Abstract
Pulmonary complication in severe Plasmodium falciparum malaria is manifested as a prolonged impairment of gas transfer or the more severe acute respiratory distress syndrome (ARDS). In either clinical presentation, vascular permeability is a major component of the pathologic process. In this report, we examined the effect of clinical P falciparum isolates on barrier function of primary dermal and lung microvascular endothelium in vitro. We showed that parasite sonicates but not intact infected erythrocytes disrupted endothelial barrier function in a Src-family kinase–dependent manner. The abnormalities were manifested both as discontinuous immunofluorescence staining of the junctional proteins ZO-1, claudin 5, and VE-cadherin and the formation of interendothelial gaps in monolayers. These changes were associated with a loss in total protein content of claudin 5 and redistribution of ZO-1 from the cytoskeleton to the membrane and the cytosolic and nuclear fractions. There was minimal evidence of a proinflammatory response or direct cellular cytotoxicity or cell death. The active component in sonicates appeared to be a merozoite-associated protein. Increased permeability was also induced by P falciparum glycophosphatidylinositols (GPIs) and food vacuoles. These results demonstrate that parasite components can alter endothelial barrier function and thus contribute to the pathogenesis of severe falciparum malaria.
Introduction
Normal endothelial barrier integrity is maintained by organized tight and adherens junctions that restrict the lateral diffusion of membrane lipids and proteins and the paracellular exchange of solutes.1 Adherens and tight junctions are composed of cytoplasmic and transmembrane proteins that assemble to form homotypic or heterotypic complexes between corresponding proteins on adjacent cells. These 2 types of junctions are found to be distinct within barriers possessing high integrity, such as in endothelial cells of the blood-brain barrier and intestinal epithelium, whereas they colocalize in dermal microvascular endothelial cells. In general, tight junctions are composed of the zona occludens family of proteins, which anchor claudins, occludins, and junctional adhesion molecules (JAMs) to the cytoskeleton primarily through PDZ (post-synaptic density-95, disc large, zonula occludens-1) domains. Adherens junctions are composed of cadherins that are anchored to the cytoskeleton by catenins. Formation of both types of junctions depends on the expression of various adherens and tight junction proteins and intracellular signaling processes that regulate their organization.2 When these junctions are disrupted, gaps may appear in the endothelium, resulting in increased vascular permeability.
Two of the most severe clinical complications of Plasmodium falciparum malaria are cerebral malaria and noncardiogenic pulmonary edema or acute respiratory distress syndrome (ARDS).3 Much attention has been focused on the possible role of vascular permeability in the etiology of cerebral malaria,4 even though the occurrence of significant edema has never been consistently demonstrated in adult or pediatric patients.5-7 Functional studies in acutely infected patients show minimal changes in blood-brain barrier integrity as indicated by albumin and immunoglobulin flux in some but not all patients with severe falciparum malaria.8,9 Together with the immunohistochemical finding that suggested an absence of junctional protein staining in microvessels containing infected red blood cells (IRBCs) in postmortem brain tissues,9,10 the loss of endothelial barrier integrity was thought to be a local rather than a generalized phenomenon. However, no associated local leakage of plasma proteins such as albumin, fibrinogen, or IgG around microvessels with IRBCs was consistently observed. In the pediatric population, interpretation of histologic results is confounded by the significant percentage of African children with intercurrent infections at autopsy.11
In comparison, edema that results from increased pulmonary capillary permeability is an integral component of the pulmonary manifestations of malaria in adults.12 ARDS is a major prognostic determinant in both African and Western adults and is associated with a high fatality rate of 60% to 70% in the absence of ventilatory support.13,14 Furthermore, impairment of gas exchange at the alveolar-capillary interface occurs in patients with severe falciparum malaria even in the absence of full-blown ARDS,15 and the abnormality can persist for as long as 2 weeks. The onset of ARDS commonly occurs a few days after the start of treatment, when parasitemia and systemic proinflammatory cytokines have decreased significantly or the infection has cleared. The etiology of the protracted pulmonary complication of severe falciparum ma-laria is currently unclear. It has been postulated that endothelial cell injury could occur as a result of a persistent local inflammatory response. The possibility that parasite components could have a direct effect on endothelial dysfunction has not been systematically investigated.
In the present study, we examined the direct effect of P falciparum components from clinical parasite isolates on the endothelial barrier integrity of primary dermal and lung microvascular endothelial cells in vitro. We show that crude parasite sonicates induced an increase in endothelial permeability in a threshold- and time-dependent manner. The increase in permeability was associated with alterations in both the morphology and the loss or redistribution of tight and adherens junctional proteins from the cytoskeleton. The effect was mediated through Src-family kinases that are known to play an important role in the regulation of endothelial barrier function. The active component in parasite sonicates was found to be a proteinase K– and trypsin-sensitive protein(s) associated with intact merozoites. Increased endothelial leakage was also induced by purified P falciparum glycophosphatidylinositols (GPIs) and food vacuoles but not purified or synthetic hemozoin. These results demonstrate that parasite products can directly alter the integrity of endothelial junctional complexes and thus may contribute to the pathologic processes in the lungs in severe falciparum malaria.
Patients, materials, and methods
The collection of blood from patients with acute P falciparum malaria at the Hospital for Tropical Diseases, Bangkok, Thailand, was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Informed consent was obtained in accordance with the Declaration of Helsinki. The collection of discarded human foreskins was approved by the Conjoint Ethics Board of the Calgary Health Region and University of Calgary, Calgary, AB.
Parasite sonicates
Infected erythrocytes (IRBCs) of clinical isolates16 containing schizonts were lysed with 0.15% saponin in PBS at 4°C. Released parasites were washed and resuspended at 108 parasites/mL in PBS. The parasite suspension was sonicated for 5 × 2 second intervals at 100 W (Braunsonic 1510; B. Braun Biotech, Allentown, PA) and stored at −80°C until use. At the time of stimulation, sonicates were thawed in a bath sonicator (Branson 2200; Branson Ultrasonic, Danbury, CT) at 4°C for 10 minutes before addition to endothelial monolayers. The concentration of sonicates was expressed as parasite equivalents/cm2. Sonicates from uninfected erythrocytes were prepared by an identical method.
P falciparum merozoites and food vacuoles
P falciparum 3D7 strain was cultured at 1% hematocrit to 30% to 40% parasitemia. After schizont burst, the culture was centrifuged at 210g at room temperature for 10 minutes. The supernatant containing merozoites, food vacuoles, and parasite membrane fragments was centrifuged at 790g for 10 minutes. The pellet was suspended in PBS and fractionated on cushions of 30%, 45%, 60%, and 90% Percoll. The merozoites and food vacuoles present in the layers on the top of the 30% and 45% Percoll cushions, respectively, were collected and refractionated on Percoll cushions and finally washed 2 times with endotoxin-free PBS (D.C.G., unpublished observation, September 2006). Microscopic examination of Giemsa-stained thin smears indicated that merozoites were more than 95% pure with occasional presence of food vacuoles, and food vacuoles were more than 98% pure (D.C.G., unpublished observation, September 2006). The identity and intactness of the purified merozoites and food vacuoles were confirmed by immunofluorescence using antibodies against the merozoite surface protein MSP-1 (anti–MSP-1.1, MR4; ATCC, Manassas, VA) and the homologue of multidrug resistance protein (anti-mdr; kind gift of A. Cowman).17 Merozoites and food vacuoles were resuspended to 50% (vol/vol) in endotoxin-free PBS. Each microliter of merozoites contained approximately 25 μg of protein.
