We thank Drs Pillai and Karandikar for their comments on our paper.1 We are pleased to learn that they do not dispute our major conclusions that the induction of FOXP3 expression in activated human T cells is TGFβ-dependent, but that such cells lack T regulatory cell function. They do question our data demonstrating that the use of the anti-FOXP3 monoclonal antibody (mAb), PCH101, may lead to erroneous conclusions about the expression of FOXP3 on human T cells. As PCH101 has been widely used in numerous published studies of human T regulatory cells, it is critical to establish its specificity. Pillai and Karandikar raise the possibility that our use of an isotype control in Figure 31 was inappropriate and suggest that it would have been preferable to use an unstimulated, FOXP3-negative population as a control. We strongly believe that resting, unactivated cells are not a good negative control to determine FOXP3 positivity on activated cells, since resting cells are very different from activated cells both in their size, physiology, and biochemical properties. If PCH101 binds nonspecifically to a different antigen that is expressed only in activated cells, then it would be invalid to use unactivated cells as a negative control.
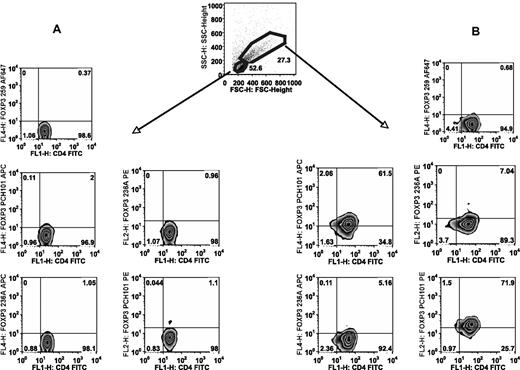
To directly compare the reactivity of a panel of anti-FOXP3 mAbs with unactivated and activated T cells, we stimulated human naive CD4+ T cells and stained both activated and unactivated populations (Figure 1). Unactivated cells were negative with all mAbs (Figure 1A). However, significant staining of the activated cells was only detected with PCH101, but not with 236A or 259 when using the unactivated population as the negative control (Figure 1B). Furthermore, identical staining profiles to that reported in Figure 3 of our paper1 were observed when the studies were repeated using mAb 206 rather than 259 (Figure 2). Thus, when one activates a FOXP3-negative population (CD4+CD25−CD127+CD45RA+T cells) in the absence of TGFβ by neutralizing TGFβ present in the serum, we do not observe significant FOXP3 induction as measured by staining with mAbs 259D, 236A/E7 (not shown), or 206D, or by real-time polymerase chain reaction (PCR). Nevertheless, cells activated under these conditions routinely stain with mAb PCH101, demonstrating that the reactivity of mAb PCH101 is partially nonspecific. This result is supported by our data that only the level of FOXP3 detected with PCH101 on activated T cells, which was induced in the presence of TGFβ1, could be knocked down with a FOXP3 siRNA. In numerous analyses of both thymic-derived FOXP3+ T regulatory cells (Tregs) as well as activated conventional T cells, we have not seen significant differences in the percentage positive (or in the MFI) of FOXP3 expressing T cells with any of the commercially available mAbs except PCH101. While PCH101 is similar to other mAbs (259D, 206D, 236A/E7) for the detection of FOXP3 on resting cells from normal, healthy donors, we question its specificity on cells from patients with autoimmune or infectious diseases whose cells are potentially activated. We strongly recommend that the use of PCH101 for the measurement of human FOXP3+ T cells be discontinued and that previous published studies using this reagent be interpreted cautiously.
PCH101 stains activated cells nonspecifically. FACS sorted human CD4+CD25−CD127+CD45RA+cells were stimulated for 5 days with plate-bound anti-CD3/CD28 and 100 IU/mL IL-2. (A) Gating on unactivated cells based on lower forward light scatter/side light scatter (FSC/SSC) and staining for FOXP3 with PCH101, 236A/E7, and 259D. (B) Gating on activated cells based on larger FSC/SSC and determining the cutoff for FOXP3 positivity from the unactivated cells. Numbers on plots are percentages of total cells.
PCH101 stains activated cells nonspecifically. FACS sorted human CD4+CD25−CD127+CD45RA+cells were stimulated for 5 days with plate-bound anti-CD3/CD28 and 100 IU/mL IL-2. (A) Gating on unactivated cells based on lower forward light scatter/side light scatter (FSC/SSC) and staining for FOXP3 with PCH101, 236A/E7, and 259D. (B) Gating on activated cells based on larger FSC/SSC and determining the cutoff for FOXP3 positivity from the unactivated cells. Numbers on plots are percentages of total cells.
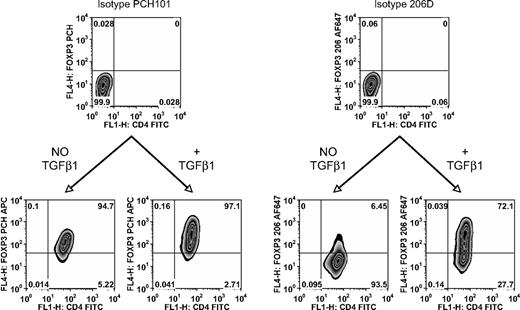
TGFβ is required for de novo induction of FOXP3. FACS sorted human CD4+CD25−CD127+CD45RA+ cells stimulated for 5 days with plate-bound anti-CD3/CD28 and 100 IU/mL IL-2 in the presence or absence of 5 ng/mL TGFβ1. Flow cytometric analysis of FOXP3 with PCH101 and 206D. FOXP3 positivity is determined by the isotype control on the same cells. Numbers on plots are percentages of total cells.
TGFβ is required for de novo induction of FOXP3. FACS sorted human CD4+CD25−CD127+CD45RA+ cells stimulated for 5 days with plate-bound anti-CD3/CD28 and 100 IU/mL IL-2 in the presence or absence of 5 ng/mL TGFβ1. Flow cytometric analysis of FOXP3 with PCH101 and 206D. FOXP3 positivity is determined by the isotype control on the same cells. Numbers on plots are percentages of total cells.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ethan M. Shevach, Laboratory of Immunology, National Institute of Allergy and Infectious Disease, National Institutes of Health, 10 Center Drive, Bldg 10, Rm 11N315, Bethesda, MD, 20892; e-mail: eshevach@niaid.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal