown that IL-15 potently up-regulates IFN-γ production in human natural killer (NK) and T cells. IL-15–induced IFN-γ mRNA synthesis and protein production is enhanced by IL-18 and IL-21.25,26 Moreover, it was previously shown that in patients with cancer treated with IL-12, production of IL-15, IL-18, and IFN-γ was induced.27
In this study, we wished to examine the possible involvement of IL-15 in the control of IFN-γ secretion by B cells. We show that IL-12 and IL-18 not only cause direct transcription of IFN-γ but also induce production of IL-15, which, in turn, stimulates IFN-γ secretion in immature B cells. This promotes autocrine inhibition of their cytoskeletal rearrangement and migration.
Methods
Mice
C57BL/6, Ii−/−,28 IL15−/− mice (Jackson Laboratory, Bar Harbor, ME) and IFN-γ−/− mice (Jackson Laboratory) were used in these experiments. All animal procedures were carried out in accordance with the Guidelines for the Care and Use of Research Animals at the Weizmann Institute of Science.
Separation of B cells
Spleen cells were obtained from the various mice, as previously described.29,30 Total B cells (Ii−/−, IL15−/−, or control) were then purified, using B220 beads (BD Biosciences, San Diego, CA). Separation of the immature and mature populations was performed as previously described.21,30 Isolation of the T1, T2, and mature populations was performed as previously described.21,31 The purity of the purified cells was analyzed by fluorescence-activated cell sorter (FACS) following each experiment, and found to be between 95% and 98%.
RNA isolation and reverse transcription
Total RNA was isolated from cells using the Tri Reagent Kit (Molecular Research Center, Cincinnati, OH). Reverse transcription was carried out using Superscript II RT (Gibco-BRL, Grand Island, NY). Primers for polymerase chain reaction (PCR) that were used included: IL-15, 5′-GACTTGCAGTGCATCTCCTTACGC-3′ and 5′-TGAAGACATGAATGCCAGCCTCA-3′; Ly49D, 5′-CCTGGCAGCTCATTGTGATAG-3′ and 5′-ATTCTGGCAGCTCTGTTTACATC-3′; IFN-γ, 5′-CATTGAAAGCCTAGAAAGTCTG-3′ and 5′-CTCATGAATGCATCCTTTTTCG-3′; and HPRT, 5′-GAGGGTAGGCTGGCCTATGGCT-3′ and 5′-GTTGGATACAGGCCAGACTTTGTTG-3′.
Quantitative real-time RT-PCR
Levels of mRNA of IFN-γ and IL-15 were analyzed by quantitative real-time reverse transcription (RT)–PCR using a Light-Cycler instrument (Roche Diagnostics, Mannheim, Germany) as described previously.32 Primer sequences were as follows: β-actin, 5′-GTGACGTTGACATCCG-3′ and 5′-CAGTAACAGTCCGCCT-3′; IFN-γ, 5′-GAACGCTACACACTGC-3′ and 5′-CTGGACCTGTGGGTTG-3′; and IL-15, 5′-ATCAACACGTCCTGACT-3′ and 5′-GTCTGAGACGAGCTCTT-3′.
β-actin levels were used to normalize samples for calculation of the relative expression levels of all the genes (IFN-γ and IL-15). All the results were evaluated for significance using the t test, and the P value was less than .05.
Antibodies, immunoflourescence, and flow cytometry
Staining was performed on freshly isolated splenocytes, as previously described.16,17,33 CD45R/B220 (RA36B2; eBioscience, San Diego, CA), IgD (217-170; BD PharMingen, San Diego, CA), IgM (11/41; eBioscience), CD21 (CR2/CR1; eBioscience), anti-C1qRp (AA4.1; eBioscience), CD23 (B3B4; eBioscience), CD24 (M1/69; eBioscience), and Annexin (BD PharMingen).
Stimulation of primary B cells with anti-Ly49D
Ly49D stimulation was performed as previously described.21
Stimulation of primary B cells with IL-15
Primary B cells (107) were suspended in 1 mL or 5 mL of RPMI medium containing 10% (vol/vol) fetal calf serum (FCS). Next, IL-15 (Peprotech, Rocky Hill, NJ) was added to each plate to a final concentration of 30 ng/mL, and the plates were then placed at 37°C for the periods of time indicated. Immediately after stimulation, the cells were washed and used for in various experiments.
Stimulation of primary B cells with IL-12 and IL-18
Stimulation of B cells with IL-12 and IL-18 was performed as previously described.21
Stimulation of primary B cells with SolIL-15Rα
Primary B cells (107) were suspended in 1 mL of RPMI medium containing 10% (vol/vol) FCS. Next, soluble IL-15 receptor α (SolIL-15Rα; R&D Systems, Minneapolis, MN) was added to each plate to a final concentration of 1 μg/mL, and the plates were then placed at 37°C overnight. After stimulation, the cells were washed and used in various experiments.
Cytoskeleton rearrangement
Transwell migration
ELISA for secreted IL-15 levels
Control B cells or Ii−/− immature B220+ cells were incubated overnight in the presence or absence of anti-Ly49D (250 ng/mL), IL-12 (20 ng/mL), or IL-18 (200 ng/mL) as described. Cell supernatants were collected, and IL-15 levels were determined by enzyme-linked immunosorbent assay (ELISA) kit (Peprotech), according to the manufacturer's instructions.
Intracellular staining
Total splenocytes (107 cells/mL) from control, Ii−/−, or IL-15−/− mice were used directly or stimulated with IL-15 or with SolIL-15Rα (1 μg/mL) for varying periods of time, as indicated. IFN-γ and IL-15 intracellular staining was performed as previously described.21 For IL-15 staining, permeabilized cells were stained with rabbit anti-mouse IL-15 (Santa Cruz Biotechnology, Santa Cruz, CA) and then with donkey anti-rabbit (Jackson Immuno-Research Laboratories, West Grove, PA).
Syk inhibition
Syk inhibition assay was carried out as previously described.21
BrdU labeling of cells
BrdU (5-bromo-2-deoxyuridine) staining was performed as previously described.30 The cells were then collected by centrifugation, washed, and stained with FITC-labeled anti-BrdU (BD Biosciences, Mountain View, CA), anti-B220 (eBioscience), and anti-493 (BD PharMingen), and were analyzed by FACS.
Calculation of relative band intensities
The ratio of band intensities in each figure was calculated as follows: the intensity of a band divided by the intensity of the control (HPRT) band in untreated or low-expressing cells was normalized to 1. The ratio for the treatment was calculated as the intensity obtained following that treatment, relative to 1.