P falciparum glycophosphatidylinositols (Pf-GPIs)
Isolation of GPIs from the P falciparum FCR-3 strain was performed as previously described.18 For incubation with cells, GPI was dissolved to 0.2 to 1 μg/mL in ethanol, water, 1-propanol (78, 20, 2; vol/vol/vol).
P falciparum hemozoin
Transwell permeability assay
Endothelial cells were grown in 12-mm Costar polyester transwells with pore size of 0.4 μm (Corning Life Sciences, Wilkes Barre, PA). The top and bottom wells contained 0.5 mL and 1.5 mL of medium, respectively. Transendothelial resistance (TER) was monitored using the Endohm voltohmeter (World Precision Instruments, Sarasota, FL). After baseline TER was measured, 100 μL of medium from the top well was replaced by the stimulating agent in PBS. Following incubation and final TER measurement, culture medium in the top well was replaced with 0.5 mL of fluorescein isothiocyanate (FITC)–labeled albumin (250 μg/mL) for 4 hours. Duplicate 20-μL and 200-μL aliquots from the top and bottom wells, respectively, were taken into wells of a 96-well plate for determination of fluorescence by a spectrophotometer (Viktor 1420; Wallac, PerkinElmer, Waltham, MA). Percentage permeability was calculated according to the following formula: % permeability = *fluorescencebottom/(*fluorescencebottom + *fluorescencetop). *Fluorescence was corrected for volume.
Immunofluorescence microscopy
Human dermal microvascular endothelial cells (HDMECs) were seeded on glass coverslips at similar densities and for similar durations as for the transwell assays. For the detection of claudin 5 and VE-cadherin, coverslips were fixed with 100% methanol for 10 minutes at 4°C. Coverslips for staining for ZO-1 and F-actin were fixed in 1% paraformaldehyde for 30 minutes at 4°C, followed by permeabilization with 0.5% Triton X-100. Cy3 (red)- or Alexa-488 (green)–labeled secondary antibodies were used for fluorescence detection. All images (Figures 2A,B, 4B, 5C, 6D, S1, S2) were acquired using Openlab 5.0.2 (Improvision, Lexington, MA) on an Olympus IX70-S8F2 inverted microscope (Center Valley, PA) using a cooled charge-coupled device (CCD) Retiga EXi camera from Q Imaging (Vancouver, BC, Canada). Image analysis was performed using the ImageJ software version 1.34r (National Institutes of Health, Bethesda, MD). All images were taken with a 60× 1.40 oil objective where each field is equal to 224.2 × 170.8 μm (1360 × 1036 pixels). Surface area of endothelial cells was calculated by tracing outlines of endothelial cells based on ZO-1 staining from 10 adjacent cells of at least 2 fields in duplicate coverslips of each experiment and expressed as μm2. Gap formation was quantitated by determining the area of interendothelial gaps between adjacent cells.
Subcellular fractionation and Western blot of junctional proteins
Detection of tight junction and adherens junction proteins was performed by Western blotting of whole-cell lysates from HDMECs grown in 35-mm tissue-culture plates. Separation of junctional proteins into cytosolic, membrane, nuclear, and cytoskeletal fractions was performed using the ProteoExtract Subcellular Proteome Extraction Kit from Calbiochem (San Diego, CA) according to the manufacturer's instructions. Protein in each fraction was concentrated by trifluoroacetic acid (TCA) precipitation, solubilized in 1 M Tris-base and Laemmli sample buffer (1:1) in a water-bath sonicator. The localization of ZO-1, claudin 5, and VE-cadherin in the fractions was analyzed by Western blot.
Statistical analysis
All data are presented as mean plus or minus SD. Data from control and treated cells were compared by Student t test for paired samples. A P value of .05 or less was considered statistically significant. Levels of significance are denoted as follows: *P < .05, **P < .01, and ***P < .001. For multiple comparisons, analysis of variance (ANOVA) followed by post hoc analysis with Tukey test was used. Levels of significance are denoted as follows: †P < .05, ††P < .01, and †††P < .001. Please also see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) for additional information.
Results
P falciparum sonicates increased endothelial permeability
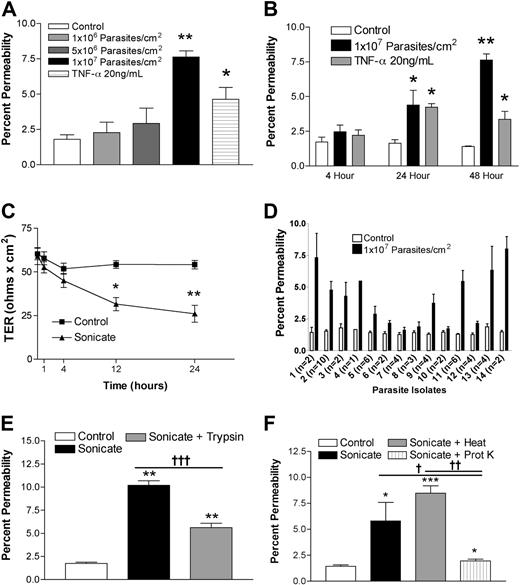
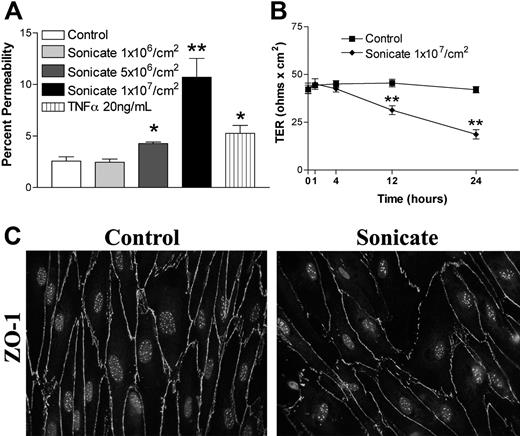
HDMECs were seeded in transwells at 105 cells/cm2. Cells were stimulated 3 days after confluence when TER was consistently maximal and maintained for at least a further 3 days. Sonicates from 4 clinical parasite isolates were added to the upper chamber of transwells at a concentration of 106 to 107 parasite equivalents/cm2, and incubation was continued for 4, 12, 24, and 48 hours. Figure 1A,B show that a concentration of 107 parasite equivalents/cm2 was associated with a significant increase in the flux of FITC-labeled albumin at 24 hours and 48 hours. There was a corresponding decrease in TER over the same time period (Figure 1C). Sonicates from uninfected erythrocytes were inactive. Based on these results, all subsequent experiments were performed with 107 parasite equivalents/cm2 for 24 hours. The percentage change in endothelial permeability varied among different clinical parasite isolates (n = 14) and was not due to differences in loading as determined by protein or hemozoin content (Figure 1D and data not shown). The permeability-enhancing activity of sonicates of various parasite isolates was remarkably consistent on different endothelial cell preparations, suggesting that it is an intrinsic property of the parasite isolate. The ability of sonicates to disrupt barrier integrity was reduced after digestion overnight with trypsin (Figure 1E) or proteinase K (n = 3; Figure 1F). Upon centrifugation at 16 000g for 15 minutes, the permeability-enhancing activity was found to reside mainly in the pellet fraction, and it was active only when applied to the apical and not the basolateral surface of endothelial cells. Finally, heating at 95°C for 15 minutes had no effect on the activity of sonicates. These results suggest that the active component of sonicates is an insoluble heat-stable component composed, at least partially, of protein(s).
Changes in endothelial permeability induced by P falciparum sonicates in HDMECs. HDMECs (105/cm2) were seeded in transwells and cultured until 3 days after confluence. (A) Increasing concentrations of parasite sonicates were added for 24 hours. An increase in the flux of FITC-albumin was observed at a threshold of 107 parasite equivalents/cm2. (B) Time course of changes in FITC-albumin flux of HDMEC monolayers incubated with 107 parasite equivalents/cm2. A significant increase in permeability was detected at 24 hours and maintained for at least 48 hours. No response was observed at 4 hours. (C) Time course of changes in transendothelial resistance (TER) of endothelial monolayers incubated with 1 107 parasite equivalents/cm2. A significant decrease in TER was observed at 12 and 24 hours. Experiments in panels A to C were performed with 4 parasite isolates. (D) Variable permeability as determined by FITC-albumin flux of 14 clinical parasite isolates at 107 parasite equivalents/cm2 for 24 hours. Parasite sonicate activity was significantly reduced after overnight (16 h) treatment with (E) trypsin (10 μg/mL) or (F) proteinase K (10 U/mL; n = 3). *P < .05; **P < .01 compared with control values by Student paired t test. For multiple comparisons †P < .05, ††P < .01, and †††P < .001 by ANOVA with post hoc analysis by Tukey test. (A-F) Results are expressed as mean (± SD).
Changes in endothelial permeability induced by P falciparum sonicates in HDMECs. HDMECs (105/cm2) were seeded in transwells and cultured until 3 days after confluence. (A) Increasing concentrations of parasite sonicates were added for 24 hours. An increase in the flux of FITC-albumin was observed at a threshold of 107 parasite equivalents/cm2. (B) Time course of changes in FITC-albumin flux of HDMEC monolayers incubated with 107 parasite equivalents/cm2. A significant increase in permeability was detected at 24 hours and maintained for at least 48 hours. No response was observed at 4 hours. (C) Time course of changes in transendothelial resistance (TER) of endothelial monolayers incubated with 1 107 parasite equivalents/cm2. A significant decrease in TER was observed at 12 and 24 hours. Experiments in panels A to C were performed with 4 parasite isolates. (D) Variable permeability as determined by FITC-albumin flux of 14 clinical parasite isolates at 107 parasite equivalents/cm2 for 24 hours. Parasite sonicate activity was significantly reduced after overnight (16 h) treatment with (E) trypsin (10 μg/mL) or (F) proteinase K (10 U/mL; n = 3). *P < .05; **P < .01 compared with control values by Student paired t test. For multiple comparisons †P < .05, ††P < .01, and †††P < .001 by ANOVA with post hoc analysis by Tukey test. (A-F) Results are expressed as mean (± SD).
Intact P falciparum–infected erythrocytes did not enhance endothelial permeability
To determine if coculture of intact IRBCs with HDMECs could disrupt endothelial barrier integrity, IRBCs at mid to late trophozoite stages from 3 clinical isolates that have been shown to adhere to HDMECs were purified on a Percoll gradient and added at 0.2%, 1.0%, and 5% hematocrit and 50% parasitemia (ie, 0.5 × 107, 2.5 × 107, or 1.25 × 108 parasites/cm2) to the upper chamber of transwells with 3-day post-confluent HDMECs in RPMI-1640 medium and 5% normal human blood group type AB serum. Normal erythrocyte (NRBC) suspensions at hematocrits identical to those for IRBCs were used for negative controls. The results show that there was no significant change in FITC-albumin flux (Figure S1A) or TER (Figure S1B) after the addition of IRBCs for 1 to 24 hours. Blood smears of IRBCs taken from duplicate transwells revealed that the parasites had matured normally in the coculture (Figure S1C,D).
Morphologic changes in endothelium associated with increased endothelial permeability
Increases in endothelial permeability can result from at least 2 processes that are not mutually exclusive, namely cell death and changes in junctional proteins. We first looked for evidence of cell death through either apoptosis or necrosis by flow cytometry. The percentage of cells positive for annexin V and propidium iodide and annexin V alone were similar in control and cells incubated with parasite sonicates (10.28% ± 3.14% vs 10.61% ± 2.32% and 6.63% ± 2.02% vs 6.35% ± 2.43%, respectively; n = 5). Similarly, there was no evidence of cytotoxicity by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (optical density [OD] at 490 nm: 0.497 ± 0.162 vs 0.469 ± 0.208 for control cells and cells incubated with parasite sonicates, respectively; n = 3).
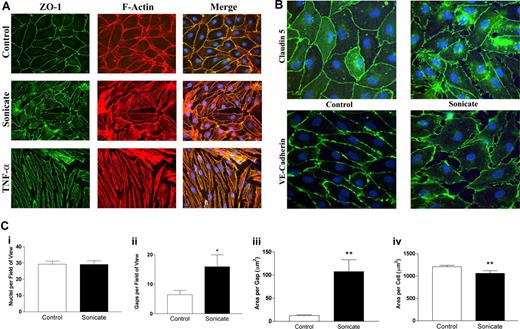
In contrast to the absence of cellular toxicity and cell death, significant morphologic changes were noted in both tight junction and adherens junction proteins by immunofluorescence microscopy. Whereas control cells stained evenly for zona-occludens 1 (ZO-1) in cortical rings at sites of cell-cell junctions that colocalized with filamentous F-actin stained with rhodamine-labeled phalloidin (Figure 2A), cells incubated with parasite sonicates displayed discontinuous staining for the protein and considerable gap formation. As well, there was a redistribution of ZO-1 from the cytoskeleton to the cytosol. Disruption of cortical staining for the tight junction protein claudin 5 and adherens junction protein VE-cadherin was also seen (Figure 2B). The above changes were distinct from the cell contraction and stress fiber formation induced by TNF-α (Figure 2A).
Morphologic changes in tight and adherens junctions proteins induced by P falciparum sonicates in HDMECs. HDMECs (105) were seeded on gelatin-coated glass coverslips of surface area 1 cm2 and incubated until 3 days after confluence. Parasite sonicates were added at 107 parasite equivalents/cm2 as for transwell assays. At the end of 24 hours, coverslips were washed in HBSS and fixed in 1% paraformaldehyde at 4°C for 30 minutes. Coverslips for staining of ZO-1 and F-actin were permeabilized with 0.5% Triton X-100. For detection of VE-cadherin, fixed cells were permeabilized with 100% methanol. (A) Staining for the tight junction protein ZO-1 (green) and F-Actin (red). In control monolayers, staining was continuous with occasional gap formation. ZO-1 colocalized with the F-actin cytoskeleton. In monolayers incubated with parasite sonicates, there was discontinuous staining of ZO-1 and increased gap formation. The overlay shows increased cytosolic ZO-1 staining as well as loss of association of ZO-1 with the F-actin cytoskeleton. These changes differed from the extensive stress fiber formation and cell contraction seen in TNF-α–stimulated monolayers. (B) Discontinuous staining of the tight junction protein claudin 5 and adherens junction protein VE-cadherin following incubation with parasite sonicates. Results in panels A and B are representative of 3 independent experiments. (C) Quantitation of morphologic changes shown in panel A by ImageJ software (n = 6). (i) Number of viable nuclei per field of view. (ii) Number of gaps between adjacent endothelial cells. (iii) Size of area per gap. (iv) Surface area of each endothelial cell. *P < .05, **P < .01 compared with control values by Student paired t test. (C) Results are expressed as mean (± SD).
Morphologic changes in tight and adherens junctions proteins induced by P falciparum sonicates in HDMECs. HDMECs (105) were seeded on gelatin-coated glass coverslips of surface area 1 cm2 and incubated until 3 days after confluence. Parasite sonicates were added at 107 parasite equivalents/cm2 as for transwell assays. At the end of 24 hours, coverslips were washed in HBSS and fixed in 1% paraformaldehyde at 4°C for 30 minutes. Coverslips for staining of ZO-1 and F-actin were permeabilized with 0.5% Triton X-100. For detection of VE-cadherin, fixed cells were permeabilized with 100% methanol. (A) Staining for the tight junction protein ZO-1 (green) and F-Actin (red). In control monolayers, staining was continuous with occasional gap formation. ZO-1 colocalized with the F-actin cytoskeleton. In monolayers incubated with parasite sonicates, there was discontinuous staining of ZO-1 and increased gap formation. The overlay shows increased cytosolic ZO-1 staining as well as loss of association of ZO-1 with the F-actin cytoskeleton. These changes differed from the extensive stress fiber formation and cell contraction seen in TNF-α–stimulated monolayers. (B) Discontinuous staining of the tight junction protein claudin 5 and adherens junction protein VE-cadherin following incubation with parasite sonicates. Results in panels A and B are representative of 3 independent experiments. (C) Quantitation of morphologic changes shown in panel A by ImageJ software (n = 6). (i) Number of viable nuclei per field of view. (ii) Number of gaps between adjacent endothelial cells. (iii) Size of area per gap. (iv) Surface area of each endothelial cell. *P < .05, **P < .01 compared with control values by Student paired t test. (C) Results are expressed as mean (± SD).
Quantitation of the changes in immunofluorescence was performed using ZO-1 as the representative protein (n = 6; Figure 2C). Compared with controls, incubation of HDMECs with parasite sonicates did not result in a difference in the number of intact nuclei but showed a significant increase in the number and size of interendothelial gaps. Consistent with these findings, the surface area of endothelial cells decreased without a concommitant change in cell volume as seen in forward scatter by flow cytometry (data not shown), suggesting that disruption of junctional proteins may result in a retraction of endothelial cells.
To confirm that sonicates did not induce the formation of stress fibers in stimulated cells, monolayers were pretreated with the myosin light-chain kinase inhibitor ML-7 at 10 μM for 30 minutes before the addition of sonicates. The inhibitor had no effect on the increase in permeability induced by parasite sonicates (control = 1.67% ± 0.49%, sonicate = 6.46% ± 2.34%, and sonicate + ML-7 = 5.59% ± 1.90%; n = 3). Cytochalasin D (1 μmol), an inhibitor of actin polymerization, also did not inhibit increases in permeability (control = 2.21% ± 0.26%, sonicate = 6.13% ± 3.68%, and sonicate + cytochalasin D = 6.55% ± 4.08%; n = 3). Together, these results indicate that the changes in permeability induced by parasite sonicates are independent of cytoskeletal remodeling or cell death.
P falciparum sonicates alter subcellular location of tight and adherens junction proteins
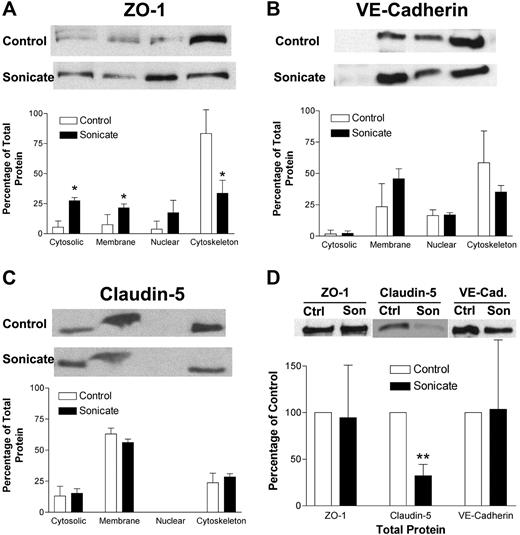
The morphologic changes in junctional proteins seen by immunofluorescence microscopy could be due to several mechanisms. There could be a reduction in total junctional proteins in HDMECs incubated with parasite sonicates, or the proteins could be redistributed to a different subcellular compartment. These possibilities were examined by Western blotting of total cell lysates and subcellular fractionation. A representative Western blot and densitometry analysis of 3 independent experiments for ZO-1 (Figure 3A), VE-cadherin (Figure 3B), and claudin 5 (Figure 3C) are shown. In unstimulated cells, ZO-1 was mainly associated with the actin cytoskeleton; VE-cadherin was associated with the membrane, nuclear, and cytoskeletal fractions; whereas claudin 5 was seen in all except the nuclear fraction. In cell lysates from HDMECs incubated with parasite sonicates, ZO-1 became more evenly distributed to all 4 fractions, indicating a loss of association with the actin cytoskeleton. Redistribution was not observed for VE-cadherin or claudin 5. In the assessment for total protein, only total claudin 5 protein was reduced following incubation with parasite sonicates (Figure 3D). These findings suggest that the disruption of junctional proteins was associated with protein degradation and redistribution away from cytoskeletal pools.
Biochemical alterations of tight and adherens junction proteins induced by P falciparum sonicates in HDMECs. (A-C) HDMECs were cultured in 35-mm dishes until 3 days after confluence with or without parasite sonicates at 107 parasite equivalents/cm2 for 24 hours. Total cell lysates were fractionated into 4 subcellular fractions: cytosolic, membrane, nuclear, and cytoskeleton (from left to right). Fractions were analyzed by Western blot for the indicated tight and adherens junction proteins. (A) ZO-1 but not (B) VE-cadherin or (C) claudin-5 was redistributed from the cytoskeleton to other subcellular fractions. (D) HDMECs were treated as in panel A. Total protein level as determined by Western blot was reduced for claudin-5 but not for ZO-1 or VE-cadherin using β-actin as a loading control. Representative Western blot results and densitometry analysis of 3 independent experiments are shown. For densitometric analysis of subcellular fractions, each fraction was expressed as a percentage of the total densitometry measurements for all 4 fractions. *P < .05, **P < .01 compared with control values by Student paired t test. (A-D) Results are expressed as mean (± SD).
Biochemical alterations of tight and adherens junction proteins induced by P falciparum sonicates in HDMECs. (A-C) HDMECs were cultured in 35-mm dishes until 3 days after confluence with or without parasite sonicates at 107 parasite equivalents/cm2 for 24 hours. Total cell lysates were fractionated into 4 subcellular fractions: cytosolic, membrane, nuclear, and cytoskeleton (from left to right). Fractions were analyzed by Western blot for the indicated tight and adherens junction proteins. (A) ZO-1 but not (B) VE-cadherin or (C) claudin-5 was redistributed from the cytoskeleton to other subcellular fractions. (D) HDMECs were treated as in panel A. Total protein level as determined by Western blot was reduced for claudin-5 but not for ZO-1 or VE-cadherin using β-actin as a loading control. Representative Western blot results and densitometry analysis of 3 independent experiments are shown. For densitometric analysis of subcellular fractions, each fraction was expressed as a percentage of the total densitometry measurements for all 4 fractions. *P < .05, **P < .01 compared with control values by Student paired t test. (A-D) Results are expressed as mean (± SD).
Role of Src-family kinases in P falciparum–induced endothelial permeability
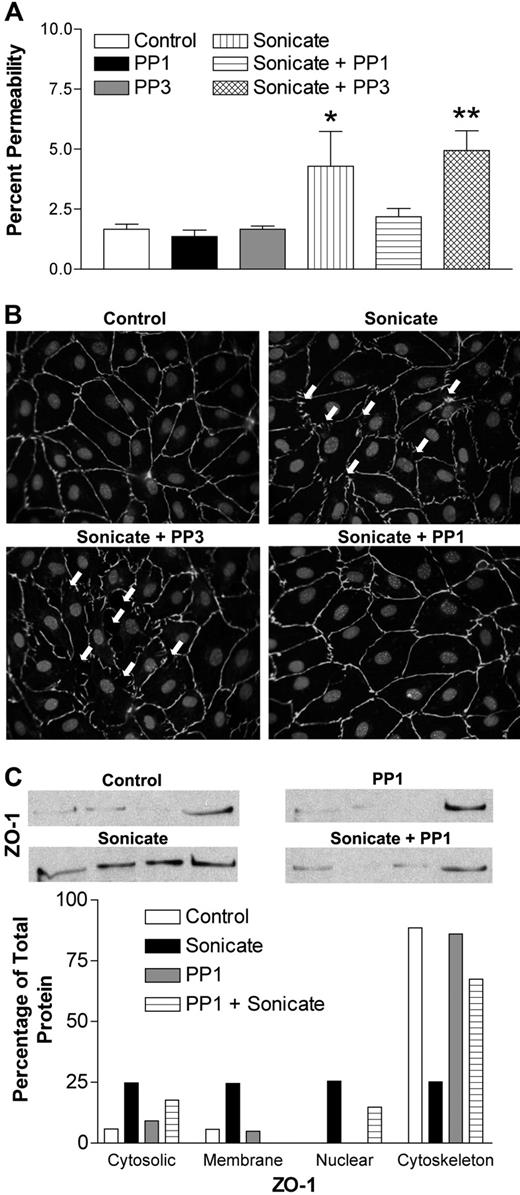
Src-family kinases are known to play a pivotal role in the regulation of endothelial permeability through phosphorylation of junctional proteins, which in turn regulates the stability of junctional complexes.21 To explore the possible involvement of Src-family kinases in the permeability-inducing effect of parasite sonicates, endothelial monolayers were stimulated in the presence of the selective inhibitor PP1 or its inactive analog PP3. The results show that 10 μM PP1 abrogated the effect of sonicates on FITC-albumin flux (Figure 4A). The inactive analog PP3 had no effect. Moreover, monolayers pretreated with PP1 before incubation with parasite sonicates retained the normal staining pattern of cellular junctions (Figure 4B). Prestimulation with PP1 also prevented ZO-1 redistribution from cytoskeletal fractions (Figure 4C). Similar inhibitory effects were not observed in cells pretreated with various concentrations of Rho kinase or pan–phosphate kinase C inhibitors (data not shown), suggesting that these pathways downstream of Src-family kinases were not involved in the permeability-inducing activity of sonicates.
Role of Src-family kinases in endothelial permeability induced by P falciparum sonicates in HDMECs. HDMECs in transwells were either untreated or pretreated for 30 minutes at 37°C with the selective Src-family kinase inhibitor PP1 and its inactive analog PP3 (10 μM). (A) PP1 but not PP3 abrogated the changes in FITC-albumin flux induced by parasite sonicates (n = 4). (B) PP1 but not PP3 inhibited the discontinuous staining for ZO-1 and gap formation induced by parasite sonicates. Arrows indicate the presence of interendothelial gaps. (C) PP1 inhibited the redistribution of ZO-1 from the cytoskeleton. Microscopy and Western blot results are representative of 4 experiments. For densitometric analysis of subcellular fractions, each fraction was expressed as a percentage of the total densitometry measurements for all 4 fractions. *P < .05, **P < .01 compared with control values by Student paired t test. (A) Results are expressed as mean (± SD).
Role of Src-family kinases in endothelial permeability induced by P falciparum sonicates in HDMECs. HDMECs in transwells were either untreated or pretreated for 30 minutes at 37°C with the selective Src-family kinase inhibitor PP1 and its inactive analog PP3 (10 μM). (A) PP1 but not PP3 abrogated the changes in FITC-albumin flux induced by parasite sonicates (n = 4). (B) PP1 but not PP3 inhibited the discontinuous staining for ZO-1 and gap formation induced by parasite sonicates. Arrows indicate the presence of interendothelial gaps. (C) PP1 inhibited the redistribution of ZO-1 from the cytoskeleton. Microscopy and Western blot results are representative of 4 experiments. For densitometric analysis of subcellular fractions, each fraction was expressed as a percentage of the total densitometry measurements for all 4 fractions. *P < .05, **P < .01 compared with control values by Student paired t test. (A) Results are expressed as mean (± SD).
P falciparum sonicates increase endothelial permeability of lung endothelial cells
To determine the relevance of our findings to the major organ affected by an increase in vascular permeability in severe falciparum malaria, namely the lung, we studied the effect of parasite sonicates on FITC-albumin flux (Figure 5A), TER (Figure 5B), and the staining for ZO-1 (Figure 5C) in primary lung blood microvascular endothelial cells. Our results confirmed that similar changes in all 3 parameters occurred in these cells as in HDMECs.
Changes in endothelial permeability induced by P falciparum sonicates in primary HLMECs. Permeability of human lung microvascular endothelial cells (HLMECs) was increased following incubation with parasite sonicates at 107 parasite equivalents/cm2 for 24 hours as determined by (A) the flux of FITC-albumin and (B) transendothelial resistance (n = 4). (C) Morphologic changes in ZO-1 staining induced by parasite sonicates as described above for HDMECs (Figure 1A) were also seen in HLMECs. *P < .05, **P < .01 compared with control values by Student paired t test. (A,B) Results are expressed as mean (± SD).
Changes in endothelial permeability induced by P falciparum sonicates in primary HLMECs. Permeability of human lung microvascular endothelial cells (HLMECs) was increased following incubation with parasite sonicates at 107 parasite equivalents/cm2 for 24 hours as determined by (A) the flux of FITC-albumin and (B) transendothelial resistance (n = 4). (C) Morphologic changes in ZO-1 staining induced by parasite sonicates as described above for HDMECs (Figure 1A) were also seen in HLMECs. *P < .05, **P < .01 compared with control values by Student paired t test. (A,B) Results are expressed as mean (± SD).
The active component of P falciparum sonicates is associated with merozoite proteins
To further define the parasite components involved in the disruption of endothelial barrier integrity, merozoites and food vacuoles were harvested from mycoplasma-free 3D7 culture supernatants and fractioned on Percoll cushions. Similar to parasite sonicates, incubation of HDMECs with intact merozoites resulted in a threshold-dependent increase in FITC-albumin flux (Figure 6A) and TER (n = 4; Figure 6B). Treatment with trypsin and proteinase K resulted in a 50% and 90% decrease, respectively, in the activity of intact merozoites (Figure 6C). Furthermore, the morphologic changes seen in HDMECs incubated with intact merozoites were similar to those observed following incubation with sonicates (Figure 6D). In contrast, incubation with purified P falciparum food vacuoles resulted in an increase in endothelial permeability (n = 4) through the induction of extensive cell death, as indicated by the complete destruction of the endothelial monolayer and apoptotic nuclei (Figure S2A-C). These morphologic changes were completely different from those following incubation of HDMECs with either parasite sonicates or merozoites, as was the ability of food vacuoles to induce IL-6 (Table 1). Collectively, these results suggest that P falciparum proteins in merozoite-enriched fractions are a major inducer of increased permeability in endothelial cells.
Changes in endothelial permeability induced by purified P falciparum merozoite proteins. HDMECs (105) seeded in transwells were incubated until 3 days after confluence. Intact merozoites from the parasite line 3D7 were added for 24 hours. Endothelial permeability was increased in a threshold-dependent manner as determined by (A) the flux of FITC-albumin and (B) transendothelial resistance (n = 4.) (C) The activity of intact merozoites was inhibited by pretreatment of merozoites with proteinase K (10 U/mL) or trypsin (10 μg/mL) overnight (16 h; n = 4). (D) Morphologic changes in ZO-1 and VE-cadherin staining as described for parasite sonicates were also seen in HDMECs incubated with intact merozoites. Results are representative of 3 independent experiments. *P < .05, **P < .01 compared with control values by Student paired t test. For multiple comparisons †P < .05 by ANOVA with post hoc analysis by Tukey test. (A-C) Results are expressed as mean (± SD).
Changes in endothelial permeability induced by purified P falciparum merozoite proteins. HDMECs (105) seeded in transwells were incubated until 3 days after confluence. Intact merozoites from the parasite line 3D7 were added for 24 hours. Endothelial permeability was increased in a threshold-dependent manner as determined by (A) the flux of FITC-albumin and (B) transendothelial resistance (n = 4.) (C) The activity of intact merozoites was inhibited by pretreatment of merozoites with proteinase K (10 U/mL) or trypsin (10 μg/mL) overnight (16 h; n = 4). (D) Morphologic changes in ZO-1 and VE-cadherin staining as described for parasite sonicates were also seen in HDMECs incubated with intact merozoites. Results are representative of 3 independent experiments. *P < .05, **P < .01 compared with control values by Student paired t test. For multiple comparisons †P < .05 by ANOVA with post hoc analysis by Tukey test. (A-C) Results are expressed as mean (± SD).
Production of IL-6 by HDMECs following incubation with P falciparum components
| Stimulant . | IL-6, pg/mL . | SEM . | n . |
|---|---|---|---|
| Experiment group 1 | |||
| Control | 0 | 0 | 6 |
| Pf-GPI, 1 μg/mL | 0 | 0 | 4 |
| IFN-γ, 20 ng/mL | 38.01 | 13.01 | 6 |
| IFN-γ + Pf-GPI | 1312.42 | 628.27 | 6 |
| IFN-γ + PAM3Cys4, 10 μg/mL* | > 20 000 | — | 3 |
| Experiment group 2 | |||
| Control | 0 | 0 | 11 |
| Pf sonicate, 1×107/cm2 | 27.92 | 7.57 | 11 |
| IFN-γ, 20 ng/mL | 0 | 0 | 4 |
| IFN-γ + Pf sonicate | 0 | 0 | 4 |
| Experiment group 3 | |||
| Control | 21.75 | 3.02 | 4 |
| Merozoite, 0.5 μL/cm | 51.38 | 10.47 | 4 |
| Experiment group 4 | |||
| Control | 0 | 0 | 4 |
| Pf food vacuole, 0.1 μL/cm2 | > 20 000 | — | 4 |
| Purified Pf hemozoin, 20 μg/cm2 | 0 | 0 | 4 |
| Stimulant . | IL-6, pg/mL . | SEM . | n . |
|---|---|---|---|
| Experiment group 1 | |||
| Control | 0 | 0 | 6 |
| Pf-GPI, 1 μg/mL | 0 | 0 | 4 |
| IFN-γ, 20 ng/mL | 38.01 | 13.01 | 6 |
| IFN-γ + Pf-GPI | 1312.42 | 628.27 | 6 |
| IFN-γ + PAM3Cys4, 10 μg/mL* | > 20 000 | — | 3 |
| Experiment group 2 | |||
| Control | 0 | 0 | 11 |
| Pf sonicate, 1×107/cm2 | 27.92 | 7.57 | 11 |
| IFN-γ, 20 ng/mL | 0 | 0 | 4 |
| IFN-γ + Pf sonicate | 0 | 0 | 4 |
| Experiment group 3 | |||
| Control | 21.75 | 3.02 | 4 |
| Merozoite, 0.5 μL/cm | 51.38 | 10.47 | 4 |
| Experiment group 4 | |||
| Control | 0 | 0 | 4 |
| Pf food vacuole, 0.1 μL/cm2 | > 20 000 | — | 4 |
| Purified Pf hemozoin, 20 μg/cm2 | 0 | 0 | 4 |
All HDMECs were incubated for 24 hours with the indicated components.
— indicates not applicable.
PAM3Cys4 is a synthetic TLR-2 ligand.
P falciparum GPIs alter endothelial permeability
Merozoite proteins are anchored by biologically active GPIs that are recognized by Toll-like receptor 2 (TLR2) on macrophages,22 and recognition of GPIs is facilitated by the proteins they anchor through micelle formation (D.C.G., unpublished observation, March 2007). To determine if Pf-GPIs alone can induce permeability, HDMECs were stimulated with 0.2 to 1 μg/mL of purified Pf-GPIs. Pf-GPIs at 1 μg/mL but not lower concentrations induced endothelial permeability (Figure 7A) and a drop in TER (n = 4; Figure 7B) in unprimed and IFN-γ–primed (20 ng/mL for 16 h) HDMEC monolayers. IFN-γ alone had a minor effect on permeability. However, there were several major differences between the effects of parasite sonicates and purified Pf-GPIs. First, Pf-GPI stimulation required priming with IFN-γ for maximum activity (Figure 7A), whereas no such effect was observed with IFN-γ and sonicate (Figure 7C). Second, incubation with parasite sonicates resulted in minimally detected IL-6 production in the supernatant, whereas Pf-GPIs at 0.5 to 1 μg/mL induced significant IL-6 production in the presence of IFN-γ (Table 1). Finally, an anti-TLR2 antibody that inhibited the response of HDMECs to the TLR2 ligand lipotechoic acid (Roxna Kapadia and M.H., unpublished observation, September 2006) did not have any effect on the activity of sonicates (n = 4; Figure 7D).
Changes in endothelial permeability induced by purified P falciparum GPIs.P falciparum GPIs from the parasite line FCR-3 at 1 μg/mL induced increased (A) FITC-albumin flux and (B) TER in unprimed and IFN-γ–primed (20 ng/mL x16 h) HDMECs (n = 4). The increase in permeability was significantly higher in primed cells. (C) IFN-γ did not enhance the effect of parasite sonicates (n = 5). (D) An inhibitory TLR2 antibody, a receptor for Pf-GPIs, had no effect on parasite sonicate activity (n = 4). *P < .05, **P < .01 compared with control values by Student paired t test. For multiple comparisons, †P < .05, †††P < .001 by ANOVA with post hoc analysis by Tukey test. (A-D) Results are expressed as mean (± SD).
Changes in endothelial permeability induced by purified P falciparum GPIs.P falciparum GPIs from the parasite line FCR-3 at 1 μg/mL induced increased (A) FITC-albumin flux and (B) TER in unprimed and IFN-γ–primed (20 ng/mL x16 h) HDMECs (n = 4). The increase in permeability was significantly higher in primed cells. (C) IFN-γ did not enhance the effect of parasite sonicates (n = 5). (D) An inhibitory TLR2 antibody, a receptor for Pf-GPIs, had no effect on parasite sonicate activity (n = 4). *P < .05, **P < .01 compared with control values by Student paired t test. For multiple comparisons, †P < .05, †††P < .001 by ANOVA with post hoc analysis by Tukey test. (A-D) Results are expressed as mean (± SD).
Lack of effect of hemozoin on endothelial permeability
Another parasite component that might contribute to disruption of endothelial barrier function is the heme polymer hemozoin. This malarial pigment was seen to be taken up into endothelial cells by transmission electron microscopy as previously reported23 and was initially thought to be the active component of parasite sonicates. However, purified or synthetic hemozoin (β-hematin) of up to 50 μg/cm2 did not produce a consistent increase in endothelial permeability (n = 4; Figure S4A,B). This concentration was well above the mean hemozoin content of parasite sonicates (7 ± 4 μg/cm2, n = 10). More importantly, the permeability-inducing effect of parasite sonicates was not affected by treatment of the endothelial cells with chloroquine (n = 3; Figure S4C), which suggests that the effect was not mediated by the direct stimulation of TLR9 by hemozoin19 or its presentation of malarial DNA to TLR9.24
Acute patient plasma induces endothelial permeability
To determine if permeability-inducing activity is detectable in the circulation of patients with severe falciparum malaria on admission, plasma from 17 patients was added to transwells at 1:10 dilution. A small but consistent increase in permeability was observed but not with plasma from 6 healthy controls (Table 2). Significant correlation of FITC-albumin flux with plasma levels of TNF-α, IL-6, and IL-10 was found. With 11 plasma samples taken 48 hours after admission, TER but not FITC-albumin flux was significantly altered. However, both measures of permeability were significantly different from control values when 8 of the 11 plasma samples were tested at 1:5 dilution (Table 2).
Effect of acute plasma on endothelial permeability
| Study group . | n . | Plasma sample, h following admission . | Dilution . | Cytokine levels in plasma samples, pg/mL . | HDMEC permeability following incubation with plasma for 24 h . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 . | IL-10 . | IL-1β . | IFN-γ . | TNF-α . | % Permeability . | % Change in TER . | ||||
| Severe malaria, median (IQR) | 17 | 0 | 1:10 | 450 (95-2950) | 1984 (1309-11641) | 0 (0-0) | 36 (21-139) | 0 (0-8) | 1.72† (1.55-2.0) | −8.92‡ (−14.64-(−)6.80) |
| Correlation to % permeability, r (P)* | 17 | 0 | 1:10 | 0.519 (.033) | 0.534 (.027) | −0.160 (.540) | −0.040 (.877) | 0.662(.004) | — | — |
| Severe malaria, median (IQR) | 11 | 48 | 1:10 | 100 (62.5-252.5) | 531 (387-1069) | ND | ND | ND | 1.47 (1.34-1.65) | -11.7† (−15.04-(−)2.43) |
| Correlation to % permeability, r (P)* | 11 | 48 | 1:10 | −0.151 (.658) | −0.4840 (.131) | — | — | — | — | — |
| Control, median (IQR) | 6 | — | 1:10 | ND | ND | ND | ND | ND | 1.40 (1.2-1.5) | 5.5 (1.5-7.2) |
| Severe malaria, median (IQR) | 8 | 48 | 1:5 | 83 (51-175) | 521 (339-718) | ND | ND | ND | 1.61† (1.43-2.33) | −13.2† (−28.5-(−)1.76) |
| Control, median (IQR) | 7 | — | 1:5 | ND | ND | ND | ND | ND | 1.24 (1.17-1.39) | 7.92 (8.43-14.3) |
| Study group . | n . | Plasma sample, h following admission . | Dilution . | Cytokine levels in plasma samples, pg/mL . | HDMEC permeability following incubation with plasma for 24 h . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 . | IL-10 . | IL-1β . | IFN-γ . | TNF-α . | % Permeability . | % Change in TER . | ||||
| Severe malaria, median (IQR) | 17 | 0 | 1:10 | 450 (95-2950) | 1984 (1309-11641) | 0 (0-0) | 36 (21-139) | 0 (0-8) | 1.72† (1.55-2.0) | −8.92‡ (−14.64-(−)6.80) |
| Correlation to % permeability, r (P)* | 17 | 0 | 1:10 | 0.519 (.033) | 0.534 (.027) | −0.160 (.540) | −0.040 (.877) | 0.662(.004) | — | — |
| Severe malaria, median (IQR) | 11 | 48 | 1:10 | 100 (62.5-252.5) | 531 (387-1069) | ND | ND | ND | 1.47 (1.34-1.65) | -11.7† (−15.04-(−)2.43) |
| Correlation to % permeability, r (P)* | 11 | 48 | 1:10 | −0.151 (.658) | −0.4840 (.131) | — | — | — | — | — |
| Control, median (IQR) | 6 | — | 1:10 | ND | ND | ND | ND | ND | 1.40 (1.2-1.5) | 5.5 (1.5-7.2) |
| Severe malaria, median (IQR) | 8 | 48 | 1:5 | 83 (51-175) | 521 (339-718) | ND | ND | ND | 1.61† (1.43-2.33) | −13.2† (−28.5-(−)1.76) |
| Control, median (IQR) | 7 | — | 1:5 | ND | ND | ND | ND | ND | 1.24 (1.17-1.39) | 7.92 (8.43-14.3) |
IQR indicates interquartile range; —, not applicable; and ND, not done.
Correlations between percentage permeability and each cytokine (r) were performed by Spearman rank sum test. P values (P) for each comparison are given in parentheses.
P < .05 compared with controls by Mann-Whitney U test.
P < .01 compared with controls by Mann-Whitney U test.
Discussion
Pulmonary edema as a result of increased vascular permeability is a cardinal feature of the impairment of lung function and ARDS in severe falciparum malaria.15,25 In this study, we show that P falciparum merozoite-associated proteins can contribute to the pathologic process by inducing alterations in junctional protein expression in primary microvascular endothelial cells in vitro, including those from the lung. These changes may serve to destabilize endothelial junctional complexes and allow the escape of macromolecules into the interstitium. Circulating plasmodial proteins have been detected for up to 2 weeks following an acute infection.26 Based on this paradigm, patients who develop ARDS would represent those with a higher load of parasite components secondary to a higher total parasite burden (not parasitemia) and/or impairment of clearance. The often-delayed onset of ARDS is not unique to falciparum malaria, as it is also known to occur in bacterial sepsis.27 In both types of infections, antimicrobial or antimalarial treatment may aggravate the situation by increasing the release of vasoactive components from the microorganisms. The occurrence of prolonged impairment of gas transfer recently demonstrated in patients with Plasmodium vivax infection would also be consistent with this hypothesis.28
A pathogenic role for parasite components in vivo is also supported by the small but reproducible effect on endothelial permeability of plasma collected on admission and at 48 hours. Of note, plasma from all 3 patients who died within the first 24 hours was among the most active. Although contribution from proinflammatory cytokines in vivo could not be excluded by these results, the levels of TNF-α and IL-6 present in the plasma samples, particularly after diluting 10-fold, are insufficient for inducing endothelial permeability in vitro.29 The incomplete correlation of permeability with cytokine levels further suggests that other factors present in the plasma might also be involved.
Disruption of junctional proteins was dependent on the activity of Src-family kinases that have been widely implicated in the negative regulation of endothelial barrier function. Human endothelial cells express 3 Src-family kinases: src, yes, and fyn. In VEGF-induced endothelial permeability, src exerts its effect via the phosphorylation and the subsequent dissociation of VE-cadherin from the cytoskeletal anchoring protein β-catenin.30 It has also been shown to mediate redistribution of ZO-1.31 VEGF-induced permeability further involves src-regulated RhoA/MLCK stress fiber formation as well as the activation of the αVβ5 integrin.32,33 In our experiments, inhibitors of both Rho kinases and MLCK had no effect on the permeability-enhancing effect of sonicates, consistent with the absence of cytoskeletal remodeling seen by immunofluorescence microscopy. The activity of TNF-α on vascular permeability is partially dependent on fyn,34 which suggests that different members of the Src-family kinases can negatively regulate endothelial permeability in response to a number of mediators.
The time delay of 4 to 12 hours required for the development of endothelial permeability in vitro suggests that either (i) a prolonged exposure to sonicates is required for the induction of endothelial permeability, or (ii) parasite components require time to settle sufficiently on the monolayers to exert their effect. In support of the latter, we observed hemozoin and other particulate matter suspended in the medium at 4 hours. Furthermore, removing the sonicates at 1 and 4 hours negated their effect on endothelial permeability measured at 24 hours (M.R.G., unpublished observation, July 2007). This situation is unlikely to occur in vivo, as schizogony occurs immediately adjacent to endothelium in microvessels that are of much smaller dimensions than the transwells. Once the parasite components become adherent, they do not appear to detach even under shear in our flow chamber system. The time lag could also be due to the requirement for a secondary mediator, as suggested by our preliminary observation that pretreatment of HDMECs with cycloheximide partially reversed the effect of sonicates. Candidate molecules that might affect endothelial integrity include chemokines, cytokines, and matrix metalloproteinases.2,35
Our results both contrasted with and significantly extended the findings of previous reports on endothelial permeability in response to P falciparum.36-38 Using transformed brain endothelium, increased endothelial permeability by intact IRBCs was found to be secondary to endothelial cell apoptosis that was dependent on prestimulation of endothelial monolayers with TNF-α and subsequent TGF-β production by adherent platelets.36,37 No effect was observed with IRBCs on unprimed endothelium in the absence of platelets. In our experiments, intact IRBCs also had no effect on endothelial barrier function of primary skin and lung cells, whereas the response to P falciparum sonicates occurred in the absence of TNF-α stimulation and/or platelets. There was no evidence of a robust proinflammatory response, direct cellular cytotoxicity, or cell death. More recently, decrease in transendothelial resistance of brain endothelial cells in response to intact IRBCs, lysates, and culture supernatant was reported.38 The effect for all preparations was early (peaked at 5 h) and transient (ie, recovery of normal barrier function within 20 h), whereas the effect of parasite sonicates in this study was found to be both delayed (12-24 h) and sustained (48 h). The discrepancy in results between the 2 studies could not be easily explained, as the exact nature of the endothelial cells used (primary versus transformed) was not specified, nor was data provided regarding the structural or biochemical changes induced in the endothelium; however, we do note a marked difference in the number of parasites added to endothelial monolayers. The 107 parasite equivalents/cm2 used in our experiments is much closer to the estimate of 4 × 106 parasites/cm2 in vivo based on an average diameter of 5 μm for an IRBC. The use of 108 parasites/0.8 cm2 as reported would have created a layer of IRBCs in the transwell that would be at least 10 cells thick or produced lysates of equally high concentrations. The importance of the IRBC-to-host cell ratio in coculture experiments on subsequent functional alterations was convincingly demonstrated in a recent study on the effect of IRBCs on dendritic cell maturation.39
The parasite components that induce the increase in permeability appear to be mainly protein in nature, found in merozites, and are insoluble or membrane bound. These parasite proteins (or membrane fragments containing proteins) are released during schizogony in close proximity to endothelial cells in the microcirculation where parasitemia has been reported to be 1.8- to 1500-fold (median 40) higher than in the periphery.40 Together with the impairment of microcirculatory blood flow, local concentration of biologically active products could be very high. Purified Pf-GPIs in the presence of IFN-γ also significantly enhanced endothelial permeability. However, they did not appear to be a major contributor to the effect of parasite sonicates. Since most P falciparum merozoite surface proteins are GPI anchored, the GPI moieties of anchored proteins may play an important role in presenting the proteins to the host cells. This suggestion is consistent with our preliminary observation that treatment of merozoites with trypsin leads to about 50% reduction in TLR2-mediated proinflammatory responses by macrophages, whereas purified merozoite surface proteins by themselves show little activity (D.C.G., unpublished observation, March 2007). Alternatively, fatty acids from P falciparum that might be present in the sonicates may down-regulate the proinflammatory activity of GPIs.41 Food vacuoles that contained reactive oxygen species, proteases, and hemoglobin breakdown products such as hemozoin were also shown to be a potent inducer of permeability. However, although biologic activities have been attributed to hemozoin,18,42,43 results with highly purified products in this study indicate that hemozoin by itself is quite inert with respect to endothelial barrier function.
In summary, we have demonstrated a potential pathogenic role for P falciparum merozoite proteins on vascular endothelium. This is in keeping with recent evidence that parasite molecules can mediate pathology independently of proinflammatory cytokines. For example, the parasite produces a homolog of human macrophage migration inhibitory factor (MIF) that has been shown to mediate the dyserythropoiesis associated with malarial anemia.44 Identification of parasite components that are directly linked to pathology in severe falciparum malaria will provide multiple targets for the development of an antidisease vaccine.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Caroline Lane (Valley View Family Practice Clinic, Calgary, AB) for providing skin specimens.
This work was supported by a grant (MT14104; M.H.) and a group grant (MGC-48374) from the Canadian Institutes of Health Research (CIHR) and a National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) grant (AI41139; D.C.G.). S.M.R. and M.H. are Scientists of the Alberta Heritage Foundation for Medical Research, Canada. M.R.G. is supported by a CIHR Training Grant in Immunity and Immunopathogenesis.
This paper is dedicated to the memory of Dr Sornchai Looareesuwan, whose untimely passing during the final stages of manuscript preparation saddened us all.
National Institutes of Health
Authorship
Contribution: M.R.G. designed and performed the research and analyzed the data. G.K. and D.C.G. contributed the parasite components and input into data interpretation. K.L. performed the research. A.G.B. and S.M.R. contributed to the design of the research. S.L. provided the parasite and plasma specimens. M.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: May Ho, Department of Microbiology and Infectious Diseases, 3330 Hospital Dr NW, Calgary, AB, Canada T2N 4N1; e-mail: mho@ucalgary.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal