Abstract
We assessed incidence and risk factors of cardiovascular events in 265 patients undergoing allogeneic hematopoietic stem-cell transplantation (HSCT) between 1980 and 2000 and who survived at least 2 years. Results were compared with a cohort of 145 patients treated during the same period with autologous HSCT. The median age of patients with allogeneic HSCT at last follow-up was 39 years, and median follow-up was 9 years. Eighteen (6.8%) patients after allogeneic and 3 (2.1%) patients after autologous HSCT experienced an arterial event. The cumulative incidence of first arterial event after allogeneic HSCT was 22.1% (95% CI, 12.0-40.9) at 25 years. The cumulative incidence 15 years after allogeneic HSCT was 7.5% as compared with 2.3% after autologous HSCT. Adjusting for age, risk of an arterial event was significantly higher after allogeneic HSCT (RR 6.92; P =.009). In multivariate analysis, allogeneic HSCT (RR: 14.5; P =.003), and at least 2 of 4 cardiovascular risk factors (hypertension, dyslipidemia, diabetes, obesity) (RR: 12.4; P =.02) were associated with a higher incidence of arterial events after HSCT. Thus, long-term survivors after allogeneic HSCT are at high risk for premature arterial vascular disease. HSCT might favor the emergence of established risk factors, such as hypertension, diabetes, and dyslipidemia.
Introduction
Allogeneic hematopoietic stem-cell transplantation (HSCT) is the treatment of choice for defined malignant and nonmalignant hematological disorders.1 Results and long-term prognosis have substantially improved; immediate survival is no longer the sole concern. Still, HSCT remains associated with considerable morbidity and mortality. Long-term health status and the development of late events related to HSCT have gained increasing interest.2-5 Some complications, such as secondary tumors, cataract development, pulmonary complications, and infertility or endocrine dysfunctions have been well described.6-10 For other organ dysfunctions or complications an unequivocal relationship with the transplant procedure is more difficult to demonstrate. In theory any organ can be the target of a late event, and the list of such late sequelae is increasing. Reasons for underreporting can be a low incidence of an event after transplantation, the relative high frequency of similar observations in a general population, or the long-time interval needed for a clinical manifestation to occur. Cardiovascular events after allogeneic HSCT could fall into such a category
Atherosclerosis is now considered an inflammatory process, where endothelial lesions occur decades before clinical manifestations such as stroke, coronary heart disease, or peripheral arterial disease become manifest.11-13 Based on such concepts, cardiovascular disease might be expected decades after transplantation. Common risk factors for arteriosclerosis are well established.14 They include smoking, arterial hypertension, obesity, diabetes, dyslipidemia, and lack of exercise. Additional transplant-related factors could result in increased risk of arterial complications after allogeneic HSCT. Endothelial damage is induced by the conditioning regimen including or not total body irradiation (TBI),15,16 and endothelial cells have been documented as a target of graft-versus-host disease (GvHD).17 In addition, a higher incidence and degree of dyslipidemia, glucose intolerance, or hypertension might be the result of posttransplant endocrine dysfunction, prolonged treatment with immunosuppressive drugs, or sedentary life style after HSCT.18
So far, only anecdotic case reports on arterial complications have been reported, most of them with a dramatic course, observed in unexpectedly young patients.19-25 Recently, a large collaborative cohort study from the United States showed that long-term survivors after allogeneic HSCT have a higher risk of diabetes and hypertension, but not an increased frequency of cardiovascular outcomes.26 We aimed to assess incidence and risk factors of cardiovascular events in long-term survivors after allogeneic HSCT and compared these to a cohort of concomitant autologous transplant recipients, treated in a single center between 1980 and 2000.
Patients and methods
Study design
This retrospective single-center cohort study was designed to estimate the cumulative incidence of cardiovascular disease in long-term survivors after allogeneic HSCT and to analyze risk factors. All consecutive patients who underwent allogeneic HSCT between January 1980 and December 2000, and who survived 2 years after transplantation, were evaluated for the development of arterial vascular events. As a control group, we used all patients treated during the same period with autologous HSCT and surviving at least 2 years. From all patients, demographic data, disease and disease state, the use of TBI in the conditioning regimen, onset and degree of acute and chronic GvHD, and survival status were assessed. All patients were seen yearly for follow-up visits. In some years, when patients could not come to the transplant center, follow-up information was obtained from his or her health care provider. The time interval between first transplantation to last follow-up, first arterial vascular event, and/or death was prospectively documented. For the study purpose, every new arterial event occurring after transplantation was recorded. Approval from the local ethical committee, Ethikkommission beider Basel, EKBB, for the transplantation procedure as well as for the retrospective data analysis was obtained. Informed consent was obtained in accordance with the Declaration of Helsinki.
Cardiovascular events were classified as cerebrovascular disease (stroke, transient ischemic attack, cerebral arterial occlusion, symptomatic lacunar infarcts), coronary heart disease (angina pectoris, myocardial infarction, chronic coronary heart disease), and peripheral arterial disease (claudication, rest pain, acute ischemia, gangrene). Risk factors for arterial vascular events evaluated were gender, patient age, use of TBI conditioning, and acute and chronic GvHD. Additionally, established cardiovascular risk factors such as arterial hypertension, diabetes mellitus, dyslipidemia, and increased body-mass index (BMI) before HSCT were collected. For patients developing a de novo cardiovascular risk factor after HSCT, the time of first appearance after HSCT was recorded. Data on smoking habits, exercise, and familial history of cardiovascular disease were not available. Hypertension was defined as increased blood pressure (> 160 mm Hg systolic or > 95 mm Hg diastolic) or the use of antihypertensive therapy. Diabetes was retained as diagnosis for patients receiving antidiabetic therapy. Dyslipidemia was defined as increased plasma lipids (total cholesterol or LDL cholesterol or triglycerides) or when patients were receiving cholesterol-lowering therapy. Patients with a BMI of more than 25 kg/m2 and more than 30 kg/m2 were considered overweight or obese, respectively.
Patients' characteristics
From 1980 to 2000, 534 patients underwent allogeneic and 252 patients underwent autologous HSCT at the transplant center of Basel. Two-hundred and sixty-five patients (49.6%) treated with allogeneic and 145 patients (57.5%) treated with autologous HSCT, alive 2 years after transplant were included in the present study. The patients' characteristics are shown in Table 1. There were significant differences between the patients treated with allogeneic and autologous HSCT. The median age at allogeneic HSCT and at last follow-up was 27 (range, 2-60) years and 39 (range, 6-66) years, respectively, with a median follow-up time of 9 (range, 2-24) years. For patients treated with autologous HSCT, the median age at transplantation (44.5 years; range, 2-69) and at last follow-up (49 years; range, 3-72) was 18 years and 10 years higher, respectively, and the median follow-up time (5 years; range, 2-16) was 4 years shorter. There were also differences with respect of the patients' disease. In both groups, most of the patients received transplants for a malignant disease (allogeneic 241; 91%; autologous 138; 95.5%). However, acute leukemia and chronic myeloid leukemia were the main indication for allogeneic, mature lymphoid neoplasia, and solid tumor for autologous transplantation (P < .001). Only few patients underwent HSCT for a nonmalignant disease. Twenty-four patients (9%) underwent allogeneic HSCT for aplastic anemia, and 7 patients underwent autologous HSCT (4.5%) for autoimmune disorders. None of the patients treated with allogeneic HSCT, and 3 of the patients treated with autologous HSCT, presented an arterial vascular disease (coronary heart disease) before transplantation. Conditioning regimen depended on the disease and stage of the disease as follows: TBI/cyclophosphamide with or without etoposide or busulfan/cyclophosphamide was used in most patients with chronic myeloid leukemia, acute leukemia, and myelodysplastic syndrome; etoposid, carmustine, Ara-C, and melphalan (BEAM conditioning) were used for lymphoma; high-dose melphalan for multiple myeloma; and cyclophosphamide for aplastic anemia and autoimmune disorders. TBI was part of the conditioning in 229 (86%) of the patients treated with allogeneic HSCT, in 53 (23%) of them with a single total dose of 10 Gy, and in 176 (77%) with 6 fractionated doses of 2 Gy. Only 19 (13%) of the patients treated with autologous HSCT were conditioned with TBI, all of them with 6 fractionated doses of 2 Gy. After allogeneic HSCT, all patients received cyclosporine A with or without methotrexate for GvHD prophylaxis. Acute GvHD of any grade was observed in 204 (78%) patients and chronic GvHD in 140 patients (54%). The occurrence of established vascular risk factors was recorded at time of HSCT and thereafter once yearly. At time of HSCT more patients treated with autologous transplantation had arterial hypertension (16% versus 4.3%; P =.0001), dyslipidemia (28.4% versus 12.6%; P =.0001), and a BMI higher than 25kg/m2 (35.2% versus 18.4%; P =.001). There was no difference for diabetes (Table 1).
Characteristics and risk factors for cardiovascular disease in patients treated with allogeneic and autologous HSCT
| Characteristics numbers . | Allogeneic HSCT n =265 . | Autologous HSCT n =145 . | P . |
|---|---|---|---|
| Male, no. (%) | 145 (55%) | 87 (60%) | .302 |
| Median age at transplantation, y (range) | 27 (2-60) | 44.5 (2-69) | <.001 |
| Median age at last follow-up, y (range) | 39 (6-66) | 49 (3-72) | <.001 |
| Median time of follow-up, y (range) | 9 (2-24) | 5 (2-16) | <.001 |
| Alive at last follow-up, no. (%) | 208 (78%) | 111 (77%) | .652 |
| Disease and transplant-related factors, no. (%) | <.001 | ||
| Acute myeloid leukemia | 79 (30%) | 5 (3.5%) | — |
| Chronic myeloid leukemia | 75 (28%) | 2 (1.5%) | — |
| Myelodysplastic or myeloproliferative syndrome | 17 (6.5%) | 3 (2%) | — |
| Acute lymphoid leukemia | 51 (19%) | 8 (5.5%) | — |
| Mature lymphoid neoplasia | 19 (7.5%) | 88 (61%) | — |
| Aplastic anemia | 24 (9%) | 0 | — |
| Solid tumor | 0 | 32 (22%) | — |
| Autoimmune disease | 0 | 7 (4.5%) | — |
| Total body irradiation for conditioning, no. (%) | <.001 | ||
| No | 36 (14%) | 126 (87%) | — |
| Yes | 229 (86%) | 19 (13%) | — |
| Risk factors for atherosclerosis at diagnosis, no. (%) | |||
| Arterial hypertension | 11/254 (4.3%) | 23/144 (16%) | <.001 |
| Diabetes | 2/250 (0.8%) | 4/145 (2.8%) | .197 |
| Dyslipidemia | 29/232 (12.6%) | 38/135 (28.4%) | <.001 |
| Body mass index over 25 kg/m2 | 45/245 (18.4%) | 50/142 (35.2%) | .001 |
| Characteristics numbers . | Allogeneic HSCT n =265 . | Autologous HSCT n =145 . | P . |
|---|---|---|---|
| Male, no. (%) | 145 (55%) | 87 (60%) | .302 |
| Median age at transplantation, y (range) | 27 (2-60) | 44.5 (2-69) | <.001 |
| Median age at last follow-up, y (range) | 39 (6-66) | 49 (3-72) | <.001 |
| Median time of follow-up, y (range) | 9 (2-24) | 5 (2-16) | <.001 |
| Alive at last follow-up, no. (%) | 208 (78%) | 111 (77%) | .652 |
| Disease and transplant-related factors, no. (%) | <.001 | ||
| Acute myeloid leukemia | 79 (30%) | 5 (3.5%) | — |
| Chronic myeloid leukemia | 75 (28%) | 2 (1.5%) | — |
| Myelodysplastic or myeloproliferative syndrome | 17 (6.5%) | 3 (2%) | — |
| Acute lymphoid leukemia | 51 (19%) | 8 (5.5%) | — |
| Mature lymphoid neoplasia | 19 (7.5%) | 88 (61%) | — |
| Aplastic anemia | 24 (9%) | 0 | — |
| Solid tumor | 0 | 32 (22%) | — |
| Autoimmune disease | 0 | 7 (4.5%) | — |
| Total body irradiation for conditioning, no. (%) | <.001 | ||
| No | 36 (14%) | 126 (87%) | — |
| Yes | 229 (86%) | 19 (13%) | — |
| Risk factors for atherosclerosis at diagnosis, no. (%) | |||
| Arterial hypertension | 11/254 (4.3%) | 23/144 (16%) | <.001 |
| Diabetes | 2/250 (0.8%) | 4/145 (2.8%) | .197 |
| Dyslipidemia | 29/232 (12.6%) | 38/135 (28.4%) | <.001 |
| Body mass index over 25 kg/m2 | 45/245 (18.4%) | 50/142 (35.2%) | .001 |
— indicates not applicable.
Statistical analysis
Patients with an arterial vascular event were compared with patients without any posttransplant cardiovascular event. Variables included into the analysis were gender and age of the recipient, disease, TBI, acute and chronic GvHD, and length of follow-up after HSCT. In addition to the patient and transplantation-specific variables, common risk factors for atherosclerosis, which were arterial hypertension, diabetes mellitus, dyslipidemia, and body mass index, were included. Pretransplant characteristics were compared using the chi-square test for categorical data and the Mann Whitney U test for continuous variables. The incidence of cardiovascular risk factors after transplantation was determined by calculating cumulative incidences with death in the absence of the risk factor as a competing risk. Similarly, cumulative incidence of vascular complications was estimated with death without a vascular event as the competing risk. Gray's test was used to compare cumulative incidences between the treatment groups.27 To identify risk factors for developing cardiovascular risk factors and cardiovascular events, univariate and multivariate Cox regression analyses were performed. Cardiovascular risk factors were incorporated as time-dependent covariates. Risk for cardiovascular events increases with age. We used multivariate models with age as the timescale to compensate for the fact that patients receiving allogeneic transplants were significantly younger than those treated with autologous transplantation. Patients entered the analysis at the age at which they received transplants and left the analysis at the age of the first cardiovascular event or at the age at last follow-up. To assess the impact of arteriovascular events on survival, an analogous model was used coding the arterial vascular event as a time-dependent variable. In all statistical procedures, P < .05 was considered as the level of significance. Statistical analysis was performed by using Stata statistical software (Release 9.2, StataCorp, College Station, TX). For cumulative risk analysis the R software package, version 2.5.0 (http://www.r-project.org) was used.
The aim of the study was to analyze the probability to develop an arterial event in patients treated with allogeneic HSCT. Patients treated during the same period of time with autologous HSCT in the same center were used as a control group.
Results
Outcome and arterial vascular events after allogeneic HSCT
At time of last follow-up, 208 (78%) of the 265 patients treated with allogeneic HSCT who survived at least 2 years after HSCT were alive, and 57 (22%) had died. The probability of overall survival at 20 years was 71% (± 4%; mean ± SD). Of 265, 18 (6.8%) patients were diagnosed with an arterial vascular event. They correspond to 2692 person years of observation. Development of a cardiovascular event was not associated with a significant increase in mortality (RR: 0.97; 95% CI, 0.43 to 2.11; P =.91). The comparison of these 18 patients with an arterial vascular event to patients without any cardiovascular disease is shown in Table 2. Patients with an arterial vascular event were significantly older at transplantation and at time of last follow-up (P < .05). Both groups were similar with respect to the use of TBI and type of TBI (single versus fractionated dose) for conditioning, and the incidence and severity of acute or chronic GvHD, but differed with respect to primary diagnosis. All 18 patients with a cardiovascular event received transplants for a myeloid neoplasm, and none of them had a lymphoid neoplasm or aplastic anemia.
Characteristics and risk factors for cardiovascular disease in patients treated with allogeneic HSCT with and without an arterial vascular event
| Characteristics numbers . | No arterial event n =247 . | With arterial event n =18 . | P . |
|---|---|---|---|
| Male gender, no. (%) | 132 (53%) | 13 (72%) | .122 |
| Median age at transplantation, y (range) | 26 (2-60) | 39 (19-59) | .002 |
| Median age at last follow-up, y (range) | 38 (6-66) | 48 (29-62) | <.001 |
| Median time of follow-up, y (range) | 9 (2-24) | 9 (2-21) | .115 |
| Alive at last follow-up, no. (%) | 196 (79%) | 12 (67%) | .20 |
| Disease and transplant-related factors, no. (%) | |||
| Acute myeloid leukemia | 71 (29%) | 8 (45%) | — |
| Chronic myeloid leukemia | 69 (28%) | 6 (33%) | .007 |
| Myelodysplastic or myeloproliferative syndrome | 13 (5%) | 4 (22%) | — |
| Acute lymphoid leukemia | 51 (21%) | 0 | — |
| Mature lymphoid neoplasia | 19 (7%) | 0 | — |
| Aplastic anemia | 24 (10%) | 0 | — |
| Total body irradiation for conditioning, no. (%) | |||
| No | 35 (14%) | 1 (6%) | .30 |
| Yes | 212 (86%) | 17 (94%) | — |
| Type of total body irradiation (n =229), no. (%) | |||
| Single fraction (10 Gy) | 48 (23%) | 5 (29%) | .52 |
| Fractionated TBI (6 × 2Gy) | 164 (77%) | 12 (71%) | — |
| Acute graft-versus-host disease (n =263), no. (%) | |||
| Grade 0 | 54 (22%) | 5 (28%) | .53 |
| Grade 1 | 72 (29%) | 5 (28%) | — |
| Grade 2 or higher | 119 (49%) | 8 (44%) | — |
| Chronic graft-versus-host disease (n=262), no. (%) | |||
| None | 111 (46%) | 11 (61%) | .57 |
| Moderate | 93 (38%) | 6 (33%) | — |
| Severe extended | 40 (16%) | 1 (6%) | — |
| Any graft-versus-host disease (n =262), no. (%) | |||
| Never | 33 (13%) | 4 (22%) | .30 |
| Yes | 211 (94%) | 14 (78%) | — |
| Characteristics numbers . | No arterial event n =247 . | With arterial event n =18 . | P . |
|---|---|---|---|
| Male gender, no. (%) | 132 (53%) | 13 (72%) | .122 |
| Median age at transplantation, y (range) | 26 (2-60) | 39 (19-59) | .002 |
| Median age at last follow-up, y (range) | 38 (6-66) | 48 (29-62) | <.001 |
| Median time of follow-up, y (range) | 9 (2-24) | 9 (2-21) | .115 |
| Alive at last follow-up, no. (%) | 196 (79%) | 12 (67%) | .20 |
| Disease and transplant-related factors, no. (%) | |||
| Acute myeloid leukemia | 71 (29%) | 8 (45%) | — |
| Chronic myeloid leukemia | 69 (28%) | 6 (33%) | .007 |
| Myelodysplastic or myeloproliferative syndrome | 13 (5%) | 4 (22%) | — |
| Acute lymphoid leukemia | 51 (21%) | 0 | — |
| Mature lymphoid neoplasia | 19 (7%) | 0 | — |
| Aplastic anemia | 24 (10%) | 0 | — |
| Total body irradiation for conditioning, no. (%) | |||
| No | 35 (14%) | 1 (6%) | .30 |
| Yes | 212 (86%) | 17 (94%) | — |
| Type of total body irradiation (n =229), no. (%) | |||
| Single fraction (10 Gy) | 48 (23%) | 5 (29%) | .52 |
| Fractionated TBI (6 × 2Gy) | 164 (77%) | 12 (71%) | — |
| Acute graft-versus-host disease (n =263), no. (%) | |||
| Grade 0 | 54 (22%) | 5 (28%) | .53 |
| Grade 1 | 72 (29%) | 5 (28%) | — |
| Grade 2 or higher | 119 (49%) | 8 (44%) | — |
| Chronic graft-versus-host disease (n=262), no. (%) | |||
| None | 111 (46%) | 11 (61%) | .57 |
| Moderate | 93 (38%) | 6 (33%) | — |
| Severe extended | 40 (16%) | 1 (6%) | — |
| Any graft-versus-host disease (n =262), no. (%) | |||
| Never | 33 (13%) | 4 (22%) | .30 |
| Yes | 211 (94%) | 14 (78%) | — |
— indicates not applicable.
Three patients had vascular symptoms but were not considered to have an arterial vascular event: one patient had cerebral symptoms due to vascular migraine, a second patient had acute arterial occlusion due to arterial emboli during pneumococcal sepsis, and a third patient presented transient ischemic attack at time of relapse of chronic myeloid leukemia (CML) with extremely high platelet count (> 1500 × 109/L). In this last case, the high platelet number, as well as platelet dysfunction, was considered to be the main cause of the cerebrovascular event.
The detailed description of the 18 patients with cardiovascular disease is shown in Table 3. There were 13 males and 5 females, with a median age at transplantation of 39 years (range, 19-59 years). The median time interval from transplantation to the first arterial vascular event was 9 years (range, 2-21 years), and the median age of the patients at time of the first arterial event of 48 years (range, 29-62 years). In total we observed 23 types of arterial vascular events in these 18 patients: 10 cerebrovascular events, 10 coronary artery events, and 3 peripheral artery events. Fifteen patients presented with a vascular event in a single territory (cerebrovascular in 8, coronary artery in 7 patients), one patient had coronary heart and peripheral arterial disease, and 2 patients had all 3 territories affected. Of the 18 patients with an arterial event, 6 (33%) died, as compared with 51 of 196 (21%) in the group without an arterial event. The cause of death of the patients with an arterial vascular event was cardiovascular disease in 2 and relapse of the primary disease in 4 of them.
Pretransplant and transplant characteristics of the 21 patients with cardiovascular disease after HSCT, and type of arterial vascular events
| UPN . | Age at HSCT, y . | Sex . | Disease . | TBI . | Acute GvHD . | Chron GvHD . | Status . | Age at first event, y . | Time interval, y . | Stop IS*, y . | Type of cardiovascular event/cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with a cerebrovascular event | |||||||||||
| 074 | 31 | F | AML | sTBI | 2 | Mod. | Alive | 52 | 20.7 | 20 | Transient ischemic attack |
| 080 | 40 | F | AML | sTBI | 0 | Mod. | Alive | 61 | 20.8 | 17 | Cerebrovascular arteriosclerosis and dementia |
| 097 | 36 | F | CML | sTBI | 2 | Mod. | Alive | 48 | 12.6 | 11 | Stroke |
| 174 | 19 | F | AML | sTBI | 0 | None | Dead | 29 | 16 | 10 | Stroke |
| 312 | 43 | M | AML | fTBI | 0 | None | Alive | 59 | 15.7 | 12 | Transient ischemic attack |
| 442 | 44 | M | CML | fTBI | 1 | None | Dead | 50 | 5.8 | 5 | Transient ischemic attack/relapse |
| 562 | 35 | F | AML | fTBI | 1 | None | Alive | 43 | 8.5 | On | Stroke |
| 832 | 44 | M | AML | fTBI | 0 | None | Dead | 46 | 2.3 | On | Lacunar infarcts/relapse |
| Patients with a coronary artery event | |||||||||||
| 192 | 19 | M | AML | fTBI | 2 | Mod. | Alive | 38 | 19.2 | 15 | Myocardial infarction |
| 326 | 41 | M | CML | fTBI | 2 | None | Alive | 58 | 16.2 | 13 | Angina pectoris |
| 394 | 44 | M | MDS | fTBI | 1 | None | Alive | 57 | 13.6 | 13 | Myocardial infarction |
| 433 | 29 | M | CML | fTBI | 2 | None | Alive | 42 | 13.3 | 10 | Angina pectoris, atrial fibrillation |
| 517 | 47 | M | AML | fTBI | 1 | None | Alive | 53 | 6.9 | 5 | Angina pectoris necessitating coronary bypass |
| 648 | 33 | M | CML | fTBI | 0 | None | Alive | 40 | 6.4 | 5 | Angina pectoris |
| 684 | 52 | M | MPS | fTBI | 2 | Mod. | Dead | 55 | 2 | On | Chronic coronary disease/relapse |
| Patients with cardiovascular events in more than one territory | |||||||||||
| 418 | 28 | M | CML | fTBI | 2 | Severe | Dead | 31 | 2.3 | On | Transient ischemic attack, angina pectoris, arterial occlusive disease, amputation. Cause of death: myocardial infarction |
| 557 | 38 | M | MDS | fTBI | 2 | Mod. | Alive | 46 | 7.6 | On | Stroke, chronic coronary disease, arterial occlusive disease, amputation |
| 770 | 59 | M | MDS | No TBI | 1 | None | Dead | 62 | 3.0 | On | Chronic coronary disease, arteriosclerosis. Cause of death: relapse |
| Patients treated with autologous HSCT with cardiovascular events | |||||||||||
| 390 | 36 | M | Hodgkin | No | NA | NA | Alive | 38 | 1.3 | NA | Chronic coronary disease |
| 670 | 50 | M | Myeloma | No | NA | NA | Alive | 63 | 3.7 | NA | Stroke |
| 695 | 59 | F | Myeloma | No | NA | NA | Dead | 69 | 0.74 | NA | Stroke |
| UPN . | Age at HSCT, y . | Sex . | Disease . | TBI . | Acute GvHD . | Chron GvHD . | Status . | Age at first event, y . | Time interval, y . | Stop IS*, y . | Type of cardiovascular event/cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with a cerebrovascular event | |||||||||||
| 074 | 31 | F | AML | sTBI | 2 | Mod. | Alive | 52 | 20.7 | 20 | Transient ischemic attack |
| 080 | 40 | F | AML | sTBI | 0 | Mod. | Alive | 61 | 20.8 | 17 | Cerebrovascular arteriosclerosis and dementia |
| 097 | 36 | F | CML | sTBI | 2 | Mod. | Alive | 48 | 12.6 | 11 | Stroke |
| 174 | 19 | F | AML | sTBI | 0 | None | Dead | 29 | 16 | 10 | Stroke |
| 312 | 43 | M | AML | fTBI | 0 | None | Alive | 59 | 15.7 | 12 | Transient ischemic attack |
| 442 | 44 | M | CML | fTBI | 1 | None | Dead | 50 | 5.8 | 5 | Transient ischemic attack/relapse |
| 562 | 35 | F | AML | fTBI | 1 | None | Alive | 43 | 8.5 | On | Stroke |
| 832 | 44 | M | AML | fTBI | 0 | None | Dead | 46 | 2.3 | On | Lacunar infarcts/relapse |
| Patients with a coronary artery event | |||||||||||
| 192 | 19 | M | AML | fTBI | 2 | Mod. | Alive | 38 | 19.2 | 15 | Myocardial infarction |
| 326 | 41 | M | CML | fTBI | 2 | None | Alive | 58 | 16.2 | 13 | Angina pectoris |
| 394 | 44 | M | MDS | fTBI | 1 | None | Alive | 57 | 13.6 | 13 | Myocardial infarction |
| 433 | 29 | M | CML | fTBI | 2 | None | Alive | 42 | 13.3 | 10 | Angina pectoris, atrial fibrillation |
| 517 | 47 | M | AML | fTBI | 1 | None | Alive | 53 | 6.9 | 5 | Angina pectoris necessitating coronary bypass |
| 648 | 33 | M | CML | fTBI | 0 | None | Alive | 40 | 6.4 | 5 | Angina pectoris |
| 684 | 52 | M | MPS | fTBI | 2 | Mod. | Dead | 55 | 2 | On | Chronic coronary disease/relapse |
| Patients with cardiovascular events in more than one territory | |||||||||||
| 418 | 28 | M | CML | fTBI | 2 | Severe | Dead | 31 | 2.3 | On | Transient ischemic attack, angina pectoris, arterial occlusive disease, amputation. Cause of death: myocardial infarction |
| 557 | 38 | M | MDS | fTBI | 2 | Mod. | Alive | 46 | 7.6 | On | Stroke, chronic coronary disease, arterial occlusive disease, amputation |
| 770 | 59 | M | MDS | No TBI | 1 | None | Dead | 62 | 3.0 | On | Chronic coronary disease, arteriosclerosis. Cause of death: relapse |
| Patients treated with autologous HSCT with cardiovascular events | |||||||||||
| 390 | 36 | M | Hodgkin | No | NA | NA | Alive | 38 | 1.3 | NA | Chronic coronary disease |
| 670 | 50 | M | Myeloma | No | NA | NA | Alive | 63 | 3.7 | NA | Stroke |
| 695 | 59 | F | Myeloma | No | NA | NA | Dead | 69 | 0.74 | NA | Stroke |
TBI indicates total body irradiation; s, single dose; f, fractionated dose; Mod., moderate; NA, not applicable; On, on immunosuppression at time of first event.
Stop IS, time interval in years between stop of immunosuppression and first arterial event.
Description of the vascular events
Among the 10 patients with cerebrovascular disease, 4 presented as stroke, 4 as transient ischemic attack, one with lacunar infarcts, and one with cerebral symptoms due to arteriovascular complications and dementia (Table 3). On carotid duplex ultrasound examination of this last patient, cerebrovascular thrombosis was found on both sides with left external carotid artery stenosis. Among the 10 patients with coronary heart disease, 2 patients had myocardial infarction, 5 angina pectoris, and 3 chronic coronary disease. All 3 patients with peripheral arterial disease also had coronary disease, and 2 of them additionally had cerebrovascular disease. At time of the first arterial event, 12 of the 18 patients were off any immunosuppressive drug since a median time of 11 years (range, 5-21 years). Six patients were still on immunosuppression when the first arterial event occurred (median time 3 years; range, 2-8 years).
The course of 2 patients is of particular interest because they developed cardiovascular disease in all 3 arterial territories, with cerebral, coronary arterial, and peripheral arterial events. The first patient (UPN 418) was a 28-year-old male who underwent allogeneic HSCT for CML in chronic phase. The posttransplant phase was uneventful with the exception of a transient acute GvHD grade 2. One year and 11 months later he presented a hematological relapse of the CML. He was treated with interferon-alfa and received 7 doses of donor lymphocyte infusions. Three months after the last infusion he developed severe extensive chronic GvHD of the skin, mucosa, and liver. At that time peripheral blood count had normalized, and peripheral blood showed full donor chimerism. Two months later the patient was in complete cytogenetic and molecular genetic remission of the CML. Five months after first donor lymphocyte infusions the patient presented with amaurosis fugax in the right eye. Within a few months he developed extensive peripheral arterial insufficiency with severe calf claudication as well as symptoms of coronary heart disease and mesenteric ischemia. An amputation of the right leg was required. The patient died from complications of myocardial infarction 12 years and 9 months after HSCT. At autopsy, generalized obliterating intimal artery proliferation and important atherosclerotic changes were described.
The second patient (UPN 557) was a 38-year-old male who underwent unrelated donor HSCT for myelodysplastic syndrome (MDS). He presented with acute GvHD grade 2 and, later, extensive moderate chronic GvHD of the skin, mucosa, and gut. Seven years after transplantation he first presented with peripheral artery disease with calf claudication. The patient was treated by iliaco-femoral bypass on both sides. A that time signs of chronic coronary heart disease were observed. During follow-up he developed cerebrovascular disease with transient ischemic attack as well as renal artery stenosis. The patient was on continuous immunosuppression with cyclosporine, steroids, and mycophenolate.
Cumulative incidence and risk factor analysis
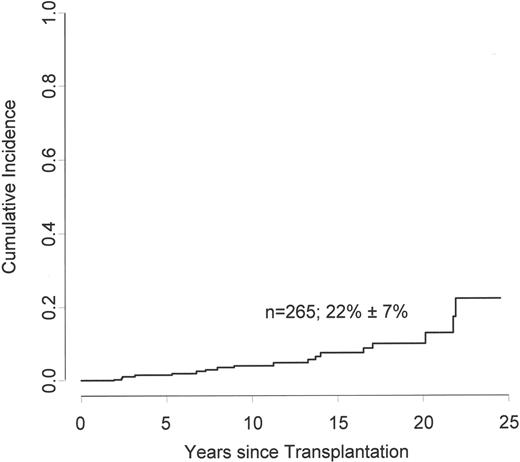
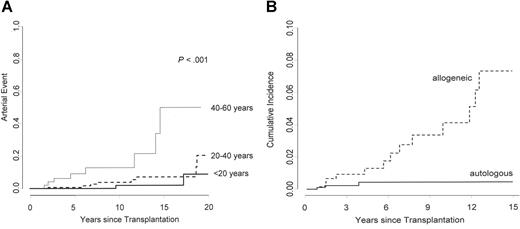
The cumulative incidence of cardiovascular disease increased from 1.5% at 5 years, to 4.1% at 10 years, to 12.8% at 20 years, and to 22.1% at 25 years (Figure 1). Patients with a higher age at time of transplant were at increased risk to develop cardiovascular events (Figure 2a), with cumulative incidences after 20 years of follow-up of 8.7% for patients younger than 20 years, 20.2% for patients between 20 and 40 years of age, and 50.1% for patients between 40 and 60 years of age at HSCT (P < .001). In univariate time-dependent Cox regression analysis, arterial hypertension (RR: 3.64; 95% CI; 1.41 to 9.44; P =.008), diabetes (RR: 9.62; 95% CI; 3.32 to 27.84; P < .001), and dyslipidemia (RR: 5.44; 95% CI; 2.02 to 14.62; P =.001) were associated with an increased risk of developing a cardiovascular complication (Figure 3). Sex, the use of TBI as conditioning regimen, the type of TBI (single dose versus fractionated dose), acute or chronic GvHD, GvHD severity, and BMI of at least 25 kg/m2 were not associated with late arterial vascular events.
The cumulative incidence of an arterial event was 1.5% (95% CI, 0.57%-3.99%; 192 patients at risk) at 5 years, 4.1% (95% CI, 2.14% to 7.83%; 120 patients) at 10 years, 12.8% (95% CI, 7.15% to 22.26%; 23 patients) at 20 years, and 22.1% (95% CI, 12.0% to 40.9%; 7 patients) at 25 years. Mean (± SD) at 25 years is shown on the figure.
The cumulative incidence of an arterial event was 1.5% (95% CI, 0.57%-3.99%; 192 patients at risk) at 5 years, 4.1% (95% CI, 2.14% to 7.83%; 120 patients) at 10 years, 12.8% (95% CI, 7.15% to 22.26%; 23 patients) at 20 years, and 22.1% (95% CI, 12.0% to 40.9%; 7 patients) at 25 years. Mean (± SD) at 25 years is shown on the figure.
Cumulative incidence of an arterial event stratified by age of the patients at time of HSCT. (A) The cumulative incidence at 20 years is 8.7% for patients younger than 20 years, 20.2% for patients between 20 and 40 years, and 50.1% for patients between 40 and 60 years of age at HSCT (P < .001). (B) The cumulative incidence of an arterial vascular event at 15 years, adjusted for age. Using an adjusted Cox model, the relative risk is significantly higher after allogeneic than after autologous HSCT.
Cumulative incidence of an arterial event stratified by age of the patients at time of HSCT. (A) The cumulative incidence at 20 years is 8.7% for patients younger than 20 years, 20.2% for patients between 20 and 40 years, and 50.1% for patients between 40 and 60 years of age at HSCT (P < .001). (B) The cumulative incidence of an arterial vascular event at 15 years, adjusted for age. Using an adjusted Cox model, the relative risk is significantly higher after allogeneic than after autologous HSCT.
Univariate analysis of risk factors for a cardiovascular event. In a time-dependent univariate Cox regression analysis, cardiovascular events are associated with the development of arterial hypertension (RR: 3.64; 95% CI, 1.41 to 9.44; P =.008), diabetes (RR: 9.62; 95% CI, 3.32 to 27.84; P < .001), and dyslipidemia (RR: 5.44; 95% CI, 2.02 to 14.62; P =.001) is increased. For a BMI greater than 25 kg/m2 and sex, the relative risk for an arterial event is not increased.
Univariate analysis of risk factors for a cardiovascular event. In a time-dependent univariate Cox regression analysis, cardiovascular events are associated with the development of arterial hypertension (RR: 3.64; 95% CI, 1.41 to 9.44; P =.008), diabetes (RR: 9.62; 95% CI, 3.32 to 27.84; P < .001), and dyslipidemia (RR: 5.44; 95% CI, 2.02 to 14.62; P =.001) is increased. For a BMI greater than 25 kg/m2 and sex, the relative risk for an arterial event is not increased.
In univariate analysis also the type of disease was associated with increased risk of cardiovascular disease. Indeed, arterial vascular event was observed only in patients with myeloid neoplasm (acute myeloid leukemia [AML], CML, MDS, or myeloproliferative disease [MPD]), and not in those with lymphoid neoplasms (acute lymphoblastic leukemia [ALL], chronic lymphocytic leukemia [CLL], non-Hodgkin lymphoma [NHL], multiple myeloma), or aplastic anemia (P =.007). Patients with myeloid neoplasms also were different in other aspects. They were older at time of transplantation (median age 32 years versus 21 years; P < .001), and at time of last follow-up (42 years versus 34 years; P < .001), and had longer follow-up time (9.3 years versus 8.2 years; P =.038).
In multivariate Cox regression analysis using age as a timescale, only the development of at least 2 cardiovascular risk factors (hypertension, diabetes, dyslipidemia, or obesity) was statistically associated with an increased risk for cardiovascular events (RR 14.6, 95% CI 1.69–126, P =.015), whereas sex and type of disease were not (P =.37 and .10, respectively).
Comparison with autologous HSCT
At time of last follow-up, 111 (77%) of the 145 patients treated with autologous HSCT who had a survival of 2 years after HSCT were alive. The probability of overall survival at 15 years was 56% ± 8%. One patient with embolic arterial occlusions during recurrent atrial fibrillation due to toxic cardiomyopathy was not considered having an arterial event. In total, 3 of 145 (2.1%) patients were diagnosed with an arterial vascular event after autologous HSCT. The characteristics of these patients are shown in Table 3. Two patients presented with stroke and one with chronic coronary heart disease. The median time interval between transplantation and first arterial event was 1.3 years (range, 0.7-4 years).
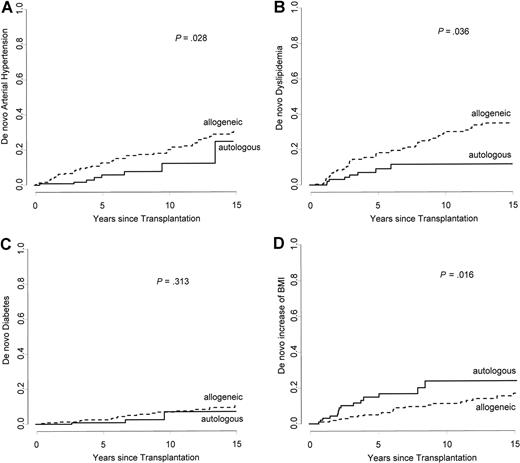
The cumulative incidence of an arterial event after autologous HSCT at 15 years was 2.3%, as compared with 7.5% for patients treated with allogeneic HSCT (P =.631). Using an age-adjusted Cox model, the relative risk is significantly higher after allogeneic HSCT than after autologous (RR 6.92; 95% CI 1.60 to 27.92; P =.009) (Figure 2B). In both treatment groups (allogeneic and autologous) there was a constant increase of de novo cardiovascular risk factors after HSCT. However, the progression of the cumulative incidence was more pronounced after allogeneic HSCT for arterial hypertension (RR: 2.50; 95% CI, 1.19 to 5.27; P =.028) and dyslipidemia (RR: 2.31; 95% CI, 1.15 to 4.65; P =.036), but not for diabetes (RR: 2.21; 95% CI, 0.64 to 7.59; P =.313). In contrast, the increase of BMI was more important in patients treated with autologous HSCT (RR: 0.50; 95% CI, 0.27 to 0.94; P =.016) (Figure 4).
Comparing patients treated with allogeneic and autologous HSCT (Gray test). Cumulative incidence of development of a de novo cardiovascular risk factor after treatment with HSCT. (A) Arterial hypertension. (B) Dyslipidemia. (C) Diabetes. (D) BMI greater than 25 kg/m2.
Comparing patients treated with allogeneic and autologous HSCT (Gray test). Cumulative incidence of development of a de novo cardiovascular risk factor after treatment with HSCT. (A) Arterial hypertension. (B) Dyslipidemia. (C) Diabetes. (D) BMI greater than 25 kg/m2.
In a multivariate model, including type of HSCT (autologous versus allogeneic), patient age, gender, and number of cardiovascular risk factors (0-1 versus 2-4, coded as a time-dependent variable), the relative risk for an arterial event was significantly higher for allogeneic HSCT (RR: 14.5; 95% CI, 2.44 to 86.3; P =.003) and higher cardiovascular risk factor score (RR: 12.4; 95% CI, 1.51 to 101; P =.02) but not gender (RR: 2.12; 95% CI, 0.80 to 5.64; P =.13).
Discussion
In this retrospective, single-center study, based on 265 long-term survivors, we found a cumulative incidence of arterial vascular events after allogeneic HSCT, such as cerebrovascular disease, coronary arterial disease, and/or peripheral arterial disease of 22% at 25 years. The median age of 49 years at the first cardiovascular event was unusually low, and some of the patients presented an event in more than one arterial territory. Adjusted for age of the patients in the control group, recipients of allogeneic HSCT had significantly more arterial events. In univariate analysis, we identified age at transplantation, arterial hypertension, diabetes, and dyslipidemia as risk factors for a cardiovascular disease after allogeneic HSCT. In multivariate analysis, allogeneic HSCT and the occurrence of 2 or more of the established cardiovascular risk factors were associated with an arterial event. Age at transplant did not impact the risk of arteriovascular events in multivariate analysis, however, this was likely due to the type of statistical model used, which aimed to correct for differences in age between the allogeneic and autologous populations at the time of follow-up and which inherently adjusted for differences in age at transplant. Finally, we showed that at time of HSCT established vascular risk factors, such as arterial hypertension and dyslipidemia, were more often present in the autologous group. However, because of more rapid progression of de novo risk factors after allogeneic HSCT, this difference between autologous and allogeneic transplantation disappeared 10 years after HSCT.
Cardiovascular disease is common in a general population and remains the leading cause of morbidity and mortality in developed countries.28 Therefore, the crucial issue here is to know whether the risk for a vascular event is increased in patients who receive transplants and not simply the consequence of a normal aging process. Autologous HSCT seems an adequate control group for the evaluation of this type of late events. After allogeneic HSCT, the cumulative incidence of an arterial event at 15 years was higher, as compared with autologous HSCT, despite a significant lower age at transplantation. When adjusted for age, patients treated with allogeneic HSCT had a 6.92-fold increased relative risk to present an arterial event. In an unselected European or US population the risk of coronary disease increases with age and is higher in males. In males with an intermediate risk profile the cumulative 10-year incidence of cardiovascular disease is about 2% at 40 years, 11% at 50 years, and 17% at 60 years. For females it is 0.7%, 1%, ands 8%, and respectively.29 In our long-term survivors, we have an overall cumulative incidence of 22% at 25 years after transplantation in a population with a median age of 27 years at transplantation, and 39 years at time of last follow-up. Together with the results from our control group, these data suggest that the risk of arterial vascular events is increased after allogeneic HSCT.
Our results are compatible with a recently published US study where diabetes and arterial hypertension were reported to be higher than in the sibling donors.26 In contrast, the authors of this study did not find an increased risk of cardiovascular events. We believe that this difference is due to design of the 2 studies. We looked at all consecutive patients in a single cohort study; the US study was based on a questionnaire and self-reporting and might be biased by a substantial number of nonreporting participating patients.
Dyslipidemia, increased blood pressure, diabetes, smoking, and obesity are established risk factors for a cardiovascular disease.30,31 In addition to these factors, the incidence of cardiovascular diseases is 3-fold higher and mortality 5-fold higher in men than in women.32 If cardiovascular diseases are more frequent after allogeneic HSCT, transplantation-related factors should be involved as well in the atherosclerotic process. GvHD has been shown to affect the endothelium, and endothelial damage has been associated with GvHD.17 The rapid progressive arterial insufficiency in one patient receiving donor lymphocyte infusions and interferon-alfa therapy is suggestive that an immunologically mediated mechanism such as extensive chronic GvHD was implied in the process. Prolonged treatment with corticosteroids is associated with increased risk for cardiovascular diseases33-35 and could be a promoting factor in the second patient with severe cardiovascular disease of all 3 territories. In organ transplantation, allograft vasculopathy is a leading cause of mortality.36-38 Despite etiologic factors implicated are still controversial, immunological mechanisms seem to be involved in the development of the graft atherosclerosis.
So far we were unable to document an association of transplant-related factors, such as type of conditioning or GvHD, with cardiovascular disease. Such a lack of association could be explained by the still low number of patients with a complication and the long time for these events to occur. In addition, the established cardiovascular risk factors have such a strong impact on the statistical model that they obscure additional factors. Finally, cardiovascular risk factors, such as hypertension and diabetes, become more frequent after allogeneic HSCT26 and therefore might be responsible for the increased risk for atherosclerosis. This last hypothesis is strongly supported by our data showing that patients treated with allogeneic HSCT have an increased risk of developing a de novo arterial hypertension and dyslipidemia after HSCT. The higher incidence of cardiovascular risk factors at HSCT in the autologous group was certainly in relation to their higher age. Despite that most of the patients had stopped their immunosuppression at time of last follow-up, post-grafting cyclosporine A used previously could play a significant role. Also compared with what we expect in a general population, our allogeneic transplant cohort has a high incidence of cardiovascular risk factors.39 Biological aging has been shown to be a contributory risk factor of coronary artery disease.40 We might therefore observe a premature aging contributing to an early onset of cardiovascular disease after allogeneic HSCT.
It is not surprising that arterial vascular events have been underestimated so far in survivors after allogeneic HSCT. A very long follow-up is needed to estimate this risk. Atherosclerosis, responsible for almost all cases of cardiovascular diseases, is an insidious process, beginning early in life, and culminating in thrombotic occlusions and vascular events in middle age and later life. After allogeneic HSCT, additional endothelial lesions would, together with the established cardiovascular risk factors, accelerate the process of atherosclerosis. Depending on the age of the patient at transplantation, the first clinical cardiovascular event will therefore appear only decades after transplantation.
Limitations of our analysis merit consideration. First, our analysis is retrospective, and our control population might not be ideal. We used autologous HSCT as a control group. Considering the difference of risk factors between patients treated with autologous and allogeneic HSCT, long-term survivors after autologous transplantation were more similar to a general population and seem therefore to be an adequate control population. Second, because of a significant difference between the autologous and allogeneic patient groups in respect to the age at transplantation and duration of follow-up, we used multivariate time-to-event models to compensate for these differences. In this regard, we believe that the methods developed here are of value to address assessment of the incidence and the risk factors for an arterial event after HSCT.
In conclusion, these data show that long-term survivors after allogeneic HSCT are at risk for cardiovascular disease of any arterial territory, as compared with patients treated with autologous transplantation. We hypothesize that allogeneic HSCT accelerates a preexisting predisposition and increases lifetime risk of cardiovascular disease by favoring the development or accentuation of established cardiovascular risk factors. These results should promote efforts in education, screening, and treatment for preventing late cardiovascular diseases in patients treated with allogeneic stem cell transplantation. Early recognition and management of hypertension, diabetes, and dyslipidemia become a mandatory part of management in allogeneic HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Hannelore Löwel, SF National Research Center for Environment and Health, Neuherberg, Germany; and Prof Felix Gutzwiller, Institute for Social and Preventive Medicine, Zürich, Switzerland, for their helpful advice concerning epidemiological aspects of the study.
This work was supported by the Swiss National Research Foundation 320B0–106105/1 and the Horton foundation.
Authorship
Contribution: Conception and design: A.T., C.B., A.R., B.B., J.R.P., and A.G. Analysis and interpretation of the data: A.T., J.R.P., M.S., and A.G. Drafting of the article: A.T. and A.G. Critical revision of the article for important intellectual content: A.T, C.B., A.R, G.S. M.S., S.M.-M., D.T., B.B., J.R.P., and A.G. Final approval of the article: A.T., C.B., A.R., G.S., M.S., M.P., J.H., S.M.M., D.H., D.T., B.B., J.R.P., and A.G. Provision of study materials or patients: J.H., D.H., and M.P. Statistical expertise: J.P., G.S., and M.S. Collection and assembly of data: C.B., A.R., and A.T.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof André Tichelli, Division of Hematology, University Hospital, Petersgraben 4, CH-4031 Basel, Switzerland; e-mail: tichelli@datacomm.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal