Abstract
The orchestration of systemic immune responses is critically dependent on coordinated lymphocyte migration and recirculation. This “homing” guides lymphocytes to the microenvironments that control their differentiation and survival, disperses the immunologic repertoire, and targets effector lymphocytes to sites of antigenic insult. Lymphocyte homing is a multistep process that requires chemotaxis and cell adhesion coupled with strategies to overcome physical barriers. At the molecular level, it is regulated by adhesion molecules and chemokines, and facilitated by intrinsic molecular programs that allow “ameboid” shape change, allowing highly effective lymphocyte traffic between different tissue compartments. In case of malignant transformation, however, the fact that lymphocytes are “licensed to move” forms a serious threat to the organism, because it permits rapid tumor dissemination irrespective of the conventional anatomic boundaries limiting early spread in most types of cancer. Thus, unlike the metastatic spread of other cancers, lymphoma dissemination generally is not a reflection of tumor progression but of conserved physiological behavior. The dissemination patterns often reflect basic rules of lymphocyte homing, explaining the strikingly tissue-specific dissemination of, for example, mucosal lymphomas, cutaneous lymphomas, and multiple myeloma. Understanding the molecular mechanisms underlying this behavior may provide novel targets for treatment of lymphoma patients.
Introduction
Lymphocyte recirculation and homing: introduction
Specific recognition of foreign antigens and effective surveillance are the 2 mainstays of the body's defense against microbial invasion. Evolution has created great antigen-receptor diversity and has equipped lymphocytes with exquisite motility and migratory properties to accomplish these tasks. As discovered more than 4 decades ago by Gowans and Knight, mature lymphocytes recirculate, moving continuously from blood to tissue and back to the bloodstream again.1 This recirculation is not random, but is guided by mechanisms allowing lymphocyte diapedesis at the correct site and directing their migration to the proper place in the tissues (reviewed in Butcher and Picker2 ; von Andrian and Mempel3 ; Kunkel and Butcher4 ; and Salmi and Jalkanen5 ). Within the tissues, lymphocytes display a characteristic “ameboid” form of cell migration,6-8 which represents a physically optimized migration mode that allows easy cell traffic toward and between different tissue compartments. Lymphocyte-endothelial recognition plays a central role in controlling the access of specialized lymphocyte subsets to particular tissues, thus influencing the nature of local immune and inflammatory responses. At the molecular level, this “homing” process is regulated by adhesion molecules in concert with chemokines: Lymphocyte subsets as well as endothelial cells specifically program their expression of adhesion molecules and chemokines/chemokine receptors, allowing lymphocytes to move selectively to specific functional compartments of the immune system, such as the MALT and the skin.2-5 This review describes the basic principles of lymphocyte trafficking and discusses emerging evidence indicating that the molecular cues regulating this process also play a critical role in lymphoma dissemination. This implies that the mechanisms underlying lymphoma dissemination are markedly different from those underlying the metastasis of most solid tumors. The focus of this review is on lymphocyte entry regulation, being the first crucial step in lymphocyte homing, but we are aware that the final distribution of (neoplastic) lymphocytes depends on the balance of entry, proliferation, and retention. However, retention and expansion per se can hardly explain the strikingly different dissemination patterns of the distinct lymphoma subtypes. Moreover, to our knowledge, there are currently no studies available that address the role of retention regulation in lymphoma dissemination, preventing a meaningful discussion of this aspect.
For the convenience of the reader, an overview of the most important adhesion molecules and chemokine receptors involved in homing, their distribution on various lymphocyte subsets and non-Hodgkin lymphoma (NHL) subtypes, their ligands, sites of interaction, and predominant role(s) in homing are given in Table 1.
Adhesion molecules, chemokine receptors, and chemokines involved in lymphocyte homing and lymphoma dissemination
| Receptor . | Expression on lymphocytes . | Expression on lymphomas . | Ligands . | Predominant sites of homing . |
|---|---|---|---|---|
| Adhesion molecules | ||||
| L-selectin | Naive T and B cells, central memory T cells | B-CLL, MCL, MZBCL, nodal FL, DLBCL, nodal PTCL | PNAd (MAdCAM-1) | PLN |
| CLA | Skin homing T cells | CTCL | E-selectin | Skin |
| α4β7 | Naive T and B cells (low), gut-homing T-cells (high), IgA plasmablasts | GI-tract MCL (MLP), GI-tract MZBCL (maltoma), GI-tract FL, GI-tract PTCL (EA-TCL) | MAdCAM-1 (VCAM-1) | Gut |
| αEβ7 | Intraepithelial T cells | GI-tract PTCL, GI-tract MZBCLs | E-cadherin | Epithelium |
| αLβ2 (LFA-1) | Broad expression on T and B cells | Broad expression on T- and B-cell lymphomas and MM | ICAM-1 ICAM-2 | Multiple sites |
| α4β1 (VLA-4) | Broad expression on T and B cells | Broad expression on T- and B-cell lymphomas and MM | VCAM-1 | Inflammatory sites and bone marrow |
| Chemokine receptors | ||||
| CCR4 | Skin-homing T cells | CTCL | CCL17* (TARC) | Skin |
| CCR7 | Naive T cells, central memory T cells | CTCL, MCL | CCL21* (SLC) | PLN |
| CCR9 | Gut-homing T cells, intraepithelial T cells, IgA plasmablasts | Unknown | CCL25* (TECK) | Intestinal mucosa and crypt epithelium |
| CCR10 | Skin-homing T cells, IgA plasma blasts | CTCL unknown | CCL27* (CTAK) CCL28* (MEC) | Skin, intestine |
| CXCR4 | Pre-B cells, B cells, plasma cells | Broad expression on T- and B-cell lymphomas and MM | CXCL12* (SDF-1) | Secondary lymphoid tissues and BM, migration to GCs |
| CXCR5 | Mature B cells | Broad expression on T- and B-cell lymphomas | CXCL13* (BLC/BCA-1) | Migration to GCs in PLN and Peyer patches |
| Receptor . | Expression on lymphocytes . | Expression on lymphomas . | Ligands . | Predominant sites of homing . |
|---|---|---|---|---|
| Adhesion molecules | ||||
| L-selectin | Naive T and B cells, central memory T cells | B-CLL, MCL, MZBCL, nodal FL, DLBCL, nodal PTCL | PNAd (MAdCAM-1) | PLN |
| CLA | Skin homing T cells | CTCL | E-selectin | Skin |
| α4β7 | Naive T and B cells (low), gut-homing T-cells (high), IgA plasmablasts | GI-tract MCL (MLP), GI-tract MZBCL (maltoma), GI-tract FL, GI-tract PTCL (EA-TCL) | MAdCAM-1 (VCAM-1) | Gut |
| αEβ7 | Intraepithelial T cells | GI-tract PTCL, GI-tract MZBCLs | E-cadherin | Epithelium |
| αLβ2 (LFA-1) | Broad expression on T and B cells | Broad expression on T- and B-cell lymphomas and MM | ICAM-1 ICAM-2 | Multiple sites |
| α4β1 (VLA-4) | Broad expression on T and B cells | Broad expression on T- and B-cell lymphomas and MM | VCAM-1 | Inflammatory sites and bone marrow |
| Chemokine receptors | ||||
| CCR4 | Skin-homing T cells | CTCL | CCL17* (TARC) | Skin |
| CCR7 | Naive T cells, central memory T cells | CTCL, MCL | CCL21* (SLC) | PLN |
| CCR9 | Gut-homing T cells, intraepithelial T cells, IgA plasmablasts | Unknown | CCL25* (TECK) | Intestinal mucosa and crypt epithelium |
| CCR10 | Skin-homing T cells, IgA plasma blasts | CTCL unknown | CCL27* (CTAK) CCL28* (MEC) | Skin, intestine |
| CXCR4 | Pre-B cells, B cells, plasma cells | Broad expression on T- and B-cell lymphomas and MM | CXCL12* (SDF-1) | Secondary lymphoid tissues and BM, migration to GCs |
| CXCR5 | Mature B cells | Broad expression on T- and B-cell lymphomas | CXCL13* (BLC/BCA-1) | Migration to GCs in PLN and Peyer patches |
Only adhesion molecules, chemokines, and their receptors with major functions in tissue-specific lymphocyte homing are listed. The αβ heterodimers belong to the integrin family.
VLA indicates very late antigen; MZBCL, marginal zone B-cell lymphoma; MAdCAM, mucosal addressin cell adhesion molecule; EA-TCL, enteropathy-associated T-cell lymphoma; MM, multiple myeloma; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; CLA, cutaneous lymphocyte antigen; ICAM, intercellular adhesion molecule; LFA, lymphocyte function–associated; MAdCAM, mucosal addressin cell adhesion molecule; PNAd, peripheral lymph node addressin; VCAM, vascular cell adhesion molecule; VLA, very late antigens; B-CLL, B-cell chronic lymphocytic leukemia; BM, bone marrow; MCL, mantle cell lymphoma; MLP, malignant lymphomatous polyposis; MZBL, marginal zone B-cell lymphoma; FL, follicular lymphoma; DLBCL, diffuse large B-cell lymphoma; PLN, peripheral lymph node; PTCL, peripheral T-cell lymphoma; EA-TCL, entheropathy-associated T-cell lymphoma; MM, multiple myeloma; and CTCL, cutaneous T-cell lymphomas.
Chemokines.
Lymphocytes: licensed to move
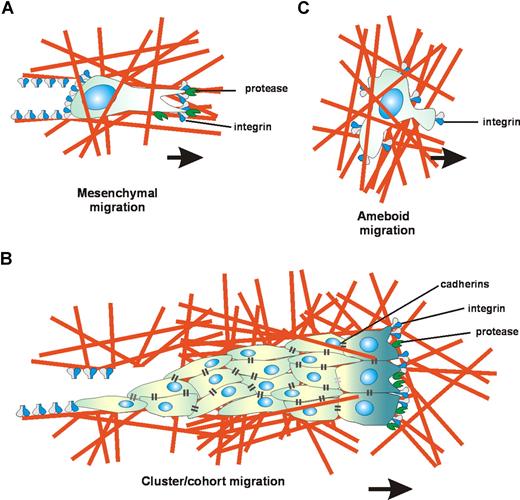
Cell migration is a universal process; most cell types in the body are capable of migrating at one or more distinct steps during their development and differentiation. This migration is essential for tissue morphogenesis and leukocyte trafficking, as well as for epithelial turnover and regeneration processes, such as wound healing. Furthermore, deregulated cell migration can take place in cancer, resulting in tumor invasion and metastasis. Given the highly diverse biologic contexts of cell migration, it is not surprising that distinct cell migration strategies have emerged. These cell-type–specific patterns of migration are acquired during differentiation and can be subdivided into at least 3 main migration modes, that is, “mesenchymal” and “ameboid” single-cell migration, and “collective” cell migration (Figure 1). The essential molecules that control and specify these different types of migration include adhesion molecules of the β1 and β3 integrin families that mediate interaction with the extracellular matrix (ECM); matrix metalloproteinases (MMPs) and serine proteinases, such as uPA/uPAR, responsible for ECM degradation; cadherins and associated molecules that mediate stable intercellular adhesions; and signaling molecules that control the actin cytoskeleton, specifically the small GTPases RhoA, Rac, and Cdc42 and their downstream effectors. In mesenchymal migration, which represents the archetype of cell migration, cells complete a migration sequence consisting of (1) cell polarization driven by localized actin polymerization causing formation of a leading pseudopod; (2) attachment of this pseudopod to ECM ligands via β1 and β3 integrin clusters called focal adhesions, interaction sites that recruit cytoplasmic adaptor, signaling, and cytoskeletal proteins as well as cell surface proteases such as MMPs and the uPA/uPAR complex; (3) local proteolysis of the ECM, widening the space for forward movement of the cell; (4) activation of contractile proteins, such as myosin II, and consequent shortening of membrane-anchored actin filaments; (5) contraction of the cell, leading to retraction of its rear end and consequent forward movement (Figure 1). This 5-step migration program is typical for single-cell migration of fibroblasts and keratinocytes as well as for single epithelial (cancer) cells that have undergone epithelial to mesenchymal transition (EMT), and represents a relatively slow process with migration velocities of 0.1 to 2 μm/min. Collective cell migration, as seen during wound healing and during the invasion of epithelial cancer cell collectives, uses the same integrin- and protease-dependent migration cycle, but in this migration type the cell junctions within the invasive collectives are stabilized by cadherins and gap-junctional cell-to-cell communication (Figure 1). Interestingly, recent studies indicate that lymphocytes do not conform to the classic 5-step paradigm of cell migration. Instead they display a characteristic form of cell migration, which has been termed “ameboid” migration, because it mimics that of the ameba Dictyostelium discoideum.6 In this migration type, integrin-mediated adhesion is partially dispensable and stable focal contacts are not formed, but cell movement is driven by short-lived relatively weak interactions with the stromal cell networks in the T- and B-cell areas of the lymphoid tissues.6-8 The lack of focal contacts and high deformability of lymphocytes allow movement at high velocity (2-30 μm/min).6-10 Moreover, the fast deformability of lymphocytes allows them to overcome matrix barriers by physical mechanisms, that is, adaptation of shape to preformed matrix structures (contact guidance), extension of lateral footholds (elbowing), and squeezing through narrow spaces (contraction rings). Thus, lymphocyte migration is shape-change driven and lymphocytes use protease-independent physical mechanisms that allow easy cell traffic toward and between structurally different tissue compartments. Among higher eukaryotes, this migration type is found only in lymphocytes and other leukocytes, hematopoietic stem cells, and certain tumor cells.6
Diversity in cell migration strategies. In the tissue microenvironment, different cell types exhibit distinct migration strategies. (A) Mesenchymal migration: Mesenchymal cells display an adhesive phenotype and develop a spindle shape. The elongated morphology is dependent on integrin-mediated adhesion and the presence of traction forces on both cell poles. Simultaneous with integrin and actin concentration at focal contacts, the cells recruit surface proteases to these substrate contact sites to digest and remodel the extracellular matrix, thus generating matrix defects that allow cell migration. Other cells may follow along the generated matrix defect creating a moving cell chain. (B) Cluster/cohort migration: Migrating cancer cell collectives use an integrin- and protease-dependent migration mode similar to mesenchymal migration, but the migrating cells within the cohorts are interconnected by cadherins and gap-junctional communication. (C) Ameboid migration: Lymphoid cells display a characteristic “ameboid” type of migration, in which integrin-mediated adhesion is dispensable and cell movement is driven by short-lived relatively weak interactions with the substrate. The lack of focal contacts and high deformability of lymphocytes allow movement at high velocity, while the fast deformability of lymphocytes allows them to overcome matrix barriers by physical mechanisms, independent of proteolytic matrix degradation.6
Diversity in cell migration strategies. In the tissue microenvironment, different cell types exhibit distinct migration strategies. (A) Mesenchymal migration: Mesenchymal cells display an adhesive phenotype and develop a spindle shape. The elongated morphology is dependent on integrin-mediated adhesion and the presence of traction forces on both cell poles. Simultaneous with integrin and actin concentration at focal contacts, the cells recruit surface proteases to these substrate contact sites to digest and remodel the extracellular matrix, thus generating matrix defects that allow cell migration. Other cells may follow along the generated matrix defect creating a moving cell chain. (B) Cluster/cohort migration: Migrating cancer cell collectives use an integrin- and protease-dependent migration mode similar to mesenchymal migration, but the migrating cells within the cohorts are interconnected by cadherins and gap-junctional communication. (C) Ameboid migration: Lymphoid cells display a characteristic “ameboid” type of migration, in which integrin-mediated adhesion is dispensable and cell movement is driven by short-lived relatively weak interactions with the substrate. The lack of focal contacts and high deformability of lymphocytes allow movement at high velocity, while the fast deformability of lymphocytes allows them to overcome matrix barriers by physical mechanisms, independent of proteolytic matrix degradation.6
Multistep lymphocyte-endothelial interaction
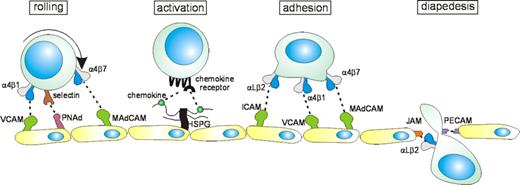
Lymphocyte exit from the bloodstream occurs via a series of interactions with vascular endothelium in specialized postcapillary venules, termed high endothelial venules (HEVs) in lymph nodes and Peyer patches.2 The initial step consists of a loose “tethering” engagement, leading to a rolling movement of the lymphocyte over the vascular endothelium (Figure 2). Generally, lymphocyte rolling is mediated by molecules of the selectin family, which are strategically localized on the tips of the cell membrane's microvilli,11,12 allowing effective interaction with their sialomucin ligands. Under certain conditions, the integrins α4β1 and α4β7, and CD44 can also mediate rolling.11,13-15 Lymphocyte rolling is transient and reversible, unless followed by a chemokine signal leading to integrin activation. Heparan sulfate proteoglycans (HSPGs) expressed on endothelium or extracellular matrix contribute to integrin activation and promotes diapedesis by concentrating and presenting chemokines to their receptors. Heparan sulfate chain modifications can affect cytokine-binding specificity and may add an additional level of complexity to the lymphocyte-endothelial cell interaction cascade. By interacting with their G protein–coupled receptor and activation of various signaling molecules including PI3K, Tec/Btk-family kinases, phospholipase C, and Ras-family GTPases,16,17 chemokines such as CXCL12 (SDF-1), CXCL13 (BCA/BLC), CCL21 (SLC), CXCL10 (IP10), and CCL17 (TARC) trigger affinity and avidity of lymphocyte integrins, resulting in rapid (milliseconds) lymphocyte arrest under flow conditions.18-20 These integrins, specifically lymphocyte function-associated antigen 1 (LFA-1) (αLβ2), α4β1, and α4β7, mediate stable adhesion and promote migration of lymphocytes across the vessel wall (Figure 2). This transmigration is further promoted by interaction with junctional adhesion molecules (JAMs), PECAM-1(CD31), and CD99. 21-23 Once in the tissue, the lymphocytes engage chemoattractant gradients, directing their migration to the correct microenvironment. The “exit decision” of a given lymphocyte in the multistep lymphocyte-endothelial cell interaction model (Figure 2) is determined by the unique combination of adhesion molecules and chemokine receptors on its cell surface. This “homing signature” enables the cell to recognize and leave the blood at specialized endothelial “address” sites expressing the relevant ligands, thus allowing tissue-specific homing.2-5,20,24,25
Lymphocyte interaction with endothelium. In the postcapillary venules, selectin-sialomucin interactions (or interactions mediated by integrin α4β1 or α4β7) mediate “rolling” of lymphocytes on the endothelium. Chemokines, presented by heparan sulfate proteoglycans (HSPGs) expressed on the endothelium, bind to chemokine receptors, which are G protein–coupled receptors, leading to increased affinity/avidity integrins on the surface of lymphocytes. Interaction of these integrins with their ligands results in stable adhesion of lymphocytes to endothelium and in diapedesis, involving engagement with junction adhesion molecules (JAMs) and PECAM-1 (CD31).
Lymphocyte interaction with endothelium. In the postcapillary venules, selectin-sialomucin interactions (or interactions mediated by integrin α4β1 or α4β7) mediate “rolling” of lymphocytes on the endothelium. Chemokines, presented by heparan sulfate proteoglycans (HSPGs) expressed on the endothelium, bind to chemokine receptors, which are G protein–coupled receptors, leading to increased affinity/avidity integrins on the surface of lymphocytes. Interaction of these integrins with their ligands results in stable adhesion of lymphocytes to endothelium and in diapedesis, involving engagement with junction adhesion molecules (JAMs) and PECAM-1 (CD31).
Tissue-specific lymphocyte homing
The homing signature of a lymphocyte is dependent on differentiation stage and antigenic experience. Mature naive lymphocytes display a remarkable tropism for the various secondary lymphoid organs, and are generally excluded from nonlymphoid sites.26 During the late stages of their ontogeny in the thymus and bone marrow, T-cell and B-cell precursors up-regulate L-selectin as well as the chemokine receptor CCR7.25 The ligands for these receptors, the peripheral lymph node addressin (PNAd) and the chemokine CCL21, are expressed on the luminal surface of HEVs (Figure 3).24,25 Naive lymphocytes also express the integrin α4β7, an important mediator of lymphocyte rolling and adhesion in the gut-associated lymphoid tissues. The combined expression of L-selectin, α4β7, LFA-1, and CCR7 allows naive lymphocytes access to both the peripheral and gut-associated lymphoid tissues, resulting in efficient surveillance of these crossroads of the immune system (Figure 3).
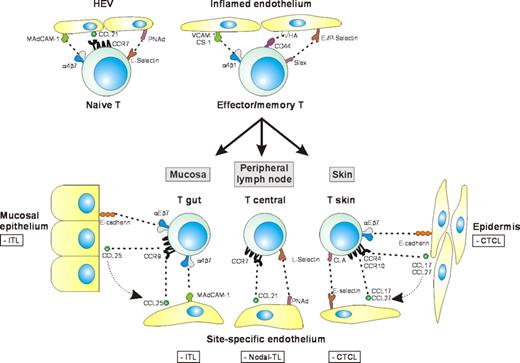
Lymphocyte trafficking and the tissue-specific dissemination of T-cell lymphomas. Lymphocyte migration is strictly regulated by adhesion molecules and chemokine receptors on lymphocytes and their ligands expressed by the endothelium. Naive T lymphocytes can home and recirculate via all secondary lymphoid tissues because they express both α4β7 (for mucosal homing) and L-selectin (for homing to peripheral lymph nodes). Migration of activated T lymphocytes to sites of inflammation involves several receptor-ligand pairs, including selectin-sialomucin, α4β1-VCAM-1, α4β1-CS-1, and CD44-hyaluronate interactions. Upon antigen priming by dendritic cells, T lymphocytes become memory cells and acquire a “homing signature,” that is, a specific adhesion and chemokine receptor make-up, which enables them to selectively home to specific tissue environments, thereby increasing the efficacy of immunosurveillance. The T-NHLs related to lymphocyte populations with tissue-specific homing properties are shown in the boxes. These tumors usually display tissue-specific dissemination patterns and express homing receptors corresponding to the tissue of origin.
Lymphocyte trafficking and the tissue-specific dissemination of T-cell lymphomas. Lymphocyte migration is strictly regulated by adhesion molecules and chemokine receptors on lymphocytes and their ligands expressed by the endothelium. Naive T lymphocytes can home and recirculate via all secondary lymphoid tissues because they express both α4β7 (for mucosal homing) and L-selectin (for homing to peripheral lymph nodes). Migration of activated T lymphocytes to sites of inflammation involves several receptor-ligand pairs, including selectin-sialomucin, α4β1-VCAM-1, α4β1-CS-1, and CD44-hyaluronate interactions. Upon antigen priming by dendritic cells, T lymphocytes become memory cells and acquire a “homing signature,” that is, a specific adhesion and chemokine receptor make-up, which enables them to selectively home to specific tissue environments, thereby increasing the efficacy of immunosurveillance. The T-NHLs related to lymphocyte populations with tissue-specific homing properties are shown in the boxes. These tumors usually display tissue-specific dissemination patterns and express homing receptors corresponding to the tissue of origin.
In the absence of antigen stimulation, a naive lymphocyte will exit the secondary lymphoid tissues via the efferent lymphatics and return to the recirculating lymphocyte pool.1 Antigen engagement blocks this exit and results in clonal expansion of the antigen-specific lymphocyte and its differentiation into a functionally specialized effector or memory cell. As an integral part of this differentiation process, the homing signature of the lymphocyte is revised to a unique combination of adhesion and chemokine receptors, allowing preferential exit from the blood in the type of lymphoid tissue where the initial activation took place.2,5,20,24-26 In addition, the newly acquired homing profile enables the lymphocyte to leave the blood in the nonlymphoid tissue draining to this lymph node. The molecular mechanisms controlling the reprogramming of the homing signature of lymphocytes are still incompletely understood. Recent studies indicate that interaction with dendritic cells (DCs) plays a crucial role. For example, it was demonstrated that priming of lymphocytes by mucosal DCs increases the expression of α4β7 and CCR9, molecules that promote specific homing to the gut.27-31 By contrast, activation by skin-derived DCs decreases α4β7 and CCR9 expression but enhances expression of the skin-homing receptor cutaneous lymphocyte antigen (CLA).32
Currently, the best characterized pathways of lymphocyte homing are those mediating homing to the gut-associated lymphoid tissue and skin (Figure 3). Both intestine and skin represent barrier tissues exposed to high antigenic load, and it is conceivable that these specialized pathways have evolved to segregate intestinal and cutaneous immunity, securing robust secondary immune responses to tissue-tropic pathogens. Effector/memory T cells with homing specificity for intestine and skin can be readily identified in the blood of healthy individuals by their mutually exclusive expression of α4β7 and CLA.33 These molecules mediate rolling through interaction with their endothelial counterreceptors MAdCAM-1 and E-selectin, respectively (Figure 3). After chemokine-triggered activation, α4β7 also causes the arrest of intestinal lymphocytes on the endothelium. In addition to mediating T-cell homing to the intestine, α4β7 plays a crucial role in the migration of B cells and plasmablasts to Peyer patches and the intestinal lamina propria (Figure 4).5,34 Unlike the gut- and skin-homing effector memory cell populations, the so-called central memory cells retain their expression of L-selectin (and CCR7) and continue to recirculate through peripheral lymph nodes.3,35
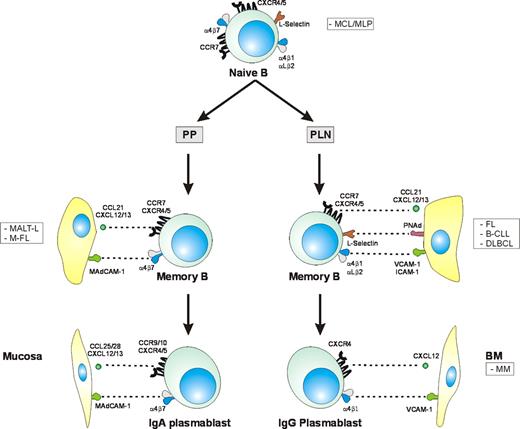
Adhesion molecule and chemokine receptor expression profiles of B-lymphocyte subsets and related lymphoid malignancies. Naive B lymphocytes coexpress L-selectin and α4β7, enabling them to migrate to the mucosa as well as to peripheral lymph nodes. Germinal center (GC) reactions in Peyer patches (PPs) lead to generation of α4β7-expressing memory B lymphocytes, which subsequently can differentiate into IgA-secreting plasma cells. Most memory B cells arising in lymph nodes (LNs), on the other hand, differentiate into IgG-secreting plasma cells. These cells express CXCR4 and the integrins α4β1 and LFA-1, which can mediate homing to the bone marrow, where these cells become long-lived plasma cells. The B-cell malignancies related to lymphocyte populations with tissue-specific homing properties are shown in the boxes.
Adhesion molecule and chemokine receptor expression profiles of B-lymphocyte subsets and related lymphoid malignancies. Naive B lymphocytes coexpress L-selectin and α4β7, enabling them to migrate to the mucosa as well as to peripheral lymph nodes. Germinal center (GC) reactions in Peyer patches (PPs) lead to generation of α4β7-expressing memory B lymphocytes, which subsequently can differentiate into IgA-secreting plasma cells. Most memory B cells arising in lymph nodes (LNs), on the other hand, differentiate into IgG-secreting plasma cells. These cells express CXCR4 and the integrins α4β1 and LFA-1, which can mediate homing to the bone marrow, where these cells become long-lived plasma cells. The B-cell malignancies related to lymphocyte populations with tissue-specific homing properties are shown in the boxes.
In addition to tissue-specific expression of adhesion molecules, site-specific production of chemokines plays a crucial role in the homing of cutaneous and intestinal T cells (Figure 3). CLA+ T cells selectively express CCR4 and CCR10, whose ligands CCL17 (TARC) and CCL27 (CTAK) are presented on the luminal surface of postcapillary venules in the skin.4 Blocking of these chemokine/receptors impairs migration of CD4+ lymphocytes to the skin.36,37 CCR9 and its ligand CCL25 (TECK) play an analogous role in T-cell recruitment to the intestine. CCR9 is expressed on a subset of T cells with strong α4β7 expression, as well as by the T cells in the epithelium and lamina propria of the small intestine,38 while CCL25 is produced by the epithelium of the small intestinal crypts and presented on the luminal surface of endothelial cells in intestinal postcapillary venules.39 Blocking of CCL25 inhibits the accumulation of antigen-specific effector T cells in the intestinal mucosa.40 B-cell recruitment into lymph nodes requires combined expression of CCR7 and CXCR4 on the B cell. Entry into Peyer patches, in addition, requires expression of CXCR5. In accordance with these receptor requirements, CCL21 and CXCL12 are displayed broadly on HEVs, while CXCL13 is found selectively on Peyer patch HEVs.41
Apart from mediating T-cell homing, CCL25/CCR9 interaction contributes to the migration of IgA plasmablasts to the small intestine. Plasmablast recruitment furthermore involves the epithelial chemokine CCL28 (MEC), which interacts with CCR10 on the plasmablasts (Figure 4).42 CCL28 is also expressed at other mucosal sites belonging to the common mucosal immune system.34 Once they left the bloodstream, lymphocytes will be recruited to specific tissue microenvironments by chemokine gradients. For example, CCL25 recruits CCR9-expressing CD8+ T cells from the intestinal lamina propria into the epithelium and enhances αEβ7 integrin–mediated adhesion to epithelial E-cadherin.38,40 CXCL12 and CXCL13 recruit B cells into distinct B-cell compartments by interacting with CXCR4 and CXCR5, respectively. CXCL13 is specifically expressed by follicular dendritic cells (FDCs) in B-cell follicles, and disruption of its function leads to a disturbed development of primary follicles and germinal centers in the spleen and Peyer patches.43-45 Within the germinal center, α4β1 in concert with LFA-1 mediates interaction of B cells with FDCs.46 These B-cell receptor–controlled integrin-mediated interactions inhibit apoptosis of germinal B cells and may be crucial for affinity maturation.47-50
The molecular basis of lymphoma dissemination
A number of clinical observations suggest that conserved homing programs mediate the dissemination of non-Hodgkin lymphomas (NHLs).51,52 For example, NHLs related to small recirculating lymphocytes, such as small-lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL) and mantle-cell lymphoma (MCL), usually show systemic dissemination at presentation, whereas NHLs related to lymphocytes undergoing active proliferation and differentiation, such as Burkitt lymphoma (BL) and diffuse large B-cell lymphomas (DLBCL), often are initially localized. Furthermore, tumor dissemination to sites of trauma and inflammation is regularly observed in lymphoma patients, implying specific recruitment of tumor cells by locally produced chemokines and activated endothelium. Most strikingly, extranodal lymphomas arising in the gut-associated lymphoid tissues or the skin show a strong preference to disseminate to mucosal sites and skin, respectively.51,52 Although they may eventually disseminate to (regional) lymph nodes, intestinal lymphomas will rarely disseminate to the skin and, vice versa, cutaneous lymphomas will seldom involve the intestine. In the following paragraphs, the molecular basis underlying the dissemination patterns of a number of distinct lymphoma subtypes will be discussed.
The dissemination of T-cell lymphomas
Virtually all mature T-lineage lymphomas are derived from memory (CD45RO+) T cells and collectively are designated “peripheral” T-cell lymphomas as opposed to “central” (thymocyte-derived) precursor T-cell neoplasms. Notwithstanding this shared derivation from antigen-experienced T cells, peripheral T-cell lymphomas represent a heterogeneous group of tumors comprising several well-defined entities with distinctive molecular, pathological, and clinical characteristics.51 For decades, the tissue of primary presentation and dissemination pattern have been recognized as important criteria for the classification of these tumors into distinctive clinicopathological entities.51 As will be discussed, this empiric practice can now be grounded on molecular data (Figure 3).
Cutaneous T cell lymphoma (CTCL) represents a striking example of a lymphoma type displaying tissue-specific dissemination. CTCLs usually express the cutaneous lymphocyte- homing receptor CLA53,54 as well as the chemokine receptor CCR4, often in combination with CCR10 and/or CXCR3.55-59 The expression of these receptors, not only on tumor cells residing in the skin but also on circulating cells, permits these cells to home effectively to the skin. Interestingly, the tumor cells of mycosis fungoides (MF) and Sézary syndrome, 2 closely related CTCL subtypes, show markedly different homing signatures, which correspond to the distinctive dissemination patterns encountered in these diseases. MF typically shows no or very little lymph node involvement during most of the disease course. In keeping with this finding, the tumor cells express low levels of the PLN homing receptor L-selectin and CCR7. During MF progression, the development of lymph node involvement is accompanied by a loss of skin-specific chemokine receptors and up-regulation of CCR7.60 Sézary syndrome, by contrast, is characterized by extensive systemic involvement. Accordingly, the tumor cells in this disease coexpress the “cutaneous-” and “PLN-”homing signatures, that is, they express L-selectin and CCR7 as well as CLA and CCR4.59 In line with the strict physiological dichotomy between the skin- and gut-homing memory T-cell populations, neither CTCL expresses the gut-homing receptor α4β7. Notably, in adult T-cell leukemia/lymphoma (ATLL), a systemic lymphoma, CCR4 expression was reported to be linked to skin dissemination.61
From the perspective of dissemination, intestinal T-cell lymphomas (ITLs) represent the mirror image of CTCLs. These lymphomas, which are most often enteropathy associated, express the mucosal-homing receptor α4β7,62 allowing them to interact with MAdCAM-1 on mucosal postcapillary venules, but lack CLA and L-selectin (Table 1).63 Moreover, they generally express the integrin αEβ7, which allows interaction with E-cadherin on the epithelial cells of the intestinal mucosa.64 At present, no comprehensive studies of chemokine receptor expression in intestinal T-cell lymphomas are available. A recent case report suggests that enteropathy-associated T-cell lymphomas (EA-TCLs) do not express the chemokine receptor CCR7,65 which is consistent with the absence of PLN dissemination in these tumors.
Nodal T-cell lymphomas represent a heterogeneous group of tumors, which can be subclassified into distinct entities based on molecular, pathological, and clinical criteria.51 At least some of these lymphomas presumably are related to the central memory T-cell subset.35 Consistent with this notion, the majority of nodal T-cell lymphomas express L-selectin, but lack the skin-homing receptor CLA as well as the mucosal-homing receptor α4β7 (Table 1).63 Anaplastic large cell lymphomas, however, are L-selectin negative, which may be related to their activated phenotype.63 The expression pattern of chemokine receptors in nodal T cells has thus far been incompletely analyzed, but the available data suggest differential expression on specific lymphoma subtypes.66,67 CCR4, the skin-homing chemokine receptor, is expressed on a minor subset of nodal T-cell lymphomas, but approximately half of these tumors express the Th1 chemokine receptors CXCR3 and/or CCR5.66,67 Expression of these receptors in peripheral T-cell lymphoma unspecified (PTCLU) has been reported to be related to a favorable prognosis.68
The dissemination of B-cell lymphomas
The trafficking pathways of T- and B-lymphocytes are markedly different, reflecting the distinctive functions of T and B cells in the immune system. Effective immunosurveillance against virally infected cells requires effector T cells to have physical access to nonlymphoid tissues. B-cell effector function, by contrast, depends primarily on antibodies produced by plasma cells. These antibodies are solubilized in body fluids and hence can act at a distance, obviating the need for B cells to migrate to peripheral sites of antigenic insult, such as the skin. Indeed, B cells are virtually undetectable in the normal skin.69 B-cell migration to the skin and other extralymphoid sites presumably occurs almost exclusively in the context of chronic inflammation driven by locally persistent antigen, including infectious agents, such as Borrelia burgdorferi in the skin and Helicobacter pylori in the stomach, or autoantigens in, for example, Sjögren disease. This persistent antigenic stimulation can lead to neoformation of lymphoid tissue,70,71 with local expression of vascular addressins and chemokines that are normally expressed in organized lymphoid tissues. These molecules are unrelated to the cues that recruit typical “skin-homing T cells” and their malignant counterparts. At these sites of chronic antigenic stimulation, in the skin or at other extralymphoid sites such as the stomach mucosa or the orbit, B-cell lymphomas, in particular marginal zone and follicular lymphomas, can eventually arise.72-74
Naive B cells need to recirculate freely through peripheral and mucosal lymphoid tissues to optimize their chances of meeting their cognate antigen, whereas B memory cells and plasmablasts have to migrate to specific lymphoid environments dedicated to their recall and effector functions. Like T cells, B cells consequently must adapt their homing signature to their specific maturational stage (Figure 4). These maturation-dependent profiles are largely conserved in B-cell lymphomas and control their dissemination. For example, naive B cells coexpress the PLN-homing receptor L-selectin and the intestinal-homing receptor α4β7.62 Combined expression of these molecules has also been reported on a subset of mantle cell lymphomas (MCLs),75 tumors that generally lack somatic hypermutations in their immunoglobulin variable genes (IgA), and are therefore presumably derived from naive B cells.76 Interestingly, α4β7 expression in MCL is associated with a clinical presentation known as malignant lymphomatous polypsis (MLP) (Figure 4), characterized by multifocal involvement of the gastrointestinal tract as well as widespread lymph node involvement.75,77 It is noteworthy that MCLs with a primary nodal presentation are often α4β7 negative but that α4β7, when present, predicts multifocal digestive tract involvement.77 In addition to the adhesion profile of MCLs, the chemokine receptor profile of these tumors, which includes expression of CCR7 and CXCR4, also allows wide dissemination to both PLNs and mucosal sites.78,79
The intestinal-homing receptor α4β7 (Figure 4) is of key importance for the homing of normal memory B cells and IgA plasmablasts to the marginal zones of Peyer patches and the intestinal lamina propria, respectively.80 In striking parallel, the malignant counterparts of gut-homing memory cells, that is, the low-grade marginal-zone B-cell lymphomas of the mucosa-associated lymphoid tissues (MALTs), express α4β7.62 These tumors are believed to arise as a result of chronic antigenic stimulation, often associated with H pylori infection. Although they coexpress L-selectin, intestinal MALT lymphomas remain localized or disseminate to other mucosal sites, but only rarely to PLNs.51,62 Low CCR7 expression, as has been found in splenic marginal-zone lymphoma and hairy-cell leukemia (HCL),78,79 B-cell neoplasms that also lack nodal dissemination, might explain the low incidence of PLN involvement in MALT-type lymphomas. Alternatively, absence of antigenic stimulation within the peripheral lymph node microenvironment may explain the lack of growth at these sites.
Similar to intestinal MALT lymphomas, primary follicular lymphomas (FLs) of the GI tract also express α4β7 (Figure 4).81 These rare lymphomas resemble nodal FLs with respect to morphology and expression of typical germinal center markers such as CD10, CD38, and BCL-6, and the presence of a BCL-2 translocation.81 However, in contrast to their nodal counterparts, they are often IgA positive, express α4β7, and remain localized to the GI tract, suggesting that they represent a distinct entity that, like low-grade intestinal MALT lymphoma, originates from local antigen-responsive B cells.81 Unlike these small cell B-cell lymphomas, however, DLBCLs and Burkitt lymphomas with a primary intestinal localization do not express α4β7 (Table 1).62 In summary, α4β7 expression is a hallmark of a number of typical GI tract B-cell lymphomas and presumably plays an important role in the characteristic mucosal dissemination of these tumors.
As already pointed out, the major chemoattractants for B cells are CXCL12 and CXCL13, and these chemokines play a role at more than one trafficking checkpoint.3-5,43-45 CXCL12 and CXCL13 are present on HEVs of lymph nodes and Peyer patches41 and are also present on HEV-like vessels at sites of lymphoid neogenesis, for example, the salivary glands in Sjögren syndrome.82 Furthermore, both CXCL12 and CXCL13 are important in the organization of germinal centers.43-45 CXCL12 guides CXCR4-positive lymphocytes to the GC dark zones, whereas CXCL13 is produced by FDCs and attracts CXCR5-positive B cells into the light zones.45 In line with their prominent function in the biology of B-cell homing, the chemokine receptors CXCR4 and CXCR5 are widely expressed in B-cell neoplasms including B-cell chronic lymphocytic leukemia (B-CLL), HCL, MCL, MALT lymphomas, FL, and DLBCL, and can drive migration of lymphoma cells79,83-85 Ectopic chemokine expression at sites of chronic inflammation with lymphoid neogenesis presumably is a key factor in the selective homing of malignant B cells to these sites. In addition, autocrine expression of CXCL13 has been reported in FL83 and primary central nervous system lymphomas.86 Apart from chemokines, other cytokines, including HGF, can also control integrin-mediated adhesion of malignant lymphocytes.87 These autocrine and paracrine interactions may contribute to lymphoma organization and promote tumor growth.
Homing of normal and malignant plasma cells
Differentiation of a B cell to a plasma cell is accompanied by a coordinated change in chemokine receptor expression.88 Whereas CXCR5 and CCR7 are down-regulated, resulting in loss of responsiveness to the B- and T-zone chemokines CXCL13, CCL19, and CCL21,34,88 CXCR4, the receptor for CXCL12 (SDF-1), is up-regulated (Figure 4). The latter chemokine is constitutively expressed by bone marrow (BM) stromal cells88-90 and, indeed, CXCL12/CXCR4 interaction is required for plasma cells homing to the BM, the major site of antibody production in adult life.88,91 Within the BM microenvironment, cytokines such as IL-6, produced by stromal cells, control the maturation and survival of plasma cells.92,93 In addition, α4β1-mediated interactions with fibronectin and VCAM-1 are also critical for plasma cell survival and longevity.94,95
Like in normal plasma cell homing, CXCL12/CXCR4 interaction is also essential in the recruitment to and retention of MM plasma cells in the BM. CXCL12 promotes transendothelial migration and induces α4β1-mediated adhesion to VCAM-1 and fibronectin.96 It should be noted that both normal and MM plasma cells also express other chemokine receptors besides CXCR4, such as CXCR6, CCR6, CCR10, and CCR3.90 Consequently, they have the potential to migrate in response to a variety of chemoattractants. Interestingly, the chemokine receptor CCR5 is expressed by MM cells but not by normal plasma cells,97 while the BM of MM patients contains high levels of the CCR5 ligand CCL3 (MIP-1α).98 CCL3, which was found to be secreted by the majority of MM cell lines as well as primary MMs,99 not only functions as a chemoattractant for MM cells97 but also possesses osteoclast stimulatory activity and may thus contribute to osteolytic bone disease.99 On top of this, CCL3 may act as an autocrine growth and survival factor for MM.97 Besides controlling proliferation and survival, several cytokines, including insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF), can induce migration of MM cells.100,101 Hence, multiple factors in the BM microenvironment may modulate MM cell homing.
Blocking antibodies that prevent interaction of α4β1 with its ligands fibronectin and VCAM-1, expressed by BM stromal cells, not only disturb chemoattractant-induced adhesion and migration of MM cells,100,101 but also inhibit growth in a murine MM model.102 This growth inhibition may be the consequence of defective MM cell homing. However, blocking of integrin-mediated interactions between MM and BM stromal cells may also disturb the niche required for MM cell expansion. Indeed, adhesion of human MM cells to BM stromal cells stimulates secretion of IL-6, which has potent proliferative and antiapoptotic effects.103 In addition, the c-Maf oncogene, besides promoting expression of cyclin D2, also promotes expression of β7-integrins, thereby enhancing MM adhesion to BM stromal cells and increasing VEGF production,104 which in turn stimulates MM growth and survival as well as angiogenesis. Although both long-lived plasma cells and MM cells are protected from apoptosis by integrin-mediated interactions with the BM stroma, the ability to proliferate in this environment is a unique property of MM cells. Cytokines including IL-6, HGF, and WNTs produced by BM stromal cells provide MM cells with proliferative and survival signals required for their expansion.105-108 Of interest, α4β1- and α5β1-mediated adhesion to fibronectin can protect MM cells from drug-induced apoptosis, a phenomenon called cell adhesion–mediated drug resistance (CAM-DR).109 Because various chemokines and growth factors produced in the BM stimulate integrin-mediated adhesion,101,110,111 these cytokines can contribute to resistance of MM cells to treatment. Hence, integrins and their regulation by chemokines play a crucial role in the homing of MM cells to the BM and, moreover, contribute to the expansion of MM cells in the BM niche by mediating interaction with the BM stroma, which via “outside-in signaling” generates growth and survival signals for the malignant plasma cells.
Conclusion and perspectives
Although most cells in the body are capable of migrating at one or more distinct steps during their development and differentiation, the trafficking propensity of lymphocytes is unrivaled among somatic cells. The unique motility program and migratory properties endowed upon lymphocytes bears witness of the great evolutionary importance of effective immunosurveillance for the survival of higher vertebrates. Lymphocytes display a physically optimized ameboid migration mode that allows flexible trafficking toward and between different tissue compartments, and use sophisticated recognition mechanisms to home to particular tissues, thus influencing the nature of local immune and inflammatory responses. At the molecular level, this homing process is regulated by adhesion molecules in concert with chemokines. Lymphomas represent the malignant counterparts of lymphocytes arrested at a specific stage of maturation. The studies discussed in this review clearly demonstrate that the selective and often strikingly tissue-specific dissemination of malignant lymphomas and MM is controlled by the same molecular mechanisms that also guide the homing of their normal lymphocyte counterparts. This implies that lymphoma dissemination, unlike the metastatic spread of other types of cancer, generally is not a reflection of tumor progression but rather of conserved physiological behavior.
Targeting receptors that are part of the homing signature of malignant lymphocytes with monoclonal antibodies or small-molecule drugs may prove a successful novel means of therapeutic intervention in lymphoma patients: By preventing the homing to and interaction of the tumor cells with their natural microenvironment, these drugs will deprive the cells of essential growth and survival signals, causing cell death by anoikis. Indeed, treatments targeting adhesion and chemokine receptors with antibodies or drugs have been shown to be effective in animal models of skin inflammation and colitis.5,112 In humans, antibodies against integrin α4 (natalizumab) and α4β7 (MLN02) have been shown to induce remissions in patients with multiple sclerosis, Crohn disease, and ulcerative colitis.113,114 In view of the important roles of α4(β7) integrins in the pathogenesis of both malignant lymphoma and multiple myeloma, these antibodies may also prove to be valuable tools for the treatment of lymphoid malignancies.
Acknowledgments
This work was supported by grants from the Dutch Cancer Society.
We thank Dr M. Snoek for critical reading of the paper.
Authorship
Contribution: S.T.P provided the concept, designed and wrote the review, and approved the final version of the paper; D.J.J.G. and M.S. contributed to the writing and original work underlying the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven T. Pals, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; email: s.t.pals@amc.uva.nl.