Results
Immature B cells express IL-15
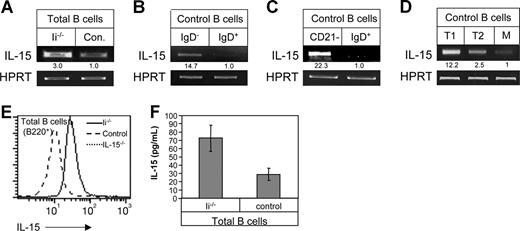
To investigate the possible involvement of IL-15 in the production of IFN-γ by immature B cells, we first compared the expression of IL-15 mRNA in immature and mature B-cell populations. Total B220+ cells were purified from either control (C57BL/6) mice enriched with mature B cells, or from invariant chain-deficient (Ii−/−) mice whose B cells are arrested at the immature stage (Figure 1A). Alternatively, B220+ IgD+ (mature; Figure 1B,C), and IgD− (immature; Figure 1B) or CD21− (immature; Figure 1C) populations were both isolated from control C57BL/6 mice. Immature B cells from both control and Ii−/− mice transcribed higher levels of IL-15 compared with the mature cells (fold increase in 3 separate experiments: Ii−/−, 2.5 ± 0.5; control, 1 ± 0). To determine at which specific stage of differentiation IL-15 transcription is up-regulated, we sorted the T1, T2, and mature populations using cell-surface markers attributed to each population,31 and followed IL-15 message by RT-PCR. As can be seen in Figure 1D, IL-15 message was down-regulated during the differentiation from T1 to T2 B cells.
Immature B cells produce and secrete higher levels of IL-15 compared with mature cells. (A-D) RT-PCR analysis. (A) Total B cells (B220+) from Ii−/− (immature) or control mice (mature). (B) Purified immature (IgD− B220+) and mature (IgD+) cells from control mice. (C) Purified immature (CD21− B220+) and mature (IgD+) B cells derived from control mice. (D) T1, T2, and mature B cells were purified as described in “Separation of B cells.” Total RNA was isolated, and reverse transcription using primers for IL-15 and HPRT was carried out using Superscript II RT. The results presented are representative of 3 different experiments. (E) Intracellular IL-15 expression. Total lymphocytes from control (mature), Ii−/− (immature), and IL-15−/− mice were stained for B220 marker and analyzed for IL-15 protein levels by intracellular staining as described in “Intracellular staining.” Histograms show IL-15 expression in B220+ cells. (F) IL-15 secretion. Total B cells (B220+) from Ii−/− (immature) or control mice (mature) were incubated overnight at 37°C, and IL-15 levels in the conditioned medium derived from both populations were analyzed by ELISA. The results presented are representative of 3 separate experiments. Data shown represent means (± SE).
Immature B cells produce and secrete higher levels of IL-15 compared with mature cells. (A-D) RT-PCR analysis. (A) Total B cells (B220+) from Ii−/− (immature) or control mice (mature). (B) Purified immature (IgD− B220+) and mature (IgD+) cells from control mice. (C) Purified immature (CD21− B220+) and mature (IgD+) B cells derived from control mice. (D) T1, T2, and mature B cells were purified as described in “Separation of B cells.” Total RNA was isolated, and reverse transcription using primers for IL-15 and HPRT was carried out using Superscript II RT. The results presented are representative of 3 different experiments. (E) Intracellular IL-15 expression. Total lymphocytes from control (mature), Ii−/− (immature), and IL-15−/− mice were stained for B220 marker and analyzed for IL-15 protein levels by intracellular staining as described in “Intracellular staining.” Histograms show IL-15 expression in B220+ cells. (F) IL-15 secretion. Total B cells (B220+) from Ii−/− (immature) or control mice (mature) were incubated overnight at 37°C, and IL-15 levels in the conditioned medium derived from both populations were analyzed by ELISA. The results presented are representative of 3 separate experiments. Data shown represent means (± SE).
To confirm that this difference is also observed at the protein level, IL-15 protein was analyzed in the immature and mature B populations by intracellular staining (Figure 1E). Total B220+ cells from control (mature) or Ii−/− mice (immature) were permeabilized, and their intracellular IL-15 was then analyzed using a specific antibody. Total B cells from mice deficient in IL-15 (IL-15−/−) were used as a negative control. Immature B cells expressed higher levels of IL-15 protein compared with mature B cells. In addition, we followed IL-15 secretion (Figure 1F). To determine IL-15 secretion, the conditioned medium collected from total B cells from control (mature) and Ii−/− mice (immature) was analyzed for IL-15 by ELISA. As can be seen in Figure 1F, immature B cells secreted higher levels of IL-15 compared with mature B cells. Thus, immature B cells express and secrete higher levels of IL-15 compared with mature B cells.
IL-15 regulates IFN-γ transcription and expression in B cells
Immature B cells express IL-15 receptor subunits (data not shown). To determine whether IL-15 regulates IFN-γ secretion from immature B cells, total B cells from Ii−/− mice (immature) were stimulated with IL-15 for various times, and IFN-γ transcription following stimulation was analyzed (Figure 2A,B). IFN-γ transcript levels were elevated as early as 30 minutes after IL-15 addition (Figure 2A; fold increase in 3 different experiments: t = 0, 1 ± 0; t = 30 minutes with no IL-15, 0.9 ± 0.2; t = 30 minutes + IL-15, 3.7 ± 1.5; t = 60 minutes with no IL-15, 0.8 ± 0.4; t = 60 minutes + IL-15, 2.1 ± 0.4) and sustained for at least 9 hours (Figure 2B). To demonstrate these results in a more quantitative manner, IFN-γ mRNA levels were analyzed by quantitative real-time RT-PCR. As shown in Figure 2C,D, IL-15 stimulation leads to increased IFN-γ transcription. Next, to determine whether IL-15 stimulation up-regulates IFN-γ protein levels, IFN-γ levels were followed in IL-15–stimulated and nonstimulated total B cells from Ii−/− (immature) mice by intracellular staining. As shown in Figure 2E, IFN-γ protein levels were significantly increased in Ii−/− mice (immature B cells) after IL-15 stimulation.
IL-15 induces IFN-γ production by B cells. (A,B) Total B cells (B220+) from Ii−/− mice (immature) were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for various time periods as indicated. RT-PCR using primers for IFN-γ and HPRT was performed as described in “RNA isolation and reverse transcription.” (C,D) Total B cells (B220+) from Ii−/− mice (immature) were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for various time periods as indicated. Quantitative real-time PCR was performed using primers for IFN-γ and HPRT as described in “Quantitative real-time RT-PCR.” Results shown are representative of 3 separate experiments. (E,F) Total lymphocytes from Ii−/− mice (E) or control mice (F) were incubated in the presence or absence of IL-15 (30 ng/mL) overnight. The cells were stained for B220, and IFN-γ protein levels were analyzed by intracellular staining as described in “Intracellular staining.” The graphs show the percentage of B220+ and IFN-γ+ B cells minus the background of cells stained with an isotype control antibody. (G) Total B cells from control (mature) and Ii−/− (immature) mice were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for 1 hour. RNA was isolated and subjected to RT-PCR was preformed using primers for IFN-γ and HPRT as described in “RNA isolation and reverse transcription.” (H) Total lymphocytes from control and Ii−/− mice were incubated overnight in the presence or absence of IL-15 (30 ng/mL) and stained for B220, and IFN-γ levels were analyzed by intracellular staining. The graph shows the fold increase in IFN-γ protein levels following treatment in the 2 populations. The results are representative of 3 separate experiments. Error bars represent SD.
IL-15 induces IFN-γ production by B cells. (A,B) Total B cells (B220+) from Ii−/− mice (immature) were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for various time periods as indicated. RT-PCR using primers for IFN-γ and HPRT was performed as described in “RNA isolation and reverse transcription.” (C,D) Total B cells (B220+) from Ii−/− mice (immature) were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for various time periods as indicated. Quantitative real-time PCR was performed using primers for IFN-γ and HPRT as described in “Quantitative real-time RT-PCR.” Results shown are representative of 3 separate experiments. (E,F) Total lymphocytes from Ii−/− mice (E) or control mice (F) were incubated in the presence or absence of IL-15 (30 ng/mL) overnight. The cells were stained for B220, and IFN-γ protein levels were analyzed by intracellular staining as described in “Intracellular staining.” The graphs show the percentage of B220+ and IFN-γ+ B cells minus the background of cells stained with an isotype control antibody. (G) Total B cells from control (mature) and Ii−/− (immature) mice were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for 1 hour. RNA was isolated and subjected to RT-PCR was preformed using primers for IFN-γ and HPRT as described in “RNA isolation and reverse transcription.” (H) Total lymphocytes from control and Ii−/− mice were incubated overnight in the presence or absence of IL-15 (30 ng/mL) and stained for B220, and IFN-γ levels were analyzed by intracellular staining. The graph shows the fold increase in IFN-γ protein levels following treatment in the 2 populations. The results are representative of 3 separate experiments. Error bars represent SD.
Mature B cells normally do not express IFN-γ. To determine whether IL-15 stimulation can elevate IL-15 expression even in the mature population, total B cells from control mice (mature) were stimulated with IL-15, and IFN-γ levels were measured by intracellular staining. As shown in Figure 2F, IL-15 elevated IFN-γ protein levels in the mature population. To compare the effect of IL-15 in the immature and mature populations, IFN-γ mRNA levels in IL-15–stimulated total control B cells (mature) were compared with their levels in stimulated total Ii−/− B cells (immature cells). As shown in Figure 2G,H, IL-15 up-regulated IFN-γ message and protein in mature cells, although to a lesser degree than in immature B cells.
IL-15 stimulation inhibits cytoskeleton rearrangement in an IFN-γ–dependent manner
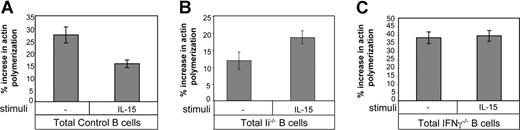
IFN-γ secreted from immature B cells inhibits their cytoskeleton rearrangement and homing in an autocrine manner.12,14 To determine whether IL-15 participates in this autocrine loop, total control B cells (mature; Figure 3A), total Ii−/− B cells (immature; Figure 3B) or total IFN-γ−/− B cells (mature; Figure 3C) were incubated for 1 hour with or without IL-15. The cells were then stimulated with CXCL12, and their ability to polymerize their actin was assessed. IL-15 treatment dramatically down-regulated the ability of control B cells (mature) to polymerize their actin due to increased IFN-γ levels (Figure 3A),16 while the levels of polymerized actin in IFN-γ–deficient cells were not affected by this cytokine (Figure 3C), showing that IL-15 induces actin polymerization in an IFN-γ–dependent manner. Interestingly, Ii−/− B cells (immature) showed increased actin polymerization (Figure 3B), probably due to the artificially elevated levels of IFN-γ, which are above the inhibitory range.16
IL-15 inhibits the migration of immature B cells in an IFN-γ–dependent manner. (A-C) Total B cells from control (mature; A) Ii−/− (immature; B) or IFN-γ−/− (C) mice were suspended in 1 mL RPMI plus 10% FCS and incubated for 1 hour in the presence or absence of IL-15 (30 ng/mL); their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” The results are representative of 3 separate experiments. Error bars represent SD.
IL-15 inhibits the migration of immature B cells in an IFN-γ–dependent manner. (A-C) Total B cells from control (mature; A) Ii−/− (immature; B) or IFN-γ−/− (C) mice were suspended in 1 mL RPMI plus 10% FCS and incubated for 1 hour in the presence or absence of IL-15 (30 ng/mL); their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” The results are representative of 3 separate experiments. Error bars represent SD.
IL-15 regulates in vivo homing of immature B cells
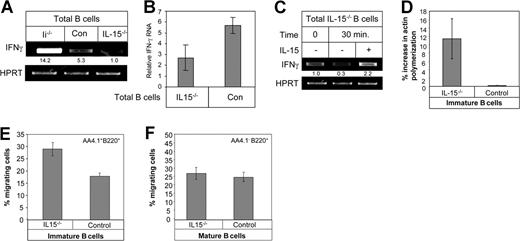
To directly show that IL-15 regulates IFN-γ production in B cells, we analyzed the expression of IFN-γ in B cells deficient for IL-15. Total control and IL-15−/− B cells were purified, and their mRNA was analyzed by RT-PCR and by quantitative real-time RT-PCR. As shown in Figure 4A-B, IL-15–deficient B cells produced significantly lower levels of IFN-γ transcript compared with control B cells. These low levels of IFN-γ could be elevated by the addition of exogenous IL-15 (Figure 4C), further demonstrating that IL-15 up-regulates IFN-γ production in B cells.
IL-15–deficient B cells produce low levels of IFN-γ. (A) Total B cells from Ii−/− (immature) control (mature) or IL-15−/− (mature) mice were purified. Total RNA was isolated, and RT-PCR was carried out using primers for IFN-γ and HPRT as described in “RNA isolation and reverse transcription.” (B) Total B cells from control (mature) or IL-15−/− mice were purified. Total RNA was isolated, and quantitative real-time PCR was carried out using primers for IFN-γ and HPRT as described in “Quantitative real-time RT-PCR.” (C) Total B cells from IL-15−/− mice were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for various periods of time, as indicated. RT-PCR using primers for IFN-γ and HPRT was performed. (D) Immature B cells (CD21−, B220+) from control and IL-15−/− mice were purified, and their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” (E,F) Transwell migration assay. Total cells from control and IL15−/− mice were placed in the upper well of a 24-well transwell plate in the presence or absence of CXCL12 (1 μg/mL). After 4 hours, the total and migrating cells were immunostained: immature (B220+AA4.1+; E); mature (B220+AA4.1−; F), and the percentage of each population was evaluated by FACS analysis. Percentage of migration was calculated as the number of migrating cells in the lower chamber in the presence of CXCL12 minus the number of migrating cells in the lower chamber without CXCL12, as a fraction of the input cells in the upper chamber. The results presented are representative of 3 separate experiments. Error bars represent SD.
IL-15–deficient B cells produce low levels of IFN-γ. (A) Total B cells from Ii−/− (immature) control (mature) or IL-15−/− (mature) mice were purified. Total RNA was isolated, and RT-PCR was carried out using primers for IFN-γ and HPRT as described in “RNA isolation and reverse transcription.” (B) Total B cells from control (mature) or IL-15−/− mice were purified. Total RNA was isolated, and quantitative real-time PCR was carried out using primers for IFN-γ and HPRT as described in “Quantitative real-time RT-PCR.” (C) Total B cells from IL-15−/− mice were suspended in 5 mL RPMI plus 10% FCS and incubated in the presence or absence of IL-15 (30 ng/mL) for various periods of time, as indicated. RT-PCR using primers for IFN-γ and HPRT was performed. (D) Immature B cells (CD21−, B220+) from control and IL-15−/− mice were purified, and their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” (E,F) Transwell migration assay. Total cells from control and IL15−/− mice were placed in the upper well of a 24-well transwell plate in the presence or absence of CXCL12 (1 μg/mL). After 4 hours, the total and migrating cells were immunostained: immature (B220+AA4.1+; E); mature (B220+AA4.1−; F), and the percentage of each population was evaluated by FACS analysis. Percentage of migration was calculated as the number of migrating cells in the lower chamber in the presence of CXCL12 minus the number of migrating cells in the lower chamber without CXCL12, as a fraction of the input cells in the upper chamber. The results presented are representative of 3 separate experiments. Error bars represent SD.
Because low-dose IFN-γ inhibits immature B-cell migration and homing, we next followed the effect of IL-15 on cytoskeleton rearrangement in these cells. Purified immature B cells derived from control or IL-15–deficient mice were stimulated with CXCL12, and their cytoskeletal rearrangement was analyzed. It can be seen (Figure 4D) that while there was almost no increase in actin polymerization in control immature B cells following CXCL12 stimulation, immature B cells derived from IL-15−/− mice were able to increase their actin polymerization after stimulation.
To further demonstrate the role of IL-15 in stimulating IFN-γ production, and hence its role in regulating migration of immature B cells, we next evaluated the migratory response of control and IL-15–deficient immature cells toward CXCL12 in a transwell assay. Migrating cells were collected from the lower well and stained, and the proportion of immature and mature cells in the migrating population was determined. A substantial and specific elevation in the migration of the IL15−/− immature (AA4.1+B220+; Figure 4E) population toward CXCL12 compared with control immature cells was observed, while there was no difference in the migratory response of mature (AA4.1−B220+) wild-type versus IL-15−/− B cells toward CXCL12 (Figure 4F). Thus, the lack of IL-15 down-regulates IFN-γ production in immature B cells, resulting in elevation of cytoskeleton rearrangement and migratory response of immature B cells.
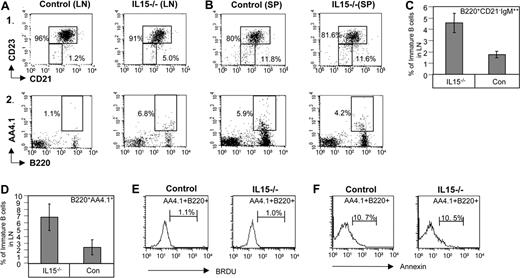
Our previous studies have established a role for IFN-γ in B-cell homing to the lymph nodes. Elevated levels of immature B cells could be detected in the lymph nodes of IFN-γ−/− mice compared with their numbers in control lymph nodes.12 Because immature B cells are restricted from such areas, an increased number of immature B cells in the lymph nodes would suggest abrogation of homing inhibition. Having shown that IL-15 regulates IFN-γ secretion, we next examined homing of immature B cells to the lymph nodes of IL-15−/− mice. To this end, we compared the percentage of the immature B population (B220+CD23−CD21−; Figure 5Ai,C or AA4.1+B220+; Figure 5Aii,D), in control and IL-15−/− mice. A substantial elevation in the percentage of immature B cells was observed in the lymph nodes of IL-15−/− mice, while there was almost no difference in the levels of immature cells in spleens of IL-15−/− versus control mice (Figure 5B). This phenotype, similar to that found in IFN-γ−/− mice12 and IL12−/− mice,21 suggests the involvement of IL-15 in IFN-γ secretion and in homing inhibition in vivo. It could be argued that the increased immature population in the LN of IL-15−/− mice resulted from alterations in the survival or proliferation capacities of these immature cells,22,23 and not from differences in homing. We therefore analyzed proliferation and survival of control and IL-15–deficient peripheral splenic immature B cells by BrdU incorporation (Figure 5E) and Annexin staining (Figure 5F). No difference in the proliferation and survival of these 2 populations was observed, suggesting that the accumulation of immature B cells in the lymph nodes of IL-15−/− mice stems from defective inhibition of lymph node migration.
IL-12 regulates in vivo homing of immature B cells. (A-D) Lymphocytes from control and IL15−/− mice from lymph node (A) or spleen (B) were triple stained with anti-B220, anti-CD23, and anti-CD21 (panel Ai and graph in panel C) or anti-AA4.1 and anti-B220 (panel Aii, and graph in panel D). Dot plots show the expression of the various markers on B220+ cells. The results presented are representative of 3 separate experiments. (E) Mice were fed with drinking water containing 1 mg/mL BrdU for 3 days. Cells were collected and stained with FITC-labeled anti-BrdU, anti-B220, and anti-AA4.1 as described in “BrdU labeling of cells,” and were analyzed by FACS. The results show the percentage of BrdU+ cells in the AA4.1+B220+ population. (F) Splenocytes from control and IL-15−/− mice were triple stained with anti-B220, anti-AA4.1, and anti-Annexin. Histograms show the expression of Annexin on B220+ AA4.1+cells. The results presented are representative of 3 separate experiments. Error bars represent SD.
IL-12 regulates in vivo homing of immature B cells. (A-D) Lymphocytes from control and IL15−/− mice from lymph node (A) or spleen (B) were triple stained with anti-B220, anti-CD23, and anti-CD21 (panel Ai and graph in panel C) or anti-AA4.1 and anti-B220 (panel Aii, and graph in panel D). Dot plots show the expression of the various markers on B220+ cells. The results presented are representative of 3 separate experiments. (E) Mice were fed with drinking water containing 1 mg/mL BrdU for 3 days. Cells were collected and stained with FITC-labeled anti-BrdU, anti-B220, and anti-AA4.1 as described in “BrdU labeling of cells,” and were analyzed by FACS. The results show the percentage of BrdU+ cells in the AA4.1+B220+ population. (F) Splenocytes from control and IL-15−/− mice were triple stained with anti-B220, anti-AA4.1, and anti-Annexin. Histograms show the expression of Annexin on B220+ AA4.1+cells. The results presented are representative of 3 separate experiments. Error bars represent SD.
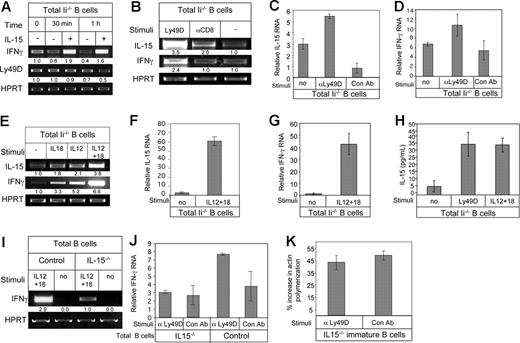
Ly49D, IL-12, and IL-18 cause elevation in IL-15 expression
We previously demonstrated that stimulation of the Ly49D receptor expressed on immature B cells by its ligand, MHC class I, induces IFN-γ secretion.16 Ly49D stimulation induces the autocrine secretion of IL-12 and IL-18, which elevates transcription of IFN-γ.21 To determine the position of IL-15 in this pathway, we first examined the possibility that IL-15 operates upstream of the Ly49D cascade. To that end, we stimulated total B cells derived from Ii−/− mice (immature) with IL-15 for various periods of time and analyzed Ly49D transcription by RT-PCR. As shown in Figure 6A, while IL-15 elevated levels of IFN-γ mRNA, there was no difference in the level of Ly49D transcript. This ruled out IL-15 as an upstream stimulator of the IFN-γ cascade.
Ly49D, IL-12, and IL-18 stimulation elevates IL-15 transcription. (A) Total B cells from Ii−/− (immature) mice were incubated in the presence or absence of IL-15 (30 ng/mL) for various periods of time, as indicated, and RT-PCR was carried out using primers for IFN-γ, Ly49D, and HPRT. (B) Total B cells from Ii−/− mice (immature) incubated in the presence or absence of anti-Ly49D antibody or an isotype control antibody RT-PCR was carried out using primers for IL-15, IFN-γ, and HPRT. (C,D) Total B cells from Ii−/− mice (immature) incubated in the presence or absence of anti-Ly49D antibody or an isotype control antibody. Quantitative real-time PCR was carried out using primers for IL-15 (C) or for IFN-γ (D). (E) Total B cells from Ii−/− mice (immature) were incubated in the presence or absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL). RT-PCR was carried out using primers for IL-15, IFN-γ, and HPRT. (F,G) Total B cells from Ii−/− mice (immature) were incubated in the presence or absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL), and quantitative real-time PCR was carried out using primers for IL-15 (F) or for IFN-γ (G). (H) Total B cells from Ii−/− (immature) mice were incubated overnight in the presence or absence of anti-Ly49D antibody, or IL-12 (20 ng/mL) and IL-18 (200 ng/mL). IL-15 levels in the conditioned medium of the cells was analyzed by ELISA. The results presented are representative of 3 separate experiments. Standard error was calculated. (I) Total B cells from control and IL-15−/− mice were incubated in the presence of absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL), and RT-PCR was carried out using primers for IFN-γ and HPRT. The results are representative of 3 separate experiments. (J) Total B cells from control and IL-15−/− mice were incubated in the presence or absence of Ly49D, and quantitative real-time PCR was carried out using primers for IFN-γ and HPRT. The results are representative of 3 separate experiments. (K) Purified immature B cells (CD21−, B220+) from IL-15−/− mice were incubated in the presence of anti-Ly49D for 3 hours. After incubation, their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” Error bars represent SD.
Ly49D, IL-12, and IL-18 stimulation elevates IL-15 transcription. (A) Total B cells from Ii−/− (immature) mice were incubated in the presence or absence of IL-15 (30 ng/mL) for various periods of time, as indicated, and RT-PCR was carried out using primers for IFN-γ, Ly49D, and HPRT. (B) Total B cells from Ii−/− mice (immature) incubated in the presence or absence of anti-Ly49D antibody or an isotype control antibody RT-PCR was carried out using primers for IL-15, IFN-γ, and HPRT. (C,D) Total B cells from Ii−/− mice (immature) incubated in the presence or absence of anti-Ly49D antibody or an isotype control antibody. Quantitative real-time PCR was carried out using primers for IL-15 (C) or for IFN-γ (D). (E) Total B cells from Ii−/− mice (immature) were incubated in the presence or absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL). RT-PCR was carried out using primers for IL-15, IFN-γ, and HPRT. (F,G) Total B cells from Ii−/− mice (immature) were incubated in the presence or absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL), and quantitative real-time PCR was carried out using primers for IL-15 (F) or for IFN-γ (G). (H) Total B cells from Ii−/− (immature) mice were incubated overnight in the presence or absence of anti-Ly49D antibody, or IL-12 (20 ng/mL) and IL-18 (200 ng/mL). IL-15 levels in the conditioned medium of the cells was analyzed by ELISA. The results presented are representative of 3 separate experiments. Standard error was calculated. (I) Total B cells from control and IL-15−/− mice were incubated in the presence of absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL), and RT-PCR was carried out using primers for IFN-γ and HPRT. The results are representative of 3 separate experiments. (J) Total B cells from control and IL-15−/− mice were incubated in the presence or absence of Ly49D, and quantitative real-time PCR was carried out using primers for IFN-γ and HPRT. The results are representative of 3 separate experiments. (K) Purified immature B cells (CD21−, B220+) from IL-15−/− mice were incubated in the presence of anti-Ly49D for 3 hours. After incubation, their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” Error bars represent SD.
We next examined the possibility that IL-15 is a downstream component of the Ly49D-induced cascade. Total B cells derived from Ii−/− mice (immature) were stimulated with anti-Ly49D antibody or a control antibody (anti-CD8); mRNA was purified, and we analyzed IL-15 mRNA levels by RT-PCR. This stimulation activates mostly the immature B population, because marginal zone B cells do not express Ly49D.16 As shown in Figure 6B, a significant elevation of IL-15 message was detected after anti-Ly49D stimulation (fold increase in 3 different experiments for IL-15: αLy49D, 3.8 ± 0.3; αCD8, 1.6 ± 0.4; and no treatment, 1 ± 0. Fold increase in 3 different experiments for IFN-γ: αLy49D, 2.3 ± 0.5; αCD8, 1.4 ± 0.4; and no treatment, 1 ± 0). These results were repeated in a more quantitative way using quantitative real-time PCR analysis. As shown in Figure 6C,D, Ly49D stimulation specifically elevated IL-15 (Figure 6C) and IFN-γ (Figure 6D) mRNA levels. We then determined whether IL-12 and IL-18, whose expression was also shown to be elevated by Ly49D stimulation,21 regulate IL-15 expression. Total B cells purified from Ii−/− mice (immature) were incubated in the presence or absence of IL-12, IL-18, or both cytokines, and IL-15 mRNA was examined by RT-PCR. As shown in Figure 6E, incubation with both IL-12 and IL-18, independently or together, resulted in an elevation of IL-15 mRNA levels (fold increase in 3 different experiments for IL-15: IL-18, 1.5 ± 0.3; IL-12, 1.8 ± 0.4; IL-12 + IL-18, 3.2 ± 0.8; and no treatment, 1 ± 0. Fold increase in 3 different experiments for IFN-γ: IL-18, 3.4 ± 0.5; IL-12, 4.3 ± 1; IL-12 + IL-18, 7.2 ± 1.2; and no treatment, 1 ± 0). Quantitative real-time PCR analysis further showed that stimulation with IL-12 and IL-18 elevated IL-15 (Figure 6F) and IFN-γ (Figure 6G) mRNA levels.
To examine whether Ly49D or IL-12 and IL-18 stimulation elevate IL-15 protein levels, total B cells from Ii−/− (immature) mice were incubated overnight in the presence or absence of anti-Ly49D antibody or IL-12 and IL-18. Conditioned medium was then collected from the cells and analyzed by ELISA for IL-15 secretion. As shown in Figure 6H, following Ly49D or IL-12 and IL-18 stimulation, a substantial increase in IL-15 secretion was detected.
To further support our findings that IL-12 and IL-18 induce IFN-γ production via IL-15, we examined IFN-γ production in total B cells derived from control and IL-15−/− mice following IL-12 and IL-18 stimulation. Control and IL-15−/− B cells were stimulated for 3 hours with IL-12 and IL-18, and IFN-γ mRNA was analyzed by RT-PCR (Figure 6I). While both cell types showed an elevation in IFN-γ mRNA following IL-12 plus IL-18 stimulation, the increase observed in control B cells was much greater than that observed for B cells derived from IL-15−/− mice.
To further demonstrate that Ly49D induces IFN-γ production via IL-15, we examined IFN-γ production in total B cells derived from control and IL-15−/− mice following Ly49D stimulation. Control and IL-15−/− total B cells were stimulated for 3 hours with anti-Ly49D or nonspecific Ab (αCD8), and IFN-γ mRNA was analyzed by quantitative real-time RT-PCR (Figure 6J). While control B cells showed an elevation in IFN-γ mRNA following Ly49D stimulation, no significant increase was observed in B cells derived from IL-15−/− mice.
Because low-dose IFN-γ inhibits immature B-cell migration and homing, we next followed the effect of IL-15 on cytoskeleton rearrangement in these cells. Purified immature B cells derived from IL-15–deficient mice were stimulated for 3 hours with anti-Ly49D. The cells were then stimulated with CXCL12, and their cytoskeleton rearrangement was analyzed. As shown in Figure 6K, no significant change was observed in the ability of IL-15−/− immature B cells to polymerize their actin following CXCL12 stimulation in the presence or absence of Ly49D stimulation. Thus, Ly49D stimulation, in immature B cells induces IL-15 expression, which elevates IFN-γ levels.
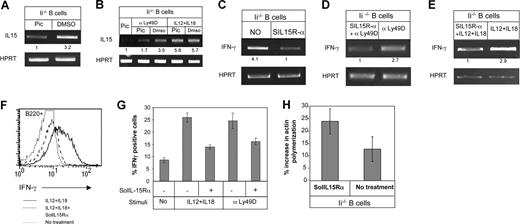
Finally, we previously showed that stimulation of Ly49D expressed on immature B cells leads to Syk phosphorylation. Blocking of Syk activity by its specific inhibitor, piceatannol, results in the down-regulation of Ly49D-induced IFN-γ expression.16,21 To determine whether IL-15 transcription is regulated by a Syk-dependent signaling event, total B cells from Ii−/− mice (immature) were incubated in the presence or absence of piceatannol for 3 hours; the transcription level of IL-15 was then analyzed by RT-PCR. As shown in Figure 7A, piceatannol treatment down-regulated the transcription of IL-15 (fold increase in 3 different experiments for IL-15: piceatannol treatment, 1 ± 0; DMSO treatment, 3 ± 0.3). To determine whether Syk-dependent IL-15 transcription regulates IFN-γ transcription through IL-12 and IL-18 or through an Ly49D-dependent pathway, total Ii−/− B cells (immature) were incubated with IL-12 and IL-18 or anti-Ly49D (Figure 7B) in the presence or absence of piceatannol. While addition of piceatannol to IL-12– and IL-18–stimulated cells did not affect IL-15 transcription, Syk-inhibited cells could not up-regulate IL-15 transcription following Ly49D stimulation (Figure 7B).
IFN-γ induction is IL15 dependent. (A) Total B cells from Ii−/− (immature) mice were incubated in the presence or absence of 120 μM of Syk inhibitor (piceatannol; pic) or with 6 μL of DMSO for 3 hours, and RT-PCR was carried out using primers for IL-15 and HPRT. (B) Total B cells from Ii−/− (immature) were incubated in the presence or absence of piceatannol (120 μM) or 6 μL of DMSO with or without IL-12 (20 ng/mL), IL-18 (200 ng/mL), and anti-Ly49D for 3 hours. RT-PCR was carried out using primers for IL-15 and HPRT. (C) Total B cells from Ii−/− (immature) mice were incubated in the presence or absence of 1 μg/mL of the IL-15 inhibitor SolIL-15Rα overnight, and RT-PCR was carried out using primers for IFN-γ and HPRT. (D,E) Total B cells from Ii−/− (immature) were incubated in the presence or absence of 1 μg/mL of SolIL-15Rα overnight with or without anti-Ly49D (D), or IL-12 (20 ng/mL; E) and IL-18 (200 ng/mL; E). RT-PCR was carried out using primers for IFN-γ and HPRT. (F) Total lymphocytes from Ii−/− mice (immature) were incubated overnight in the presence or absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL) in the presence or absence of SolIL-15Rα. The cells were then stained with anti-B220, and IFN-γ protein levels were analyzed by intracellular staining. Histograms show IFN-γ expression in B220+ cells. (G) Graph showing the fold increase in IFN-γ+ cells following the various treatments minus the background of the isotype control. (H) Total B cells from Ii−/− mice (immature) were incubated in the presence or absence of SolIL-15Rα (1 μg/mL) overnight. Their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” The results presented are representative of 3 separate experiments. Error bars represent SD.
IFN-γ induction is IL15 dependent. (A) Total B cells from Ii−/− (immature) mice were incubated in the presence or absence of 120 μM of Syk inhibitor (piceatannol; pic) or with 6 μL of DMSO for 3 hours, and RT-PCR was carried out using primers for IL-15 and HPRT. (B) Total B cells from Ii−/− (immature) were incubated in the presence or absence of piceatannol (120 μM) or 6 μL of DMSO with or without IL-12 (20 ng/mL), IL-18 (200 ng/mL), and anti-Ly49D for 3 hours. RT-PCR was carried out using primers for IL-15 and HPRT. (C) Total B cells from Ii−/− (immature) mice were incubated in the presence or absence of 1 μg/mL of the IL-15 inhibitor SolIL-15Rα overnight, and RT-PCR was carried out using primers for IFN-γ and HPRT. (D,E) Total B cells from Ii−/− (immature) were incubated in the presence or absence of 1 μg/mL of SolIL-15Rα overnight with or without anti-Ly49D (D), or IL-12 (20 ng/mL; E) and IL-18 (200 ng/mL; E). RT-PCR was carried out using primers for IFN-γ and HPRT. (F) Total lymphocytes from Ii−/− mice (immature) were incubated overnight in the presence or absence of IL-12 (20 ng/mL) and IL-18 (200 ng/mL) in the presence or absence of SolIL-15Rα. The cells were then stained with anti-B220, and IFN-γ protein levels were analyzed by intracellular staining. Histograms show IFN-γ expression in B220+ cells. (G) Graph showing the fold increase in IFN-γ+ cells following the various treatments minus the background of the isotype control. (H) Total B cells from Ii−/− mice (immature) were incubated in the presence or absence of SolIL-15Rα (1 μg/mL) overnight. Their actin polymerization was analyzed as described in “Cytoskeleton rearrangement.” The results presented are representative of 3 separate experiments. Error bars represent SD.
These results show that Ly49D induces a Syk-dependent cascade that elevates IL-12 and IL-18 expression. These cytokines up-regulate IL-15 expression, which in turn promotes IFN-γ secretion and inhibits immature B-cell homing.
IL-15 stimulation regulates IFN-γ production in an autocrinic manner
To determine whether secreted IL-15 regulates IFN-γ secretion and function in B cells, we blocked IL-15–regulated pathways using a soluble recombinant form of the IL-15 receptor subunit, SolIL-15Rα. SolIL-15Rα is a naturally occurring protein which exhibits high-affinity binding to IL-15 and therefore neutralizes its biologic activity by competing with the natural cell-surface receptor.34 To determine whether secreted IL-15 regulates IFN-γ mRNA levels, total Ii−/− (immature) B cells were incubated overnight with SolIL-15Rα (1 μg/mL), and IFN-γ message levels were then analyzed by RT-PCR. As shown in Figure 7C, SolIL-15Rα incubation down-regulated the IFN-γ mRNA levels. This result suggests that in immature B cells, IL-15 induces IFN-γ production in an autocrine manner (fold increase in 3 different experiments for IFN-γ: no treatment, 2.8 ± 1.0; SolIL-15Rα, 1 ± 0). SolIL-15Rα treatment also inhibited Ly49D– (Figure 7D) and IL-12– and IL-18– (Figure 7E) induced IFN-γ transcription. We next analyzed IFN-γ protein levels under these conditions. Treatment with SolIL-15Rα down-regulated the steady-state levels of IFN-γ protein (Figure 7F) induced by IL-12 and IL-18 (Figure 7F,G) or anti-Ly49D stimulation (Figure 7G).
Finally, we determined the function of SolIL-15Rα in regulating cytoskeleton rearrangement of immature B cells. Ii−/− B cells (immature) were incubated overnight with SolIL-15Rα, and actin polymerization assay was preformed. As shown in Figure 7H, cells incubated with SolIL-15Rα lowered their IFN-γ levels (Figure 7C), resulting in an increased ability to polymerize their actin.
Taken together, these results show that Ly49D stimulation induces Syk phosphorylation, resulting in IL-12 and IL-18 expression, which in turn up-regulate IL-15 expression and secretion. IL-15 expression is essential for IFN-γ expression and function.
Discussion
Immature B cells leave the bone marrow and migrate to the spleen to complete their maturation process. Before arriving at the spleen, immature B cells are excluded from entry into nonsplenic secondary lymphoid organs, and from sites of infection and inflammation. Despite our detailed understanding of the steps involved in lymphocyte entry to lymph nodes, relatively little is known about how the cells enter the spleen and how they are directed to the white pulp of the splenic compartment. The entry of B cells to the spleen was regarded until recently as a process for which integrin activation was not required,35,–37 but recent results indicated that integrin activation also has a role in the entry of mature B cells into the splenic white pulp. However, while integrin inhibition causes a decrease in B-cell entry to the white pulp, it has no influence on the total B-cell number in the spleen.38 In addition, while inhibition of a single integrin (LFA-1 or α4β7) results in a dramatic inhibition of T- and B-cell homing to the lymph nodes, it has only a minor effect on their entrance to the white pulp of the spleen. Only dual integrin inhibition can inhibit the entrance to the white pulp.38
Previous studies in our group have demonstrated that to prevent their arrival to antigen-enriched compartments that would lead to their death, immature B cells down-regulate their own migration into nonsplenic sites. This inhibition is mediated by 2 independent pathways. The first involves the secretion of low levels of IFN-γ,12 and the second involves the expression of the chemokine receptor CCR2.13
The IFN-γ–induced pathway is transmitted through the IFN-γ receptor and down-regulates the homing ability of B cells in response to CXCL12.12,14,15 The process of IFN-γ secretion was shown to be highly regulated and controlled by 2 receptors: Ly49D and Ly49G2,16,17 and the cytokines IL-12 (the p40 subunit) and IL-18.21
In this study, we followed the role of IL-15 in the secretion of IFN-γ by B cells. Our results show that immature B cells from both control and Ii-deficient mice produce and secrete higher levels of IL-15 compared with mature cells. Stimulation of B cells with IL-15 induces IFN-γ production. We show that IL-15 regulates B-cell migration both in vitro and in vivo. Stimulation of B cells with IL-15 reduces their ability to polymerize their actin. In addition, IL-15–deficient immature B cells are able to polymerize their cytoskeleton and home to the lymph nodes more efficiently compared with control immature B cells. Therefore, regulation of IFN-γ production by IL-15 results in inhibition of their homing. In mice lacking IL-15, IFN-γ,12 or IL-12,21 or in the absence of the Ly49D ligand, H2-D,16 this restriction is removed, and immature B cells migrate more efficiently to the lymph node than immature B cells from control mice. This elevation does not result from a total increase in the immature B-cell subset.
In B cells, IL-15 stimulates proliferation of anti-IgM– and PMA-activated B cells and inhibits apoptosis.39 Besides its antiapoptotic activity, IL-15 is also widely considered as a classical “first-line-of-defense” and “danger-signal” cytokine.23
Our results show that IL-15 is a downstream effector of the Ly49D, IL-12, and IL-18 cascade. The interplay between IL-12, IL-18, and IL-15 and IFN-γ production is not entirely clear. Stimulation of IL-15−/− B cells with IL-12 and IL-18 or Ly49D can induce production of low levels of IFN-γ, indicating that IL-12 and IL-18 do not induce IFN-γ production exclusively via IL-15, although this is the major pathway. This finding is plausible, since the IFN-γ gene has multiple binding sites for different transcription factors, including AP-1, NF-κB, and NFAT, and also contains binding sites for various STATs (signal transducers and activators of transcription).25 It is therefore possible that IL-12 and IL-18 induce IFN-γ transcription through more than a single pathway. It has been previously shown that in T cells, IL-12 and IL-18 synergistically directly enhance IFN-γ gene expression through interaction between STAT4 (induced by IL-12) and AP-1 (induced by IL-18).40 In addition, they can induce IFN-γ production indirectly by elevation of IL-15 expression, which was shown to activate binding of STAT1, STAT3, STAT4, and STAT5 to the IFN-γ promoter in NK and T cells.25
The tight regulation on IFN-γ production indicates the great significance of low-level IFN-γ secretion in maintaining an efficient maturation process of immature B cells. Secretion of specific low levels of IFN-γ insures the targeting of immature B cells to the spleen. Only after final maturation can mature B cells, which do not secrete IFN-γ, recirculate freely and home to various compartments to exert their function.
In summary, our model (Figure 8) suggests that the activating Ly49D receptor recognizes MHC class I on peripheral tissues. Activation of the Ly49D receptor triggers a signaling cascade that increases transcription of IL-12 (p40) and IL-18, which interact with their specific receptors expressed at nonlimiting levels on these immature B cells. IL-12 and IL-18 stimulate the production of IL-15, and the 3 cytokines together regulate the secretion of specific low doses of IFN-γ. IFN-γ then exerts autocrine inhibition on the migration of immature B cells to the lymph node and to sites of infection and inflammation.
Proposed model for the pathway regulating IFN-γ secretion in immature B cells. Ly49D stimulation by its ligand, MHC class I, activates a cascade leading to the secretion of IL-12 and IL-18. Both IL-12 and IL-18 bind to their receptor, expressed on immature B cells, resulting in the main (direct pathway, via IL-15) or less effective (indirect) activation of IFN-γ secretion. IFN-γ secretion results in the inhibition of the migration of immature B cells to the lymph node and sites of inflammation.
Proposed model for the pathway regulating IFN-γ secretion in immature B cells. Ly49D stimulation by its ligand, MHC class I, activates a cascade leading to the secretion of IL-12 and IL-18. Both IL-12 and IL-18 bind to their receptor, expressed on immature B cells, resulting in the main (direct pathway, via IL-15) or less effective (indirect) activation of IFN-γ secretion. IFN-γ secretion results in the inhibition of the migration of immature B cells to the lymph node and sites of inflammation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge members of the Shachar laboratory for helpful discussions.
This work was supported by the Israel Science Foundation (founded by the Academy of Sciences and Humanities); the Minerva Foundation; a European Union grant from the Migration and Targeting Cell Migration in Chronic Inflammation (MAIN); and a grant for stem cell research from Ruth and Allen Ziegler. I.S. is the incumbent of the Dr Morton and Ann Kleiman Professorial Chair.
Authorship
Contribution: G.H. and T.A.-W. designed and performed the experiments, analyzed results, and helped write the paper; and I.S. designed the experiments, analyzed results, and helped write the paper.
G.H. and T.A.-W. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Idit Shachar, Department of Immunology, Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: idit.shachar@weizmann.ac.il.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal