Umbilical cord tissue provides a unique source of cells with potential for tissue repair. Umbilical cord tissue–derived cells (UTCs) are MHC class I (MHCI) dull and negative for MHC class II (MHCII), but can be activated to increase MHCI and to express MHCII with IFN-γ stimulation. Mesenchymal stem cells with similar characteristics have been inferred to be nonimmunogenic; however, in most cases, immunogenicity was not directly assessed. Using UTC from Massachusetts General Hospital MHC-defined miniature swine, we assessed immunogenicity across a full MHC barrier. Immunogenicity was assessed by in vitro assays including mixed lymphocyte reaction (MLR) and flow cytometry to detect serum alloantibody. A single injection of MHC-mismatched unactivated UTCs did not induce a detectable immune response. When injected in an inflamed region, injected repeatedly in the same region or stimulated with IFN-γ prior to injection, UTCs were immunogenic. As clinical cellular repair strategies may involve injection of allogeneic cells into inflamed regions of damaged tissue or repeated doses of cells to achieve the desired benefit, our results on the immunogenicity of these cells in these circumstances may have important implications for optimal success and functional improvement for this cellular treatment strategy for diseased tissues.
Introduction
Mesenchymal stem cells (MSCs) have been isolated from a number of different tissues and have potential in cellular repair therapy.1,2 Among the published sources of these cells are bone marrow, periosteum, trabecular bone, adipose tissue, synovium, skeletal muscle, deciduous teeth, and umbilical cords.1,,,,,–7 Studies have reported engraftment of mesenchymal stem cells with some functional improvement after direct implantation across both allogeneic and xenogeneic transplant barriers. These results were achieved without immunosuppression and without evidence of a cellular infiltrate that would indicate an immune response.3,8,,,–12 The apparent absence of an immune response from in vivo experiments corroborates in vitro results from coculture mixed lymphocyte reaction (MLR) assays where responder cells failed to proliferate against MSC stimulators.13,14 In a recent study, however, intracardiac allogeneic porcine MSCs were shown to elicit an immune response despite their low immunogenic profile in vitro.15 The lack of MHCII and costimulatory molecule expression on the surface of unactivated MSCs may account for reduced immunogenicity of MSCs. However, upon exposure to inflammatory cytokines such as interferon-gamma (IFN-γ), MHCII and other costimulatory molecules can be up-regulated.8 Xenogeneic systems may not be optimal models to directly assess immunogenicity, as cytokines such as interferon-γ that affect MHCII expression and thus initiation of an immune response may be incompatible and ineffective across species. Clinical cellular repair strategies will likely involve injection of allogeneic cells into inflamed regions of damaged tissue, and repeated doses of cells may be necessary to achieve the desired benefit. The assessment of immunogenicity of stem-cell sources when injected repeatedly or in an inflammatory environment will be necessary for development of clinical protocols for effective cellular therapy.
A cell population isolated from the digested tissue of human umbilical cords is currently being evaluated for potential clinical application for repair in cases such as retinal disease and myocardial infarction. Preclinical functional studies using human umbilical cord tissue–derived cells (UTCs) are currently in progress, and early results show promise for these UTCs as cellular therapy of retinal disease in animal models.16 Massachusetts General Hospital (MGH) MHC-defined miniature swine17,–19 provide a unique preclinical model to assess immunogenicity of allogeneic UTCs. By approximating donor-recipient mismatch barriers often seen in clinical transplantations, these animals have been used for large animal studies of cellular and solid organ transplantation.20,–22 We now report the isolation and characterization of a porcine analog of human UTCs derived from miniature swine. We also describe the evaluation of immunogenicity of these cells using intravenous and subcutaneous injections across full allogeneic MHC barriers.
Methods
UTC isolation and culture
Umbilical cords were isolated from 4 MGH MHC-defined miniature swine leukocyte antigen (SLA)dd fetuses delivered at term through cesarean section. Porcine UTCs were extracted by the same procedure as described for isolation of human UTCs.16 Briefly, cords were drained of all blood, washed in phosphate-buffered saline, and then mechanically dissociated and digested using a mixture of the enzymes collagenase (10 U/mL; Sigma, St Louis, MO), dispase (12.6 U/mL; Roche Diagnostics, Indianapolis, IN), and hyaluronidase (1 U/mL, Vitrase; ISTA Pharmaceuticals, Irvine, CA) diluted in Dulbecco modified Eagle medium (DMEM)–low glucose medium (Invitrogen, Carlsbad, CA). After digestion at 37°C for 2 hours, the processed tissue was centrifuged, washed, and resuspended in growth medium consisting of DMEM-low glucose, 15% fetal bovine serum (FBS; Hyclone, Logan, UT), 0.001% 2-mercaptoethanol (Sigma), 1 mL per 100 mL antibiotic (10 000 U/mL penicillin, 10 000 μg/mL streptomycin). The resulting cells were seeded at a density of 3000 or 5000 viable cells/cm2 on tissue culture–treated flasks (Corning, Corning, NY) previously coated with 2% gelatin (Gelita porcine Type A: 250 Bloom; Sigma) and grown at 37°C, 5% CO2 in growth medium. Cultures were passaged regularly until reaching approximately 16 doublings and then cryopreserved.
MHC-defined miniature swine recipients
Animals receiving injections of SLAdd porcine umbilical tissue cells (pUTCs) were either SLAcc or SLAac to constitute a full, 2-haplotype mismatch. Transplantation across full, 2-haplotype MHC-mismatch barriers leads to vigorous rejection of kidney allografts in the absence of immunosuppression.23,–25 Twelve animals between 3 and 7 months of age were used for this study (Table 1). Two animals were injected with peripheral blood mononuclear cells (PBMCs) to serve as positive controls. Four animals received unactivated UTCs, either by intravenous injection (n = 2) or by subcutaneous injection (n = 2). The subcutaneous route of administration was chosen as a stringent test of immunogenicity since this route is typically highly immunogenic. Lack of an immune response following subcutaneous injection indicates that these cells would likely not induce an immune response when administered by any other, more clinically relevant route. To approximate the injection of these cells around damaged or inflamed tissue, 2 animals received unactivated UTCs injected subcutaneously around an inflammatory lesion prepared using complete Freund adjuvant (CFA/UTC), while another 2 animals received INF-γ–activated (MHCII+) UTCs (aUTCs) subcutaneously. To investigate the effect of multiple dosing in a noninflammatory environment, repeated subcutaneous injections of unactivated UTCs were given to 2 additional animals (UTCs SC×3). All animal care procedures were in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources.26 The difference in immune response rates between experimental groups was assessed by Fisher exact test; a 2-sided P value was reported.
In vitro responses by mixed lymphocyte reaction (MLR) assay
| . | Early harvest (D2 or D3) DD SI . | Standard harvest (D5) DD SI . | D2 or D3 SI ratio to before treatment . | SI ratio (D2 or D3/D5) . | Antibody response (titer) . | Cytotoxic antibody . |
|---|---|---|---|---|---|---|
| Animal 16707/PBMCs* | ||||||
| Before treatment | 4.7 | 19 | 1.000 | 0.247 | — | — |
| D 15 | 8.1 | 24.1 | 1.723 | 0.336 | — | — |
| D 30 | 8.1 | 26.1 | 1.723 | 0.310 | Yes (1:2560) | Yes |
| Animal 16792/PBMCs* | ||||||
| Before treatment‡ | N/A | N/A | N/A | N/A | — | — |
| D 15 | 13 | 79 | N/A | 0.165 | — | — |
| D 30 | 2.7 | 12.8 | N/A | 0.211 | Yes (1:2560) | Yes |
| Animal 16649/UTCs IV* | ||||||
| Before treatment | 1.4 | 8.3 | 1.000 | 0.169 | — | — |
| D 15 | 1.3 | 6.1 | 0.929 | 0.213 | — | — |
| D 30 | 1.4 | 16.6 | 1.000 | 0.084 | — | — |
| D 60 | 1.5 | 17.4 | 1.071 | 0.086 | No | No |
| Animal 16481/UTCs IV* | ||||||
| Before treatment | 0.8 | 11 | 1.000 | 0.073 | — | — |
| D 15§ | N/A | N/A | N/A | N/A | — | — |
| D 30 | 1 | 5.1 | 1.250 | 0.196 | — | — |
| D 60 | 1.6 | 7.4 | 2.000 | 0.216 | No | No |
| Animal 16650/UTCs SC* | ||||||
| Before treatment | 3.1 | 53.7 | 1.000 | 0.058 | — | — |
| D 15 | 2.5 | 7.5 | 0.806 | 0.333 | — | — |
| D 30 | 5.9 | 15.2 | 1.903 | 0.388 | No | No |
| Animal 16633/UTCs SC* | ||||||
| Before treatment‡ | N/A | N/A | N/A | N/A | — | — |
| D 15 | 5.4 | 6.8 | N/A | 0.794 | — | — |
| D 30 | 2.6 | 4.6 | N/A | 0.565 | No | No |
| Animal 16843/CFA/UTCs* | ||||||
| Before treatment | 6.9 | 123 | 1.000 | 0.056 | — | — |
| D 15 | 4.1 | 4.2 | 0.594 | 0.976 | — | — |
| D 30 | 3.3 | 7.6 | 0.478 | 0.434 | — | — |
| After skin graft | 11.4 | 8.6 | 1.652 | 1.326 | Yes (1:640) | Yes |
| Animal 16844/CFA/UTCs* | ||||||
| Before treatment | 5.4 | 37.7 | 1.000 | 0.143 | — | — |
| D 15 | 3.2 | 5.6 | 0.593 | 0.571 | — | — |
| D 30 | 3.9 | 6.1 | 0.722 | 0.639 | — | — |
| After skin graft | 13.7 | 17 | 2.537 | 0.806 | Yes (1:640) | Yes |
| Animal 16854/aUTCs† | ||||||
| Before treatment | N/A | 221 | N/A | N/A | — | — |
| D 15 | 3.7 | 248 | N/A | 0.015 | — | — |
| D 30 | 0.44 | 1.3 | N/A | 0.338 | — | — |
| D 60 | 9.5 | 409 | N/A | 0.023 | — | — |
| After skin graft | 19 | 535 | N/A | 0.036 | Yes (1:640) | Yes |
| Animal 16922/aUTCs† | ||||||
| Before treatment | N/A | 31 | N/A | N/A | — | — |
| D 15 | 12 | 31 | N/A | 0.387 | — | — |
| D 30§ | N/A | N/A | N/A | N/A | — | — |
| D 60 | 3.4 | 489 | N/A | 0.007 | — | — |
| After skin graft | 20 | 186 | N/A | 0.108 | Yes (1:640) | Yes |
| Animal 17025/UTCs SC×3† | ||||||
| Before treatment | 4.2 | 51 | 1.000 | 0.082 | — | — |
| D 15 | 6.8 | 208 | 1.619 | 0.033 | — | — |
| D 45 | 19 | 426 | 4.524 | 0.045 | — | — |
| D 75 | 56 | 87 | 13.333 | 0.644 | Yes (after 2nd inj, >1:10240) | Yes (after 2nd inj) |
| After skin graft | 90 | 27 | 21.429 | 3.333 | — | — |
| Animal 17026/UTCs SC×3† | ||||||
| 2 Before treatment | 4.6 | 165 | 1.000 | 0.028 | — | — |
| D 15 | 8.2 | 223 | 1.783 | 0.037 | — | — |
| D 45 | 30 | 125 | 6.522 | 0.240 | — | — |
| D 75 | 29 | 0.58 | 6.304 | 50.000 | — | — |
| D 96 | 487 | 39 | 105.870 | 12.487 | — | — |
| After skin graft | 21 | 2.2 | 4.565 | 9.545 | Yes (after 2nd inj, 1:640) | Yes (after 2nd inj) |
| After skin graft repeat | 166 | 1.1 | 36.087 | 150.909 | — | — |
| . | Early harvest (D2 or D3) DD SI . | Standard harvest (D5) DD SI . | D2 or D3 SI ratio to before treatment . | SI ratio (D2 or D3/D5) . | Antibody response (titer) . | Cytotoxic antibody . |
|---|---|---|---|---|---|---|
| Animal 16707/PBMCs* | ||||||
| Before treatment | 4.7 | 19 | 1.000 | 0.247 | — | — |
| D 15 | 8.1 | 24.1 | 1.723 | 0.336 | — | — |
| D 30 | 8.1 | 26.1 | 1.723 | 0.310 | Yes (1:2560) | Yes |
| Animal 16792/PBMCs* | ||||||
| Before treatment‡ | N/A | N/A | N/A | N/A | — | — |
| D 15 | 13 | 79 | N/A | 0.165 | — | — |
| D 30 | 2.7 | 12.8 | N/A | 0.211 | Yes (1:2560) | Yes |
| Animal 16649/UTCs IV* | ||||||
| Before treatment | 1.4 | 8.3 | 1.000 | 0.169 | — | — |
| D 15 | 1.3 | 6.1 | 0.929 | 0.213 | — | — |
| D 30 | 1.4 | 16.6 | 1.000 | 0.084 | — | — |
| D 60 | 1.5 | 17.4 | 1.071 | 0.086 | No | No |
| Animal 16481/UTCs IV* | ||||||
| Before treatment | 0.8 | 11 | 1.000 | 0.073 | — | — |
| D 15§ | N/A | N/A | N/A | N/A | — | — |
| D 30 | 1 | 5.1 | 1.250 | 0.196 | — | — |
| D 60 | 1.6 | 7.4 | 2.000 | 0.216 | No | No |
| Animal 16650/UTCs SC* | ||||||
| Before treatment | 3.1 | 53.7 | 1.000 | 0.058 | — | — |
| D 15 | 2.5 | 7.5 | 0.806 | 0.333 | — | — |
| D 30 | 5.9 | 15.2 | 1.903 | 0.388 | No | No |
| Animal 16633/UTCs SC* | ||||||
| Before treatment‡ | N/A | N/A | N/A | N/A | — | — |
| D 15 | 5.4 | 6.8 | N/A | 0.794 | — | — |
| D 30 | 2.6 | 4.6 | N/A | 0.565 | No | No |
| Animal 16843/CFA/UTCs* | ||||||
| Before treatment | 6.9 | 123 | 1.000 | 0.056 | — | — |
| D 15 | 4.1 | 4.2 | 0.594 | 0.976 | — | — |
| D 30 | 3.3 | 7.6 | 0.478 | 0.434 | — | — |
| After skin graft | 11.4 | 8.6 | 1.652 | 1.326 | Yes (1:640) | Yes |
| Animal 16844/CFA/UTCs* | ||||||
| Before treatment | 5.4 | 37.7 | 1.000 | 0.143 | — | — |
| D 15 | 3.2 | 5.6 | 0.593 | 0.571 | — | — |
| D 30 | 3.9 | 6.1 | 0.722 | 0.639 | — | — |
| After skin graft | 13.7 | 17 | 2.537 | 0.806 | Yes (1:640) | Yes |
| Animal 16854/aUTCs† | ||||||
| Before treatment | N/A | 221 | N/A | N/A | — | — |
| D 15 | 3.7 | 248 | N/A | 0.015 | — | — |
| D 30 | 0.44 | 1.3 | N/A | 0.338 | — | — |
| D 60 | 9.5 | 409 | N/A | 0.023 | — | — |
| After skin graft | 19 | 535 | N/A | 0.036 | Yes (1:640) | Yes |
| Animal 16922/aUTCs† | ||||||
| Before treatment | N/A | 31 | N/A | N/A | — | — |
| D 15 | 12 | 31 | N/A | 0.387 | — | — |
| D 30§ | N/A | N/A | N/A | N/A | — | — |
| D 60 | 3.4 | 489 | N/A | 0.007 | — | — |
| After skin graft | 20 | 186 | N/A | 0.108 | Yes (1:640) | Yes |
| Animal 17025/UTCs SC×3† | ||||||
| Before treatment | 4.2 | 51 | 1.000 | 0.082 | — | — |
| D 15 | 6.8 | 208 | 1.619 | 0.033 | — | — |
| D 45 | 19 | 426 | 4.524 | 0.045 | — | — |
| D 75 | 56 | 87 | 13.333 | 0.644 | Yes (after 2nd inj, >1:10240) | Yes (after 2nd inj) |
| After skin graft | 90 | 27 | 21.429 | 3.333 | — | — |
| Animal 17026/UTCs SC×3† | ||||||
| 2 Before treatment | 4.6 | 165 | 1.000 | 0.028 | — | — |
| D 15 | 8.2 | 223 | 1.783 | 0.037 | — | — |
| D 45 | 30 | 125 | 6.522 | 0.240 | — | — |
| D 75 | 29 | 0.58 | 6.304 | 50.000 | — | — |
| D 96 | 487 | 39 | 105.870 | 12.487 | — | — |
| After skin graft | 21 | 2.2 | 4.565 | 9.545 | Yes (after 2nd inj, 1:640) | Yes (after 2nd inj) |
| After skin graft repeat | 166 | 1.1 | 36.087 | 150.909 | — | — |
D 2 or D 3 (early) detectable response defined as follows: (1) SI more than 10; or (2) ratio more than 2.5 of early harvest (D 2 or D 3) SI compared with pretreatment assay; or (3) ratio of more than 1 of early harvest to conventional (D 5) harvest.
IV indicates intravenous; SC, subcutaneous; inj, injection; and —, undetectable.
CFSE-based MLR assay with early harvest on D 3 and conventional D 5 harvest.
Thymidine-based MLR assay with early harvest on D 2 and conventional D 5 harvest.
Technical problem with incomplete CFSE labeling.
Technical difficulty: no third-party response or high self-background.
Flow cytometry
Porcine UTCs were analyzed by surface staining and flow cytometry to determine surface phenotype as previously described for human UTCs.16 Mouse monoclonal antibodies to CD31(WM59), CD44(MAC329), CD90(5E10), SLA class I (1E3), SLA class II DR (1053h2–18-1), SLA class II DQ (BL4H2), HLA-A, -B, and -C (G46–2.6), HLA-DR, -DP, and -DQ (TU39), and rat anti–mouse IgG secondary antibodies were purchased from BD Biosciences (San Jose, CA). Porcine-specific CD45RA (STH267)27 was kindly provided by Mitsugu Shimizu (National Institute of Animal Health, Tsukuba, Japan). Cells were acquired on a flow cytometer (FACSCalibur; BD Biosciences, Tsukuba, Japan).
Immunocytochemistry
Analysis of intracellular proteins and confirmation of cell surface receptor expression was performed using standard immunocytochemistry on 4% paraformaldehyde-fixed cultures of porcine UTCs. For staining of umbilical cord tissue, sections of porcine umbilical cord, and for comparison, human umbilical cord (NDRI, Philadelphia, PA), were obtained for analysis. Antibodies used for immunocytochemistry were as follows: CD90 (5E10; BD Biosciences), vimentin (V9; Sigma), anti-α smooth muscle actin (1A4; Sigma) and pig endothelial marker (MIL11; Serotec, Raleigh, NC). Following primary antibody incubation, fixed cells were washed and incubated with the appropriate isotype-specific antibody: goat anti–mouse IgG (Texas Red or Alexa 488), goat anti–mouse IgG1 (Texas Red or Alexa 488), and/or goat anti–rabbit IgG (Texas Red or Alexa 488) (1:250; Molecular Probes, Eugene, OR). Cultures were then counterstained with 10 μM DAPI (Molecular Probes) for 10 minutes to visualize cell nuclei. Fluorescence was visualized using the appropriate filter on either a Nikon Eclipse 80i microscope with Plan Apo 20×/0.75 objective or a Nikon Eclipse TE2000-U microscope with Plan Fluor ELWD 20×/0.45 objective (Nikon, Lake Placid, NY). Images were captured using either an Evolution QEi FAST digital color video camera (Media Cybernetics, Carlsbad, CA) or a CoolSnap ES (Roper Scientific). All images were acquired using Image Pro Plus version 5.1 with Turboscan software (Media Cybernetics) and processed with Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA).
Activation of UTCs by IFN-γ stimulation
Porcine and human UTCs were seeded after thaw at 5000 cells/cm2 in growth media and incubated at 37°C, 5% CO2. After 5 days, 80 ng/mL swine IFN-γ (PSC4030; Biosource International, Camarillo, CA) and 25 ng/mL human IFN-γ (RIFNG50; Pierce, Rockford, IL) were added to porcine and human UTC cultures, respectively. UTCs were harvested with trypsin 48 hours later and washed with media prior to flow cytometric analysis of surface MHCI and MHCII expression.
Injection of unactivated and activated UTCs
Cryopreserved porcine UTCs were thawed, cultured for 4 to 5 days, harvested, resuspended in lactated Ringer solution at a concentration of 107 cells/mL and infused into fully allogeneic SLAcc or SLAac recipient pigs at a total dose of 108 cells. For intravenous injections (n = 2), unactivated pUTCs were slowly infused at a concentration of × 107 cells/mL in a total volume of 10 cc through the intravenous catheter. For subcutaneous injections, a total of 4 skin injection sites were injected subcutaneously with 2.5 cc of the cell suspension using a 25-gauge needle. For animals receiving repeated subcutaneous injections, a total of 3 doses of cells were administered separated at 1-month intervals. The first 2 doses were injected into the same site, while the third dose was injected at a disparate site.
Injection of UTCs near an inflammatory lesion
An inflammatory lesion was created in 2 animals using complete Freund adjuvant (CFA) injected subcutaneously 1 week prior to pUTC injection. CFA (77140; Pierce) at a volume of 2 cc was injected subcutaneously using a 25-gauge needle. The pUTCs were injected subcutaneously at 4 sites placed around the periphery of the CFA lesion using a 25-gauge needle.
Antibody detection by flow cytometry
Antibody response to UTCs was assayed by flow cytometry using sera collected at serial time points from injected animals to stain peripheral blood mononuclear cells (PBMCs) that were haplotype matched to UTCs (SLAdd). Briefly, 10 μL serum from each recipient was added to 106 cells of SLAdd PBMCs. Following 30 minutes of incubation, cells were washed twice prior to incubation with a fluorescein-conjugated secondary antibodies (FITC goat anti–swine IgM and FITC goat anti–swine IgG: 02-14-03 and 02-14-02, respectively; KPL, Gaithersburg, MD). Sera from previously immunized animals were used as a positive control. Detection of antibody was reported as a difference in mean fluorescence intensity compared with the pretreatment sample. The level of detectable antibody was also titered by serial dilutions of the sera samples. For samples in which antibody was detected, specificity of binding to MHCI or MHCII was determined using PBMCs of recombinant haplotypes, SLAgg (MHCIc/MHCIId) and SLAkk (MHCId/MHCIIc).
Antibody complement–mediated cytotoxicity assay
Detection of cytotoxic antibodies to cell surface antigens was performed using an antibody/complement reaction, followed by a dye exclusion assay and fluorescence-activated cell sorting (FACS) acquisition. Sera samples were serially diluted in a 96-well round-bottom plate and incubated with 25 μL of 5 × 106 cells/mL target SLAdd PBMCs at a total volume of 50 μL per well. A negative control of media alone and a positive control consisting of serum from a previously immunized animal were used for each assay. Following 15 minutes of sera incubation at 37°C, each well was washed with 125 μL media and then centrifuged at 100 g for 5 minutes at 4°C. Diluted rabbit complement was added to each well and incubated at 37°C for 30 minutes prior to addition of 10 μL of 10 μg/mL 7-amino-actinomycin D (7-AAD, A9400; Sigma) for 30 minutes at 4°C. The percentage of 7-AAD–positive cells was measured by flow cytometry.
Mixed lymphocyte reaction (MLR)
Responder PBMCs were plated in triplicate in 96-well flat-bottom plates (Costar, Cambridge, MA) at a final concentration of 4 × 105 cells/well and were stimulated by an equal number of irradiated (25 Gy) stimulator PBMCs. The medium consisted of RPMI 1640 supplemented with 6% fetal pig serum (FPS), 10 mM N-2-hydroxyethylpiperazine-N-2-ethansulfonic acid, 1 mM glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL gentamicin, and 2 × 105 M 2-mercaptoethanol. Cultures were incubated for 2 and 5 days at 37°C in 6% CO2 and 100% humidity. 3H-thymidine was added for the last 6 hours of culture and wells were harvested onto glass fiber filters (WALLAC, Turko, Finland) and counted for beta emission.
For CFSE MLR, porcine PBMC responders were labeled using 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) in dimethylsulfoxide and the reaction was quenched with fetal bovine serum prior to MLR setup. Wells were harvested at days 3 and 5 of incubation to assess proliferation based on CFSE intensity by flow cytometry. With each successive generation in a population, proliferation is marked by a decrease in cellular fluorescence intensity that is readily followed by flow cytometry.28
Skin grafting
A pUTC haplotype-matched SLAdd skin graft was placed at least 30 days following pUTC injection to confirm whether the animal was sensitized by the pUTC injection. Split-thickness skin grafts were obtained from both a donor animal and the experimental animal using a dermatome. Skin was then placed on a graft bed, also prepared with the dermatome on the dorsum of the recipient animal. SLAdd and self–skin grafts were monitored daily to determine acceptance or rejection of the skin based on 3 characteristics: texture, color, and temperature. The Wilcoxon rank-sum test was used to assess for an association between antibody response prior to skin graft and the time to skin graft rejection. The exact 2-sided P value was computed by StatXact (Cytel, Cambridge, MA).
Results
Comparison of porcine and human umbilical cord structure and phenotype
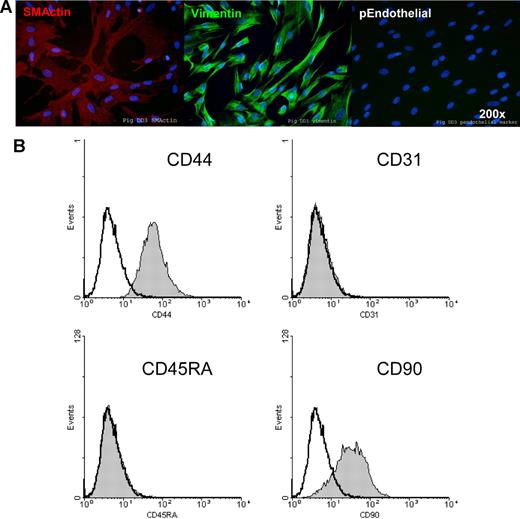
Miniature swine umbilical cords were found to have a similar anatomy and structure as human umbilical cords with 2 exceptions: (1) the observance of an allantoic duct in pigs, and (2) increased vasculature within the connective tissue of the porcine cord as previously described.29 The lack of an allantoic duct in the cross section of term human cord was not surprising as it is well known that this feature retracts during development, and is observable only at the fetal end of the cord. Otherwise, both cords consist of 2 arteries and 1 vein surrounded by Wharton, a proteoglycan-rich matrix. Phenotyping using immunohistochemistry also showed substantial similarities including numerous cells within both miniature swine (Figure 1A) and human (Figure 1B) cords staining positive for CD90 and vimentin.
Comparison of human and porcine umbilical cords. While differing in overall diameter, cross sections of miniature swine (A) and human (B) umbilical cord tissue share anatomic similarities (hematoxylin and eosin stain; scale bar = 1 mm). In addition, immunohistochemical staining for CD90 (red) and vimentin (green) expression, markers that identify both pig and human UTCs in culture, indicates that these cells are found in abundance and share phenotypic similarity (DAPI: nuclear counterstain, IHC; scale bar represents 100 μm). See “Immunocytochemistry” for image acquisition information.
Comparison of human and porcine umbilical cords. While differing in overall diameter, cross sections of miniature swine (A) and human (B) umbilical cord tissue share anatomic similarities (hematoxylin and eosin stain; scale bar = 1 mm). In addition, immunohistochemical staining for CD90 (red) and vimentin (green) expression, markers that identify both pig and human UTCs in culture, indicates that these cells are found in abundance and share phenotypic similarity (DAPI: nuclear counterstain, IHC; scale bar represents 100 μm). See “Immunocytochemistry” for image acquisition information.
Phenotype of isolated porcine UTCs
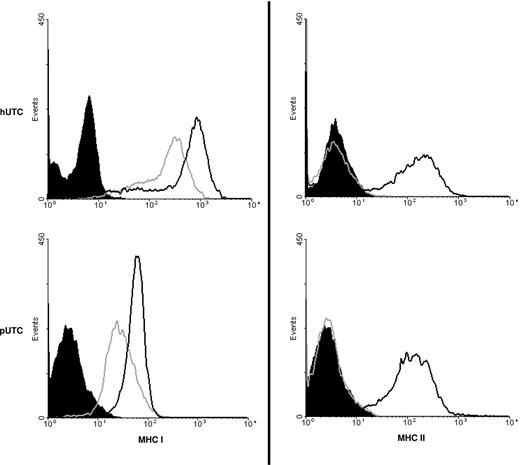
The phenotype of pUTCs was determined by immunohistochemistry and flow cytometry of cultured cells. Isolated pUTCs were found to be positive for smooth muscle actin and vimentin, but negative for a porcine-specific endothelial cell marker (Figure 2A). Similar to the published phenotype of human UTCs,16 surface staining and flow cytometry of pUTCs revealed positive cell-surface expression of CD44 and CD90, with a lack of CD31 and CD45RA (Figure 2B). Also similar to human UTCs, unactivated pUTCs express MHCI on their surfaces, but do not express MHCII (Figure 3). After exposure to IFN-γ for 48 hours, MHCII surface expression is induced on both human and porcine UTCs and MHCI surface expression is increased (Figure 3).
Immunohistochemical and flow cytometric phenotyping of UTCs. Porcine UTCs were phenotyped using available porcine-specific and known cross-reactive reagents by immunohistochemistry (A) and by flow cytometry (B). Filled curve represents UTCs; hollow curve, isotype negative control. See “Immunocytochemistry” for image acquisition information.
Immunohistochemical and flow cytometric phenotyping of UTCs. Porcine UTCs were phenotyped using available porcine-specific and known cross-reactive reagents by immunohistochemistry (A) and by flow cytometry (B). Filled curve represents UTCs; hollow curve, isotype negative control. See “Immunocytochemistry” for image acquisition information.
Inducible expression of MHCII and increased MHCI with IFN-γ. Unactivated UTCs (gray, hollow curves) of both human (top panels) and porcine (bottom panels) origin express MHCI (left), but no MHCII (right). After 48 hours of incubation with IFN-γ, human and porcine activated UTCs (black, hollow curves) have increased expression of MHCI (left) and induced expression of MHCII (right). In all cases, staining was compared with an unstained negative control (black, filled).
Inducible expression of MHCII and increased MHCI with IFN-γ. Unactivated UTCs (gray, hollow curves) of both human (top panels) and porcine (bottom panels) origin express MHCI (left), but no MHCII (right). After 48 hours of incubation with IFN-γ, human and porcine activated UTCs (black, hollow curves) have increased expression of MHCI (left) and induced expression of MHCII (right). In all cases, staining was compared with an unstained negative control (black, filled).
Assessment of induced antibody to SLAdd cells
Antibody response to SLAdd cells was assessed by flow cytometry. Serum IgM to SLAdd PBMCs was not easily detectable in any animal at the weekly time points of serum collection (data not shown), but serum IgG production could be assessed. Animals injected with SLAdd PBMCs demonstrated production of anti-SLAdd IgG antibody within the first 3 weeks after injection, indicating sensitization (Figure 4A). Animals receiving a single injection of the same dose of pUTCs failed to demonstrate any detectable levels of serum antibody production of IgG directed toward SLAdd cells in a similar time period (Figure 4B). Animals injected with pUTCs activated by IFN-γ to express MHCII (Figure 4C) and animals injected with unactivated pUTCs around a CFA-induced inflammatory lesion (Figure 4D) demonstrated production of IgG antibody to SLAdd cells within the first 3 weeks after injection. In the animals injected with repeated doses of unactivated pUTCs, induced antibody to SLAdd could be detected only after reinjection into the same site one month later (Figure 4E). Following a third injection one month later into a separate site, one animal showed a boost in IgG levels, while the other maintained high levels of IgG.
Serum antibody to SLAdd cells after single administration of cells. Control animals injected with 1 × 108 PBMCs (A) demonstrated production of IgG antibody to SLAdd PBMCs within 2 weeks of injection. Animals receiving one injection of 108 unactivated UTCs either intravenously or subcutaneously (B) did not have detectable antibody. IgG antibody production was also seen in animals injected with 108 activated UTCs (aUTCs) expressing MHCII (C) and in animals receiving 108 UTCs injected around an inflammatory lesion (CFA/UTC) (D), as well as after in animals receiving repeated doses (108 cells/dose) of UTCs (E).
Serum antibody to SLAdd cells after single administration of cells. Control animals injected with 1 × 108 PBMCs (A) demonstrated production of IgG antibody to SLAdd PBMCs within 2 weeks of injection. Animals receiving one injection of 108 unactivated UTCs either intravenously or subcutaneously (B) did not have detectable antibody. IgG antibody production was also seen in animals injected with 108 activated UTCs (aUTCs) expressing MHCII (C) and in animals receiving 108 UTCs injected around an inflammatory lesion (CFA/UTC) (D), as well as after in animals receiving repeated doses (108 cells/dose) of UTCs (E).
Of the 8 animals with detectable SLAdd antibody, 5 had titers up to 1:640 dilution, while the remaining 3 had higher titers more than 1:640 (Table 1). In addition, these serum samples were tested for MHC specificity of the SLAdd (MHCId, MHCIId) binding using recombinant haplotype PBMC targets. Serum from animals with induced SLAdd antibody after UTC injection—either unactivated or activated—bound to SLAkk (MHCId, MHCIIc) PBMC targets, with little to no binding of SLAgg (MHCIc, MHCIId) targets. These results indicate that the antibody produced after cellular injection is directed against MHCI and not MHCII. Serum was also evaluated for the presence of cytotoxic antibodies by complement-mediated cytotoxicity using samples taken before treatment and at days 15 to 20 after injection. Cytotoxic antibody was detected in sera of all animals that had detectable levels of IgG directed at SLAdd PBMCs. No cytotoxic antibody was seen in sera of animals that had no detectable IgG (Table 1).
In vivo responses to skin graft challenge
Skin obtained from SLAdd animals was placed on animals injected with unactivated UTCs, activated UTCs, and unactivated UTCs after CFA injection (Table 2). In comparison with self–skin grafts that were accepted, animals injected with unactivated UTCs first demonstrated signs of rejection by days 5 to 6 after graft. Rejection was complete by fulfillment of the 3 criteria of texture, temperature, and color by day 8 after graft. Based on comparison with historical controls,30 these fully mismatched skin grafts were rejected in a normal tempo, and this clinical pattern of rejection was consistent with a primary immune response and with no prior sensitization after UTC injection. Animals that received the activated UTCs also accepted self–skin grafts, but demonstrated earlier signs of rejection of SLAdd skin grafts by postoperative days 1 to 2. Rejection by all criteria was complete by day 6 after graft. In the animals injected with unactivated UTCs around a CFA inflammatory lesion, self-grafts were accepted, while SLAdd skin grafts began to show evidence of rejection by days 2 to 4 after graft, with complete rejection by day 6. For these animals, the patterns of skin graft rejection were consistent with a second-set rejection confirming prior sensitization from UTC injection. Antibody response prior to skin graft was associated with earlier skin graft rejection according to both the start of clinical signs and the completion of rejection (P = .036). In all cases, the rejection of SLAdd skin grafts confirmed normal immunocompetence in UTC recipients with no evidence for tolerance induction.
Tempo of UTC haplotype–matched skin graft rejection after injection
| Animal . | UTC source . | Antibody prior to skin graft . | Clinical pattern of skin graft rejection (start to completion day) . |
|---|---|---|---|
| 16649 | Unactivated UTCs | No | Normal (6-8) |
| 16481 | Unactivated UTCs | No | Normal (5-8) |
| 16843 | Unactivated UTCs around CFA | Yes | Accelerated (4-6) |
| 16844 | Unactivated UTCs around CFA | Yes | Accelerated (2-6) |
| 16854 | Activated UTCs | Yes | Accelerated (2-6) |
| 16922 | Activated UTCs | Yes | Accelerated (1-6) |
| 17025 | Unactivated UTCs × 3 | Yes | Accelerated (1-5) |
| 17026 | Unactivated UTCs × 3 | Yes | Accelerated (1-6) |
| Animal . | UTC source . | Antibody prior to skin graft . | Clinical pattern of skin graft rejection (start to completion day) . |
|---|---|---|---|
| 16649 | Unactivated UTCs | No | Normal (6-8) |
| 16481 | Unactivated UTCs | No | Normal (5-8) |
| 16843 | Unactivated UTCs around CFA | Yes | Accelerated (4-6) |
| 16844 | Unactivated UTCs around CFA | Yes | Accelerated (2-6) |
| 16854 | Activated UTCs | Yes | Accelerated (2-6) |
| 16922 | Activated UTCs | Yes | Accelerated (1-6) |
| 17025 | Unactivated UTCs × 3 | Yes | Accelerated (1-5) |
| 17026 | Unactivated UTCs × 3 | Yes | Accelerated (1-6) |
In vitro responses to SLAdd PBMC stimulation
In vitro responses to SLAdd stimuli were evaluated using MLR assays, either by a 3H-thymidine–pulsed or CFSE-based method. Assays were harvested at an early incubation time point at day 2 for 3H-thymidine assays or at day 3 for CFSE assays to distinguish sensitized from normal alloimmune responses. Proliferation at these early harvests may be indicative of sensitization.31 All assays were also harvested at day 5, the conventional time point for assessing the presence of alloresponses. All of these results are summarized in Table 1. A detectable response at the early harvest was defined by fulfillment of one of the following criteria: (1) stimulation index (SI) more than 10; (2) ratio early harvest SI to day-5 SI more than 1; or (3) ratio of early harvest SI more than 2.5 compared with that of the pretreatment assay (applicable only to postinjection assays). From these early harvests, a detectable sensitized response was seen after injection in both animals injected with activated, MHCII+ UTCs, in both animals receiving repeated injections of UTCs after the second injection, and in only 1 of the 2 control animals injected with SLAdd PBMCs. No other animals had a detectable early proliferative response by these same methods prior to rejection of a SLAdd skin graft. Following rejection of SLAdd skin, however, strong proliferative responses at day-2 or -3 MLR harvests could be detected. Taken together, these data suggest that early MLR harvest can detect strong sensitization responses but may not detect mild sensitization, which may occur with cellular injections. Detection of alloantibody responses may be a more reliable indicator of sensitization following cellular injection. All animals evaluated had normal proliferative responses to SLAdd PBMCs when harvested after 5 days of in vitro incubation.
Discussion
Human UTCs have been reported as having potential for cellular repair.16 Here we report the isolation and characterization of a porcine analog of human UTCs and assess immunogenicity of this unique cell source using clinically relevant MGH MHC-inbred miniature swine. Characterization of isolated porcine UTCs confirmed that these cells are phenotypically similar to human UTCs and could be used as an analog for human cells. Immunogenicity studies revealed that a single injection of unactivated UTCs across a full MHC barrier does not elicit a detectable adaptive immune response. Animals injected once either systemically or subcutaneously with unactivated UTCs had no detectable alloantibody production and a normal rejection pattern for unsensitized SLAac or SLAcc animals following in vivo SLAdd skin graft challenge. It seems unlikely that the UTCs are simply being cleared and removed from the host without being seen by its immune system, as injection of PBMCs and activated UTCs under similar conditions leads to a discernible response. The lack of MHCII expression and/or the low expression of MHCI on unactivated UTCs may play a role in the reduced immunogenicity. Similar results would be expected with injection of any cell type lacking MHCII and having a low level of MHCI expression. Another possibility is that UTCs may play a direct role in modulating immune responses; however, our studies were not designed to assess this.
Our data demonstrate that under certain circumstances, UTCs can elicit an immune response. Following injection of UTCs previously activated by IFN-γ stimulation, injection of unactivated UTCs near an inflammatory lesion created by CFA, or repeat injection of unactivated UTCs in the same site, alloantibody production is detected within a week and accelerated SLAdd skin graft rejection occurs. Although statistical comparisons are limited by the small animal numbers, the consistency of observed data across experimental conditions assessed by different in vitro assays supports the interpretation on immunogenicity. If a single injection of MHC-mismatched unactivated UTCs were truly capable of eliciting an immune response, specifically more than 90% of the time, it is very unlikely no immune response would be detected in 2 of 2 recipient animals (P = .010) in each of the unactivated intravenous and subcutaneous groups. Pooling the animals receiving unactivated pUTCs (n = 4) for comparison, the induction of an immune response is significantly associated (P = .067) with each of the other experimental conditions, namely injection in an inflamed region, repeated injection in the same region, or IFN-γ stimulation of UTCs prior to injection.
Surface expression of MHCI is increased and MHCII is induced following incubation with IFN-γ in vitro on human and porcine UTCs. Since IFN-γ is an inflammatory cytokine typically present in damaged tissues and sites of inflammation, MHCI and MHCII may be up-regulated on UTCs injected near an inflammatory lesion, affecting immunogenicity. In the animals receiving repeated injections of UTCs in the same site, multiple injections may have caused slight mechanical irritation leading to an inflammatory environment. Another possibility is that the first UTC injection may have caused a mild immune response undetectable by our assays and the second injection resulted in a detectable secondary immune response. Following a third dose of UTCs administered to a disparate site in these animals, levels of serum IgG increased in one animal and remained elevated in the other, suggesting a systemic secondary immune response.
Overall, these findings suggest that care must be taken in the clinic to avoid sensitization against the cell therapy product. The environment into which UTCs are injected is an important clinical consideration, especially if these cells are used for repairing damaged, inflamed tissues. Repeated administration into the same location may cause rejection of previously engrafted cells and may negate the previously acquired benefits of therapy. Although we investigated only IFN-γ, it is also possible that other cytokines present in damaged tissues might affect expression of costimulatory molecules in addition to MHC on UTCs, thereby increasing immunogenicity. Clinical treatments using UTCs may require careful timing to ensure inflammation at the planned site of injection has subsided in order to avoid an immune response and the possible rejection of therapeutic cells. Alternatively, concurrent immunosuppression and/or anti-inflammatory agents may have to be used to avoid sensitization. The effect of these additional treatments in this model has not yet been assessed and is an area of active investigation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully appreciate the technical assistance of Jennifer Goodrich on the in vitro assays, Ahmed Ghazi on the antibody cytotoxicity assay, Patricia Rafferty on the histology of the pig and human umbilical cords, and Dr Beow Yeap for the statistical analysis of our data. We thank Drs Kazuhiko Yamada and Shuji Nobori for harvesting the porcine umbilical cords, as well as Drs Benjamin Horner, Adam Griesemer, Masayoshi Okumi, and Hisashi Sahara for providing the SLAdd skin grafts. We also appreciate the assistance of Dr Krzysztof Wikiel in the care and monitoring of our animals. Finally, we acknowledge Dr Yong-Guang Yang and Dr Henry Winn for their critical review of this paper.
This work was supported by Centocor R&D, Stem Cell Internal Venture–sponsored research agreement.
Authorship
Contribution: P.S.C., I.R.H., S.H.P., D.H.S., and C.A.H. designed research; P.S.C., D.J.M., E.L.H., N.C., S.N.G., D.P.L., I.R.H., and S.H.P. performed research and collected data; P.S.C., D.J.M., N.C., S.H.P., and C.A.H. analyzed data; P.S.C., D.J.M., N.C., S.H.P., D.H.S., and C.A.H. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christene A. Huang, Transplantation Biology Research Center, Massachusetts General Hospital, 149-9019 13th St, Boston, MA 02129; e-mail: huangc@helix.mgh.harvard.edu.