We conducted a cohort-study among 518 female 5-year Hodgkin lymphoma (HL) survivors, aged 14 to 40 years (median: 25 years) at treatment (1965-1995). Multivariable Cox regression was used to quantify treatment effects on risk of premature menopause, defined as cessation of menses before age 40 years. After a median follow up of 9.4 years, 97 women had reached menopause before age 40 years. Chemotherapy was associated with a 12.3-fold increased risk of premature menopause compared with radiotherapy alone. Treatment with MOPP (mechlorethamine, vincristine, procarbazine, prednisone)/ABV (doxorubicine, bleomycine, vinblastine) significantly increased the risk of premature menopause (hazard ratio [HR]: 2.9), although to a lesser extent than MOPP treatment (HR: 5.7). Alkylating agents, especially procarbazine (HR: 8.1) and cyclophosphamide (HR: 3.5), showed the strongest associations. Ten years after treatment, the actuarial risk of premature menopause was 64% after high cumulative doses (> 8.4 g/m2) and 15% after low doses (≤ 4.2 g/m2) of procarbazine. The cumulative risk of menopause at age 40 years did not differ much according to age, but time to premature menopause was much longer in women treated at early ages. As long as alkylating agents will be used for curing HL, premature menopause will remain a frequent adverse treatment effect, with various clinical implications.
Introduction
The introduction of modern radiotherapy and chemotherapy has dramatically improved the prognosis of patients with Hodgkin lymphoma (HL).1 Unfortunately, improved prognosis has been accompanied by long-term toxicity. Both chemotherapy and radiotherapy may induce gonadal failure.2 In female patients, treatment may induce acute ovarian failure or chemotherapy-related amenorrhea, shortly after completion of cancer therapy, or deplete the nonrenewable pool of primordial follicles, resulting in earlier menopause.3 Premature menopause not only may impact upon a patient's quality of life because of reduced fertility, it may also increase the risks of cardiovascular disease4,5 and osteoporosis.6,7 On the other hand, it may decrease the future risk of breast cancer as a second malignancy.8,9
The radiosensitivity of the human ovary has been studied in great detail. Wallace et al were able to make a model predicting the age at which ovarian failure is likely to develop after radiation to a field that includes the ovary in women treated for cancer, depending on radiation dose and age at treatment.10 The information on the effects of chemotherapy on early menopause, however, is still limited. Studies suffer from several shortcomings, including small sample size11,,,,,,,,,,,,,,,,–28 and lack of detailed information on treatment exposures.29,30 Although in most cases only acute gonadotoxic effects and chemotherapy-related amenorrhea have been studied rather than long-term effects on premature menopause, it can be concluded that alkylating agents are the most hazardous chemotherapeutic agents, and that gonadotoxicity is dose dependent. Recently, Sklar et al31 published a large study on early menopause in female survivors of childhood cancer in which extensive information on treatment was available. However, the proportion of women who had reached early menopause at the end of follow-up (4%) and the proportion of women with an attained age of at least 40 years (8%) were relatively small, rendering it difficult to draw firm conclusions. An interesting finding from the latter study31 was that the risk of early menopause was much higher among the 404 women treated for HL than among those treated for other childhood malignancies.
In this study, we assessed treatment-related risk factors for premature menopause, defined as cessation of menses before age 40 years, in a cohort of 518 female patients treated for HL. Unique features of this cohort are the availability of extensive treatment data (cumulative doses of specific chemotherapeutic agents, radiation treatment fields, dates of all treatments), information on confounders (smoking, body mass index [BMI], use of oral contraceptives) and prolonged follow up (up to 35 years) resulting in an attained age of at least 40 years in more than 60% of the cohort. These factors allowed us to study treatment effects in more detail than previous studies (eg, the dose-dependent effect of various alkylating agents).
Methods
Data collection procedures
From our previously described late-effects HL cohort comprising 2689 patients with HL as the first malignancy treated between 1965 and 1995 in The Netherlands Cancer Institute, Amsterdam or Erasmus MC-Daniel den Hoed Cancer Center, Rotterdam,32,–34 we selected all female 5-year survivors first treated between the ages of 14 and 40 years (n = 658). Patient selection and methods of data collection have been described in detail previously.32,,–35 In short, data were collected on date of birth; sex; date of HL diagnosis; pathologic stage; histology; date of first recurrence; date of most recent medical information or date of death; vital status and cause of death; menstrual status 1 year after treatment, after salvage therapy, and at the end of follow-up; age at menopause; and number of live-born children before and after diagnosis of HL. Smoking was scored positive when the patient was smoking at the end of follow-up or had stopped smoking less than 1 year before the end of follow-up. Overweight was scored positive when the patient's BMI was more than 25 at end of follow-up, or when overweight was mentioned in the medical record, in case of missing information on height or weight. When information on smoking status and overweight at end of follow-up was lacking, information from time at diagnosis was used. Women were defined as users of hormonal replacement therapy (HRT) or oral contraceptives (OCs) when they had ever used these drugs. Data were collected directly from the medical records, through treating physicians, and through general practitioners (GPs). We succeeded in obtaining medical status up to at least January 1, 2002, for 635 women (97%).
Of 556 (84%) women, data on menopausal status at the end of follow-up could be obtained from medical records. For 19 women known to have reached menopause, the exact age at menopause was unknown. For 9 of these women, whose attained age at end of follow-up was younger than 40 years, attained age was assigned as age at menopause. The other 10 women were assumed not to have reached menopause before age 40 years. We excluded 38 women because they were postmenopausal at study entry (10 surgical menopause, 4 natural menopause, 24 roentgen-castration), leaving 518 women available for analyses.
Compared with the 140 women who were excluded, these 518 women were both born and treated somewhat more recently (average year of birth: 1954 vs 1951, P < .001; average year of first treatment: 1980 vs 1978, P = .002). However, no significant differences in treatment (initial and relapse chemotherapy, initial and relapse radiotherapy), age at first treatment, smoking, and BMI were observed. For 37 postmenopausal women, information on age at menopause was also available from a mailed questionnaire.9 The mean age at menopause obtained from the medical records did not differ significantly from the age reported by the women themselves (mean difference: 0.5 years, P = .60), and correlation was good (Pearson r = 0.90).
Treatment
Since treatment for HL has changed considerably over the last decades, a variety of treatment regimens was used in the study population.36 Primary treatment was usually given according to treatment protocols of the European Organisation of Research and Treatment of Cancer (EORTC)37 ; treatment for recurrences was generally not standardized.34
To evaluate the risk of various late complications related to treatment, we collected detailed information on both chemotherapy and radiotherapy from medical records. Dates of primary as well as first salvage treatment with radiotherapy and chemotherapy were recorded. Cumulative doses of the various drugs were calculated by multiplying the daily dose times duration of use for single agents, and by multiplying the number of cycles by the standard dose per cycle for the specific agent in a certain treatment regimen in case of coordinated regimens. To disentangle the effects of multiple chemotherapeutic agents on menopause, we divided them into 6 therapeutic classes: alkylating agents, antimetabolites, antimitotic agents, antitumor antibiotics, topoisomerase inhibitors, and other chemotherapeutics. The individual alkylating agents that were used in our study population were as follows: procarbazine, mechlorethamine, carmustine, dacarbazine, ifosfamide, chlorambucil, lomustine, and cyclophosphamide. In the dose-response analyses for procarbazine and mechlorethamine, women with missing information on exact dose were analyzed as a separate category.
For all women, radiation fields for primary treatment and all relapses were recorded. For 25% of the cohort, exact radiation dose to the ovaries was quantified in a previous study.9 Among these women, 3 categories of radiotherapy dose to the ovaries could be identified: women who received only supradiaphragmatic radiation (low dose to the ovaries; mean: 0.2 Gy), those who were irradiated to the paraaortic nodes and/or to the spleen (medium dose to the ovaries; mean: 1.2 Gy), and women who received direct radiation to the ovaries without oophoropexy (relatively high dose to the ovaries; mean: 31 Gy) (fields: inverted Y, iliacal, inguinal, other abdominal, total body). Those with oophoropexy and abdominal irradiation received only a mean ovary dose of 4.6 Gy. The women for whom the dose had not been quantified previously9 were assigned to these categories of radiation dose to the ovaries based on recorded radiation fields.
Statistical analyses
The outcome of interest of our study was premature menopause, defined as cessation of menses before age 40 years. We performed a cohort analysis, in which women were followed from day of first treatment for HL until menopause (n = 97), age 40 years (n = 219), last known date of menopausal status (n = 122 including date of death), second primary malignancy (n = 26), date of surgical menopause (n = 7), or date of direct radiation to the ovaries (n = 47), whichever came first.
Absolute risks (ARs) of menopause according to treatment category were calculated as the number of events per category divided by the number of person-years in that category. Cox regression techniques38 were applied to quantify the relative effects of different treatments, with time since first treatment or attained age during the follow-up period as the time scale39 and adjustment for several confounders (smoking, overweight, use of oral contraceptives [OCs], age at first treatment). To address the overlap in chemotherapeutic exposures, all treatment-related variables were time dependent, and we carefully adjusted one regimen (or drug) for other potentially correlated treatments. Exposure to radiotherapy was considered in the analyses by censoring subjects when they received direct radiation to the ovaries (n = 47), and by adjusting all multivariate analyses for dose level of radiotherapy (supradiaphragmatic radiation only, or irradiation to paraaortic nodes and/or spleen with or without supradiaphragmatic radiation). Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using SAS 9.1 (SAS Institute, Cary, NC). Modification of treatment-related effects by age at first treatment and smoking were evaluated by including interaction terms in the regression model, and were tested according to likelihood ratio tests. We evaluated the proportional hazards assumption by fitting interaction terms between treatment and the logarithm of attained age, which was used as the time scale. Trend tests were based on tests of the slope of continuous number of cycles or dose among patients with nonzero values.
Results
After a median follow up of 9.4 years, 97 of the 518 women had reached menopause before age 40 years (range: 19-39 years; median: 33.5 years). Only 3 women reached menopause within the first year after treatment started. Table 1 shows the distribution of the patient population by age at first treatment for HL, year of first treatment, stage of HL, treatment category, and the presence of other risk factors for premature menopause.
Patient characteristics of Hodgkin lymphoma population
| . | Patients, no. . | % . |
|---|---|---|
| Total | 518 | 100 |
| Age at first treatment, y | ||
| 14 to 21 | 172 | 33.2 |
| 22 to 28 | 170 | 32.8 |
| 29 to 39 | 176 | 34.0 |
| Year of first treatment | ||
| 1966 to 1973 | 146 | 28.2 |
| 1974 to 1982 | 134 | 25.9 |
| 1983 to 1995 | 238 | 45.9 |
| Initial stage of HL | ||
| I | 92 | 17.8 |
| II | 273 | 52.7 |
| III | 64 | 12.4 |
| IV | 36 | 6.9 |
| Unknown | 53 | 10.2 |
| Total treatment* | ||
| RT only | 187 | 36.1 |
| CT only | 52 | 10.0 |
| Initial RT plus CT (first year), no further treatment | 192 | 37.1 |
| RT plus CT, including follow-up treatment† | 87 | 16.8 |
| Lifestyle factors | ||
| Smoking | 139 | 26.8 |
| Use of oral contraceptives | 340 | 65.6 |
| Overweight | 120 | 23.2 |
| . | Patients, no. . | % . |
|---|---|---|
| Total | 518 | 100 |
| Age at first treatment, y | ||
| 14 to 21 | 172 | 33.2 |
| 22 to 28 | 170 | 32.8 |
| 29 to 39 | 176 | 34.0 |
| Year of first treatment | ||
| 1966 to 1973 | 146 | 28.2 |
| 1974 to 1982 | 134 | 25.9 |
| 1983 to 1995 | 238 | 45.9 |
| Initial stage of HL | ||
| I | 92 | 17.8 |
| II | 273 | 52.7 |
| III | 64 | 12.4 |
| IV | 36 | 6.9 |
| Unknown | 53 | 10.2 |
| Total treatment* | ||
| RT only | 187 | 36.1 |
| CT only | 52 | 10.0 |
| Initial RT plus CT (first year), no further treatment | 192 | 37.1 |
| RT plus CT, including follow-up treatment† | 87 | 16.8 |
| Lifestyle factors | ||
| Smoking | 139 | 26.8 |
| Use of oral contraceptives | 340 | 65.6 |
| Overweight | 120 | 23.2 |
RT indicates radiotherapy; CT, chemotherapy.
Treatment until end of follow-up in this study; total treatment for HL and all recurrences may be more extensive; 99 patients were treated for recurrent disease.
Cumulative exposure switched from CT only to RT plus CT in 12 patients, and from RT only to RT plus CT in 58 patients.
Of the 331 women who were treated with chemotherapy, the majority received one or more cycles of MOPP (mechlorethamine, vincristine, procarbazine, prednisone) (n = 148) as their only chemotherapy regimen (n = 80) or in combination with other regimens or single agents (n = 68). The second most commonly used combination regimen was MOPP/ABV (mechlorethamine, vincristine, procarbazine, prednisone/doxorubicine, bleomycine, vinblastine) (n = 86), which was the only chemotherapy regimen in most of these women (n = 71). In addition, 27 and 25 women were treated with ABVD (doxorubicine, bleomycine, vinblastine and dacarbazine) and EBVP (epirubicine, bleomycin, vinblastine, prednisone), respectively.
We found that treatment with chemotherapy was associated with a 12.3-fold significantly increased risk of premature menopause (Table 2). The unadjusted cumulative risk for menopause among women treated with chemotherapy was 48% (95% CI: 40%-54%) at age 40 years, whereas for women treated with radiotherapy alone this was only 2% (95% CI: 0%-4%). Women who were treated with MOPP or MOPP/ABV were at increased risk to reach menopause before age 40 years (HR: 5.7 [95% CI: 3.6-9.1] and HR: 2.9 [95% CI: 1.6-5.2], respectively), and these risks were dose dependent (P-trend MOPP < .001, P-trend MOPP/ABV = .01). Cumulative risks for menopause at age 40 years among women treated with MOPP or MOPP/ABV were 63% (95% CI: 52%-72%) and 42% (95% CI: 25%-56%), respectively. No significant effects of treatment with ABVD or EBVP on premature menopause were observed.
Risk of premature menopause in women treated for Hodgkin lymphoma according to chemotherapy regimen, compared with women who were treated with radiotherapy only, provided the ovaries were not located in the radiation fields
| . | Patients, no.* . | % . | Events, no. . | Py . | Events/1000 Py . | HR (95% CI) . | P-trend† . |
|---|---|---|---|---|---|---|---|
| RT alone | 245 | 47 | 6 | 2426 | 2 | 1 (ref) | — |
| CT, yes/no‡ | 331 | 64 | 91 | 2760 | 33 | 12.3 (5.3-28.8) | — |
| MOPP§ | 141 | 28 | 56 | 1001 | 56 | 5.7 (3.6-9.1) | — |
| 1 to 3 cycles of MOPP | 37 | 7 | 24 | 297 | 37 | 3.8 (1.8-7.8) | — |
| 4 to 6 cycles of MOPP | 68 | 13 | 21 | 535 | 45 | 4.4 (2.5-8.0) | — |
| More than 6 cycles of MOPP | 39 | 8 | 3 | 169 | 124 | 11.6 (6.5-20.7) | .001 |
| MOPP/ABV§ | 85 | 17 | 16 | 642 | 25 | 2.9 (1.6-5.2) | — |
| 1 to 6 cycles of MOPP/ABV | 69 | 14 | 11 | 507 | 22 | 2.6 (1.3-5.2) | — |
| More than 6 cycles of MOPP/ABV | 18 | 4 | 5 | 135 | 37 | 4.8 (1.8-12.8) | .01 |
| ABVD§ | 25 | 5 | 1 | 260 | 4 | 0.3 (0.0-1.9) | — |
| EBVP§ | 25 | 5 | 5 | 217 | 23 | 1.2 (0.5-3.3) | — |
| . | Patients, no.* . | % . | Events, no. . | Py . | Events/1000 Py . | HR (95% CI) . | P-trend† . |
|---|---|---|---|---|---|---|---|
| RT alone | 245 | 47 | 6 | 2426 | 2 | 1 (ref) | — |
| CT, yes/no‡ | 331 | 64 | 91 | 2760 | 33 | 12.3 (5.3-28.8) | — |
| MOPP§ | 141 | 28 | 56 | 1001 | 56 | 5.7 (3.6-9.1) | — |
| 1 to 3 cycles of MOPP | 37 | 7 | 24 | 297 | 37 | 3.8 (1.8-7.8) | — |
| 4 to 6 cycles of MOPP | 68 | 13 | 21 | 535 | 45 | 4.4 (2.5-8.0) | — |
| More than 6 cycles of MOPP | 39 | 8 | 3 | 169 | 124 | 11.6 (6.5-20.7) | .001 |
| MOPP/ABV§ | 85 | 17 | 16 | 642 | 25 | 2.9 (1.6-5.2) | — |
| 1 to 6 cycles of MOPP/ABV | 69 | 14 | 11 | 507 | 22 | 2.6 (1.3-5.2) | — |
| More than 6 cycles of MOPP/ABV | 18 | 4 | 5 | 135 | 37 | 4.8 (1.8-12.8) | .01 |
| ABVD§ | 25 | 5 | 1 | 260 | 4 | 0.3 (0.0-1.9) | — |
| EBVP§ | 25 | 5 | 5 | 217 | 23 | 1.2 (0.5-3.3) | — |
Py indicates person-years; and —, not applicable.
Ten patients with missing information on number of cycles of MOPP or MOPP/ABV were excluded; patients may contribute person-time to more than one category.
Based on the slope of continuous number of cycles of a given regimen among those treated with that regimen.
Adjusted for smoking, oral contraceptive use, dose level of radiotherapy, and age at first treatment; age was used as the time scale.
Adjusted for smoking, oral contraceptive use, dose level of radiotherapy, age at first treatment, each other, and other chemotherapy (alkylating chemotherapy only: yes/no, nonalkylating chemotherapy only: yes/no, alkylating and nonalkylating chemotherapy: yes/no, unknown chemotherapy regimen: yes/no); age was used as the time scale.
When chemotherapeutic agents were divided into different classes, only the alkylating agents increased the risk of premature menopause (Table 3). Although a considerable proportion of the women were treated with antimitotic agents (n = 301) and antitumor antibiotics (n = 150), no effects on menopause were identified for any of these agents. Within the group of alkylating agents (which mainly consisted of procarbazine and mechlorethamine), significantly increased risks of premature menopause were found for procarbazine and cyclophosphamide. As expected, there was substantial overlap between treatment with procarbazine and mechlorethamine (ie, only approximately 10% of events occurred in women treated with one drug but not the other). However, the mutually adjusted model showed a strong association for procarbazine, but not for mechlorethamine, with no indication for collinearity (eg, no inflated standard errors). The adjusted HR for procarbazine (8.1; 95% CI: 2.0-32.8) was significantly different from the HR of 1.6 (95% CI: 0.6-4.0) found for mechlorethamine (P < .001). In addition, the effect of procarbazine on menopause was dose dependent (P-trend = .02), whereas the effect of mechlorethamine was not (P-trend = .90). Among women treated with procarbazine, the risk of premature menopause increased with 11% per additional 1.4 g/m2 (equal to 1 cycle of MOPP or 2 cycles of MOPP/ABV) of cumulative exposure to the drug (95% CI: 1.02-1.22; P-trend = .02). Compared with women treated with radiotherapy only, the risk of premature menopause was largest among women treated with more than 8.4 g/m2 procarbazine (HR: 20.4; 95% CI: 4.4-93.6). This 20-fold increased risk was also significantly higher than the 5-fold increased risk (HR: 5.2; 95% CI: 1.6-17.1) observed in women treated with 4.2 g/m2 to 8.4 g/m2 procarbazine (P = .02).
Risk for premature menopause in women treated for Hodgkin lymphoma with various chemotherapeutic agents, compared with women who were treated with radiotherapy only, provided the ovaries were not located in the radiation fields
| . | Patients, no.* (n = 518) . | % . | Events, no. (n = 97) . | Py (n = 4989) . | Events/1000 Py . | HR (95% CI) . |
|---|---|---|---|---|---|---|
| RT alone | 245 | 47 | 6 | 2426 | 2 | 1 (ref) |
| CT classes‡ | ||||||
| Alkylating agents, yes/no | 276 | 53 | 85 | 2130 | 40 | 6.1 (2.7-13.7) |
| Antimetabolites, yes/no | 12 | 2 | 7 | 66 | 105 | 1.4 (0.3-7.9) |
| Antimitotic agents, yes/no | 302 | 58 | 80 | 2538 | 31 | 0.6 (0.3-1.4) |
| Antitumor antibiotics, yes/no | 150 | 29 | 29 | 1188 | 24 | 0.5 (0.3-0.9) |
| Topoisomerase inhibitors, yes/no | 20 | 4 | 10 | 98 | 102 | 1.6 (0.6-4.3) |
| Antimitotic agents§ | ||||||
| Vincristine, yes/no | 234 | 45 | 77 | 1725 | 45 | 1.6 (0.7-3.6) |
| Vinblastine, yes/no | 183 | 35 | 31 | 1567 | 20 | 0.5 (0.2-1.4) |
| Antitumor antibiotics‖ | ||||||
| Doxorubicin, yes/no | 119 | 23 | 22 | 934 | 24 | 0.4 (0.2-1.1) |
| Bleomycin, yes/no | 146 | 28 | 27 | 1170 | 23 | 1.0 (0.4-2.4) |
| Alkylating agents | ||||||
| Procarbazine, yes/no¶ | 239 | 46 | 78 | 1770 | 44 | 8.1 (2.0-32.8) |
| 4.2 g/m2 or less#** | 98 | 19 | 13 | 753 | 23 | 1.3 (0.2-6.8) |
| 4.2 to 8.4 g/m2# | 90 | 17 | 32 | 691 | 46 | 5.2 (1.6-17.1) |
| More than 8.4 g/m2#†† | 48 | 9 | 26 | 205 | 127 | 20.4 (4.4-93.6) |
| Exact amount unknown | 13 | 3 | 7 | 121 | 58 | 5.9 (1.6-22.4) |
| Mechlorethamine, yes/no¶ | 239 | 46 | 75 | 1763 | 43 | 1.6 (0.6-4.0) |
| 36 mg/m2 or less#** | 101 | 19 | 16 | 779 | 26 | 2.6 (0.6-10.5) |
| 36 to 72 mg/m2# | 93 | 18 | 32 | 701 | 46 | 1.3 (0.5-3.3) |
| More than 72 mg/m2#‡‡ | 42 | 8 | 22 | 208 | 106 | 0.8 (0.2-3.3) |
| Exact amount unknown | 13 | 3 | 5 | 76 | 66 | 1.0 (0.3-3.6) |
| Cyclophosphamide, yes/no¶ | 40 | 8 | 24 | 243 | 99 | 3.5 (2.0-5.9) |
| Dacarbazine, yes/no¶ | 27 | 5 | 2 | 269 | 7 | 0.3 (0.1-1.5) |
| Lomustine, yes/no¶ | 25 | 5 | 9 | 80 | 112 | 1.6 (0.7-3.6) |
| Chlorambucil, yes/no¶ | 16 | 3 | 10 | 77 | 130 | 2.0 (0.8-4.7) |
| Carmustine, yes/no¶ | 7 | 1 | 4 | 36 | 111 | 0.7 (0.1-3.7) |
| Ifosfamide, yes/no¶ | 4 | 1 | 2 | 18 | 111 | 1.3 (0.2-10.3) |
| Mutually exclusive CT categories‡** | ||||||
| No CT | 289 | 56 | 6 | 2248 | 3 | 1 (ref) |
| Nonalkylating CT only | 45 | 9 | 1 | 465 | 2 | 0.8 (0.1-6.9) |
| Alkylating CT, no procarbazine | 46 | 9 | 5 | 360 | 14 | 5.4 (1.6-18.2) |
| Alkylating CT, 8.4 g/m2 or less procarbazine | 183 | 35 | 44 | 1444 | 30 | 10.9 (4.6-26.1) |
| Alkylating CT, more than 8.4 g/m2 procarbazine | 48 | 9 | 26 | 205 | 127 | 41.5 (16.9-102) |
| . | Patients, no.* (n = 518) . | % . | Events, no. (n = 97) . | Py (n = 4989) . | Events/1000 Py . | HR (95% CI) . |
|---|---|---|---|---|---|---|
| RT alone | 245 | 47 | 6 | 2426 | 2 | 1 (ref) |
| CT classes‡ | ||||||
| Alkylating agents, yes/no | 276 | 53 | 85 | 2130 | 40 | 6.1 (2.7-13.7) |
| Antimetabolites, yes/no | 12 | 2 | 7 | 66 | 105 | 1.4 (0.3-7.9) |
| Antimitotic agents, yes/no | 302 | 58 | 80 | 2538 | 31 | 0.6 (0.3-1.4) |
| Antitumor antibiotics, yes/no | 150 | 29 | 29 | 1188 | 24 | 0.5 (0.3-0.9) |
| Topoisomerase inhibitors, yes/no | 20 | 4 | 10 | 98 | 102 | 1.6 (0.6-4.3) |
| Antimitotic agents§ | ||||||
| Vincristine, yes/no | 234 | 45 | 77 | 1725 | 45 | 1.6 (0.7-3.6) |
| Vinblastine, yes/no | 183 | 35 | 31 | 1567 | 20 | 0.5 (0.2-1.4) |
| Antitumor antibiotics‖ | ||||||
| Doxorubicin, yes/no | 119 | 23 | 22 | 934 | 24 | 0.4 (0.2-1.1) |
| Bleomycin, yes/no | 146 | 28 | 27 | 1170 | 23 | 1.0 (0.4-2.4) |
| Alkylating agents | ||||||
| Procarbazine, yes/no¶ | 239 | 46 | 78 | 1770 | 44 | 8.1 (2.0-32.8) |
| 4.2 g/m2 or less#** | 98 | 19 | 13 | 753 | 23 | 1.3 (0.2-6.8) |
| 4.2 to 8.4 g/m2# | 90 | 17 | 32 | 691 | 46 | 5.2 (1.6-17.1) |
| More than 8.4 g/m2#†† | 48 | 9 | 26 | 205 | 127 | 20.4 (4.4-93.6) |
| Exact amount unknown | 13 | 3 | 7 | 121 | 58 | 5.9 (1.6-22.4) |
| Mechlorethamine, yes/no¶ | 239 | 46 | 75 | 1763 | 43 | 1.6 (0.6-4.0) |
| 36 mg/m2 or less#** | 101 | 19 | 16 | 779 | 26 | 2.6 (0.6-10.5) |
| 36 to 72 mg/m2# | 93 | 18 | 32 | 701 | 46 | 1.3 (0.5-3.3) |
| More than 72 mg/m2#‡‡ | 42 | 8 | 22 | 208 | 106 | 0.8 (0.2-3.3) |
| Exact amount unknown | 13 | 3 | 5 | 76 | 66 | 1.0 (0.3-3.6) |
| Cyclophosphamide, yes/no¶ | 40 | 8 | 24 | 243 | 99 | 3.5 (2.0-5.9) |
| Dacarbazine, yes/no¶ | 27 | 5 | 2 | 269 | 7 | 0.3 (0.1-1.5) |
| Lomustine, yes/no¶ | 25 | 5 | 9 | 80 | 112 | 1.6 (0.7-3.6) |
| Chlorambucil, yes/no¶ | 16 | 3 | 10 | 77 | 130 | 2.0 (0.8-4.7) |
| Carmustine, yes/no¶ | 7 | 1 | 4 | 36 | 111 | 0.7 (0.1-3.7) |
| Ifosfamide, yes/no¶ | 4 | 1 | 2 | 18 | 111 | 1.3 (0.2-10.3) |
| Mutually exclusive CT categories‡** | ||||||
| No CT | 289 | 56 | 6 | 2248 | 3 | 1 (ref) |
| Nonalkylating CT only | 45 | 9 | 1 | 465 | 2 | 0.8 (0.1-6.9) |
| Alkylating CT, no procarbazine | 46 | 9 | 5 | 360 | 14 | 5.4 (1.6-18.2) |
| Alkylating CT, 8.4 g/m2 or less procarbazine | 183 | 35 | 44 | 1444 | 30 | 10.9 (4.6-26.1) |
| Alkylating CT, more than 8.4 g/m2 procarbazine | 48 | 9 | 26 | 205 | 127 | 41.5 (16.9-102) |
Patients may contribute person-time to more than one category; numbers do not add up.
Adjusted for smoking, oral contraceptive use, dose-level of radiotherapy, age at first treatment, and other variables; age was used as the time scale.
§Adjusted for smoking, oral contraceptive use, dose-level of radiotherapy, age at first treatment, other variables, and other chemotherapy (alkylating agents: yes/no, antimetabolites: yes/no, antitumor antibiotics: yes/no, topoisomerase inhibitors: yes/no, other chemotherapy: yes/no); age was used as the time scale.
Adjusted for smoking, oral contraceptive use, dose level of radiotherapy, age at first treatment, other variables, and other chemotherapy (alkylating agents: yes/no, antimetabolites: yes/no, antitumor antibiotics: yes/no, antimitotic agents: yes/no, topoisomerase inhibitors: yes/no, other chemotherapy: yes/no); age was used as the time scale.
Adjusted for smoking, oral contraceptive use, dose level of radiotherapy, age at first treatment, other variables, and other chemotherapy (antimitotic agents: yes/no; antitumor antibiotics: yes/no; unknown chemotherapy regimen: yes/no; antimetabolites, topoisomerase inhibitors, or other chemotherapy: yes/no); age was used as the time scale.
Adjusted for smoking, oral contraceptive use, dose level of radiotherapy, age at first treatment, other variables, and other chemotherapy (cyclophosphamide: yes/no, other alkylating chemotherapy: yes/no, other nonalkylating chemotherapy: yes/no, unknown chemotherapy regimen: yes/no); age was used as the time scale.
One cycle of MOPP contains 1.4 g/m2 procarbazine and 12 mg/m2 mechlorethamine; one cycle of MOPP/ABV contains 0.7 g/m2 procarbazine and 6 mg/m2 mechlorethamine; one cycle of BEACOPP (baseline or escalated) contains 0.7 g/m2 procarbazine.
P-trend is .02. Based on the slope of continuous number of cycles of a given regimen among those treated with that regimen.
P-trend is .90. Based on the slope of continuous number of cycles of a given regimen among those treated with that regimen.
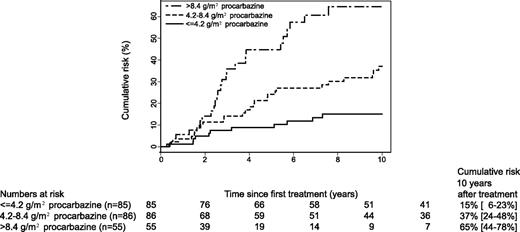
As cumulative procarbazine dose was most strongly associated with premature menopause, we divided treatment into 5 mutually exclusive categories based on procarbazine dose. As expected, in this latter model an elevated risk of premature menopause was observed only in women treated with alkylating chemotherapeutic agents, and especially with high doses of procarbazine. The unadjusted cumulative risk of premature menopause according to procarbazine dose is graphically presented in Figure 1; 10 years after first treatment it was as high as 65% (95% CI: 44%-78%) in those women treated with high doses of procarbazine.
Cumulative risk of premature menopause by cumulative dose of procarbazine among women treated for Hodgkin lymphoma with procarbazine-containing chemotherapy regimens.
Cumulative risk of premature menopause by cumulative dose of procarbazine among women treated for Hodgkin lymphoma with procarbazine-containing chemotherapy regimens.
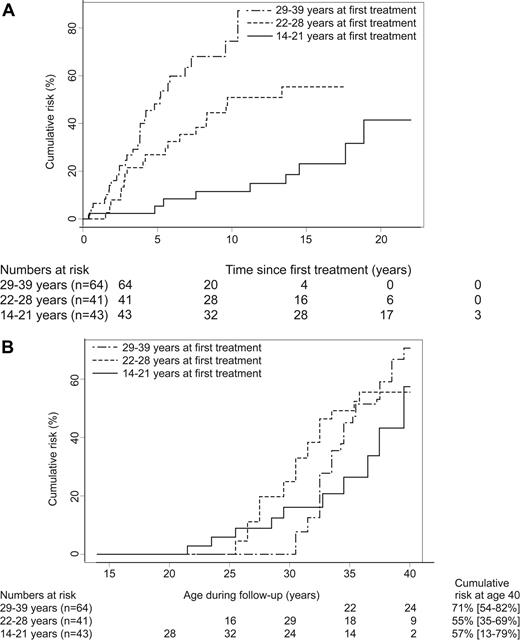
Age at first treatment was an independent risk factor for premature menopause in our study. Compared with age at first treatment of 14 to 21 years, HRs (and 95% CIs) adjusted for chemotherapy were 2.6 (1.5-4.5) for 22 to 28 years and 5.2 (2.8-9.4) for 29 to 39 years (P-trend < .001). We found no significant interaction between treatment with chemotherapy (yes/no) and age at first treatment, either as a continuous variable (P = .14) or as a categoric variable (P = .07).
Figure 2 graphically presents the influence of age at first treatment on risk for premature menopause among women treated with MOPP. Figure 2A demonstrates that women who were older at first treatment developed premature menopause sooner after treatment than the younger ones. However, Figure 2B, in which age during follow-up is used as a time scale, shows that the cumulative risk of premature menopause at age 40 years was not much different according to age at first treatment.
Cumulative risk of premature menopause according to age at first treatment among women treated for Hodgkin lymphoma with MOPP. (A) Cumulative risk of premature menopause versus time since first treatment. (B) Cumulative risk of premature menopause versus age during follow-up (attained age).
Cumulative risk of premature menopause according to age at first treatment among women treated for Hodgkin lymphoma with MOPP. (A) Cumulative risk of premature menopause versus time since first treatment. (B) Cumulative risk of premature menopause versus age during follow-up (attained age).
In addition, we did not observe a significant difference between the effect of chemotherapy among smokers (HR: 18.9) and nonsmokers (HR: 10.8) (P = .6). We neither identified differences in the effects of chemotherapy between those treated with combined modality therapy or chemotherapy only (P = .14), nor found differences between those receiving supradiaphragmatic radiation and those irradiated to the paraaortic nodes and/or spleen (P = .50). The data showed no evidence of nonproportional hazards (P = .24 for procarbazine dose; P = .51 for mechlorethamine dose).
Discussion
This study, which is one of the largest studies on gonadotoxicity in female HL survivors, provides new information regarding therapy-related risk factors for premature menopause. Because of the size of the study population and the detailed characterization of treatment-specific exposures, we can provide precise estimates of risk for premature menopause.
Our results indicate that among 5-year survivors of HL who were treated with chemotherapy between the ages of 14 and 40 years, the risk of entering menopause before age 40 years was 12-fold higher than for those who were treated with supradiaphragmatic radiotherapy or with irradiation to the paraaortic nodes and/or to the spleen alone; cumulative risk was 48% at age 40 years. The chemotherapeutic agents responsible for the induction of premature menopause were the alkylating agents, and especially procarbazine. For procarbazine, we observed a significant dose-response effect. Although the cumulative dose of procarbazine per cycle of MOPP/ABV is halved compared with a cycle of MOPP, women who were treated with MOPP/ABV still experienced a significantly increased risk for premature menopause (HR: 2.9). The cumulative risk for menopause at age 40 years for women treated with MOPP/ABV was 42%. The majority of these women (76%) was treated with 6 cycles of MOPP/ABV, containing 4.2 g/m2 procarbazine.
Nowadays, the most frequently used chemotherapy regimens for curing HL are ABVD and (escalated) BEACOPP (bleomycine, etoposide, doxorubicine, cyclophosphamide, vincristine, procarbazine, prednisone). In our population, there were only 27 women who were treated with ABVD and none with BEACOPP. Our results do not suggest, however, that ABVD is associated with an increased risk (HR: 0.3; 95% CI: 0.0-1.9) for premature menopause.
In accordance with this finding, Hodgson et al found no significant subfertility in their case-control study in female HL survivors treated with ABVD.40 Behringer et al describe that more than 50% of the patients who have received 8 cycles of BEACOPP had continuous amenorrhea.41 This confirms our concerns regarding gonadotoxicity of BEACOPP since patients treated with 8 cycles of BEACOPP baseline or escalated receive a cumulative dose of 5.6 g/m2 procarbazine. However, since the number of patients exposed to these regimens in our study population was limited, no firm conclusions should be drawn.
We were able to separate the gonadotoxic effect of procarbazine from the effect of mechlorethamine, which is often given together with procarbazine. Dose-response effects of alkylating chemotherapeutic agents on premature menopause have been described previously at the group level. Chiarelli et al calculated an alkylating agent score by adding up the number of alkylating agents received by each patient multiplied by the number of months each drug was taken.30 Sklar developed a more sophisticated alkylating agent score, which also took into account whether a drug was given in a high, medium, or low dose compared with the rest of the study population.3 In both methods, all alkylating agents are assumed to have the same gonadotoxic effects. Our study is the first to report risks for specific agents separately, and we found that there were significant differences between the various alkylating agents with respect to their risks of premature menopause.
In concordance with studies on ovarian failure among patients with systemic lupus erythematosus42 and breast cancer,43 we found a significantly increased risk of premature menopause following treatment with cyclophosphamide (HR: 3.4; 95% CI: 2.0-5.8). Unfortunately, we were not able to assess dose-response effects of cyclophosphamide and several other agents, because the range of doses used was narrow.
The dose-related effects of radiotherapy on premature menopause have been described previously in great detail,10,31 and therefore were not the main focus of our present study. However, we performed additional analyses in the group of women treated with radiotherapy alone, to confirm whether our population of HL patients responded to the effects of radiotherapy as expected. Women who were initially treated with radiotherapy and later with chemotherapy were also included in this analysis up to the moment they received chemotherapy. In this population of 549 women (including 31 women initially treated with radiotherapy to a field including the ovaries), 17 reached menopause before the age of 40 years. Thirteen of the 17 premature menopauses occurred after radiation to a field including the ovaries, which therefore was associated with a strongly increased risk for premature menopause compared with supradiaphragmatic irradiation (adjusted HR: 24.4; 95% CI: 6.2-95.5). No difference was found between irradiation restricted to the paraaortic nodes and/or to the spleen compared with supradiaphragmatic irradiation (P = .79).
It has been shown previously that age at treatment influences the risk of premature menopause.29,–31 Because the number of oocytes decreases with age, it is generally believed that at older ages less harm to the oocytes is needed to induce menopause. Our results confirm these findings. Our results show that it takes longer before women treated at an earlier age reach menopause, but their cumulative risk at age 40 years is comparable with that of women treated at older ages. One has to be aware, however, that these observations are based on a limited number of patients and power may be insufficient to draw generalizable conclusions from Figure 2. To adjust for the underlying increasing hazard for menopause, we also performed Cox analyses using attained age during follow up as the time scale. Using that approach, a woman who reaches menopause is compared with all women at risk who are of the same age.39 We chose the occurrence of menopause before the age of 40 years for assessment of treatment effects for 2 reasons. First, the occurrence of menopause at such a young age has more clinical implications compared with older ages at menopause, including family planning and influence on future risks of cardiovascular disease4,5 and osteoporosis.6,7 Second, the fact that natural menopause in the general population starts to occur after the age of 40 years44,45 may render it more difficult to study therapy-induced menopause after the age of 40 years. This is reflected by weaker associations observed in our study for chemotherapy (adjusted HR: 3.7; 95% CI: 2.5-5.7), when the outcome of interest was any occurrence of menopause, regardless of age.
In agreement with the literature,41,46 we found that women who used oral contraceptives experienced less often premature menopause (HR: 0.6; 95% CI: 0.4-0.9); in the multivariate models the association weakened to 0.8 (95% CI: 0.5-1.3). It is possible either that oral contraceptives mask the occurrence of menopause, or that women who lost ovarian function after treatment did not start using oral contraceptives. Unfortunately, we did not collect information on timing of oral contraceptive use. We also found that smoking was associated with a 1.5-fold increased risk for premature menopause; the association lost statistical significance in some of the multivariate models. It has been shown before that smoking decreases the age of natural menopause in the general population.46,47 Overweight, defined as a body mass index of more than 25 kg/m2, was not associated with premature menopause in our population. Epidemiologic studies on overweight and menopause have shown mixed results, and 2 opposite effects of higher estrogen levels in overweight women have been proposed: they may delay menopause,48 or the increased stimulation of follicular growth may result in exhaustion of follicles.46
When interpreting the results of this study, certain limitations should be taken into account. As in other studies in this field, the analyses disentangling the effects of specific chemotherapeutic agents were complicated due to the combination of a limited number of drugs into treatment regimens. Further, we relied on information from medical records to determine menopausal status. The occurrence of menopause, especially menopause occurring years after first treatment for HL, likely was not the main topic of interest for treating physicians. Information on the occurrence of menopause was missing for nearly 16% of the women. It is possible that information on menopause for these women was not recorded in the notes merely because they had not reached menopause yet. The exclusion of these women from our analysis could have led to an overestimation of the incidence of premature menopause in our population. However, when all women with unknown menopausal status were assumed to experience menopause after the age of 40 years, the cumulative risk of premature menopause would still be 28%, instead of the 33% we observed now. Nevertheless, we found no differences in treatment between the women with known and unknown menopausal status, suggesting that women with missing menopause information are a random subset of all women, and excluding them is unlikely to cause systematic overestimation or underestimation of the effects of chemotherapy. In addition, the measurement of occurrence of premature menopause as such does not provide information on the underlying mechanism of premature ovarian failure. Future studies including additional information on hypothalamic-pituitary-ovarian axis function would be informative.
In our study, we included women treated for HL after the age of 14 years. One may argue that not all women have reached menarche at that age. From 22% of our population, we had information on age at menarche. In this subpopulation, 84% of the women had started menarche before the age of 14 years, and none of the women was treated for HL before menarche. We therefore assume that the proportion of women that may have been treated before menarche was too low to have influenced our results.
In conclusion, based on a large cohort of female HL survivors with detailed treatment data, we have demonstrated that female HL survivors treated with chemotherapy (particularly the alkylating agents procarbazine and cyclophosphamide) are at an increased risk for premature menopause compared with women treated with radiotherapy only, provided the ovaries were not located in the radiation fields. Patients treated with both MOPP and MOPP/ABV experience high risks, and a strong dose-response effect was found for cumulative procarbazine dose. Reduction of the use of alkylating agents may decrease the risk of premature menopause in the future. However, as long as alkylating agents (and especially procarbazine) will be used for curing HL, premature menopause will remain a substantial problem in female HL survivors, with implications for the risks for other diseases such as cardiovascular disorders4,5 and osteoporosis.6,7
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant no. NKI 98-1833 and NKI 04-3068 from the Dutch Cancer Society, Amsterdam, the Netherlands.
We thank S. Grivell and L. D. Dorresteijn for their efforts to collect data. We are indebted to thousands of physicians from throughout the Netherlands who provided follow-up data for the study.
Authorship
Contribution: M.L.D.B., B.M.P.A., and F.E.L. contributed to the design of the study; M.L.D.B., J.H., M.H., B.M.P.A., and F.E.L. were involved with the data analysis and interpretation; M.A.K. and G.M.O. contributed to collection of data and administrative support; M.L.D.B., M.H., M.B.V., B.M.P.A., and F.E.L. contributed to the writing of the report. All authors approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Flora E. van Leeuwen, Department of Epidemiology, the Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, the Netherlands; e-mail: f.v.leeuwen@nki.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal