Introduction
Medicine is gradually adapting to the challenges presented by a rapidly aging society. Although “aging” itself is not a disease, older people are more likely to have illnesses, and these are more likely to be chronic and debilitating. Geriatric medicine, the discipline responsive to the special needs of older patients, involves participation of practitioners from multiple disciplines including nursing, social work, physical and occupational therapy, nutrition, and pharmacy. Although management of chronic illness occupies much of the effort, the goals of geriatric medicine are global and include maintenance of physical and cognitive function, utilization of community resources, and disease prevention. These are in alignment with the overarching desire of older people to maintain autonomy. From this perspective, venous thrombosis and its consequences have been remarkably underinvestigated.
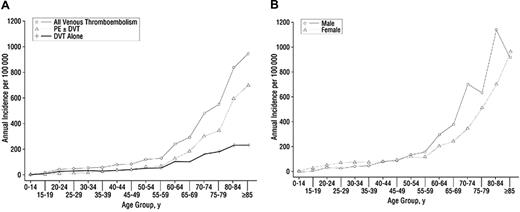
Each year, approximately 1 000 000 Americans1 suffer a venous thromboembolic (VTE) event—deep vein thrombosis (DVT) and/or pulmonary embolism (PE). Despite significant knowledge of risk factors, such as surgery, trauma, neurologic disability, cancer, hospitalization for medical illness, oral contraceptives, and pregnancy and the puerperium,2-4 annual death rates from VTE remain high and VTE is likely the most common cause of preventable death in hospitalized patients.5 One of the least understood aspects of venous thrombosis risk is advancing age. Incidence rates of VTE (Figure 1A) increase dramatically at about age 55 and by age 80 are nearly 1 in 100 per year, approximately 1000-fold higher than for those aged 45 or younger.6 Furthermore, rates of PE rise faster than DVT in the elderly (Figure 1B) so that the disease has greater fatal impact.
Annual incidence of venous thromboembolism among redidents of Olmsted County, Minnesota, from 1966 to 1990. (A) All VTE, deep vein thrombosis (DVT) alone, and pulmonary embolism (PE) with or without deep vein thrombosis (PE ± DVT) by age. (B) All VTE by age and sex. Reproduced with permission.6
Annual incidence of venous thromboembolism among redidents of Olmsted County, Minnesota, from 1966 to 1990. (A) All VTE, deep vein thrombosis (DVT) alone, and pulmonary embolism (PE) with or without deep vein thrombosis (PE ± DVT) by age. (B) All VTE by age and sex. Reproduced with permission.6
Pathophysiology of VTE in the elderly
Pathophysiologic mechanisms underlying venous thrombosis were described by the 19th century pathologist Virchow as a triad of vascular injury, venous stasis, and inherent problems of blood fluidity.7 We know now that vascular injury can be crude (traumatic) or subtle (endothelial cell “activation” by cytokines or components of pathogens) and that blood fluidity is influenced by a delicate balance among a large array of procoagulant and anticoagulant molecules.8 Studies of patients with inherited thrombophilias have led to a model in which even subtle perturbations of this balance can increase thrombotic risk by several fold.8-12 People with chronically elevated levels of procoagulant proteins, including coagulation factors VIII, IX, and XI, and prothrombin are at increased risk, as are people with chronically lower levels of anticoagulant proteins, including protein C, antithrombin, and protein S. A mutation in the coagulation factor V gene (factor V Leiden) that renders it resistant to proteolytic inactivation by activated protein C is the most common inherited thrombophilia in white populations, but is uncommon in African and Asian populations.13-16 Recently a role for cell-derived microparticles in hemostasis has been proposed.17,18 These are fragments of cell membranes that bud off of blood cells, endothelial cells, and cancer cells during cellular activation and/or apoptosis and can serve as sites for assembly of coagulation enzymes and as carriers of tissue factor procoagulant, the most important initiator of thrombin generation. Since microparticles are generated during many chronic diseases, including inflammatory disorders and cancer, they may provide a mechanistic link between common age-associated comorbidities and thrombosis.
There is surprisingly little known about how prothrombotic factors relate specifically to age-related increases in thrombosis risk. In part, this is because research has been hindered by the lack of animal models of spontaneous venous thrombosis. VTE is a disease that appears to be unique to the human condition, perhaps related to bipedalism and to evolutionary pressures from birth trauma associated with large neonatal cranial size. Nevertheless, recent studies of thrombosis in mice induced by chemical or light injury19 suggest that clot formation is a dynamic process and that platelets and microparticles play a role in addition to tissue factor–driven thrombin generation.17,19 Whether these models can yield insight into age-related thrombosis risk remains to be determined. If the molecular and genetic mechanisms responsible for age-dependent increases in thrombosis in mice are similar to those in humans, it could provide a model for investigating mechanisms and potential interventions. The dynamic nature of venous thrombi observed in mouse models may impact the question of how and why clots in only a subset of patients with DVT embolize. While DVT is often associated with serious complications, only PE is capable of causing death; thus greater understanding of what contributes to the risk of embolization is needed.
Among important research questions that remain unanswered are the specific roles that blood cells, plasma components, microparticles, and endothelium may play in age-related VTE risk; whether chronic inflammation shifts the hemostatic balance—and if so, how much is required; and the role of age-related changes in the microcirculation and in cellular responses to drugs, such as estrogens. Age-related increases in circulating D-dimer, factor VIII, and thrombin fragment F1+2 have been noted,11,20 but their relationship to VTE is not known. Age-associated loss of vein structural integrity as a contributor to VTE risk, postthrombotic syndrome, and bleeding on anticoagulation therapy should also be studied.
Epidemiology of VTE
In contrast to the array of studies of cardiovascular disease risk in populations, such as Framingham and the Northwick Park Heart Study, there is markedly less information available regarding VTE. Most available information comes from single institution experiences or from studies of subjects and families with known thrombophilia or prior thrombosis, such as the Leiden Thrombophilia Study.12,21,22 One large prospective population study is the Longitudinal Investigation of Thromboembolism Etiology (LITE), which identified VTE in 2 cohorts totaling approximately 21 000 people aged 45 to 100: ARIC (Atherosclerosis Risk in Communities) and CHS (Cardiovascular Health Study).15,20,23 The age-standardized incidence of first VTE reported in LITE was 1.92 per 1000 person years, with men having slightly higher risk than women and incidence rates increasing markedly as a function of age. Autopsy data, however, suggest that reported incidence rates are markedly underestimated.5
Studies have revealed few specific insights as to the etiologies of age-dependent thrombotic risk, other than presence of comorbid conditions, general frailty,24 and associated common acquired risk factors such as surgery,25 hospitalization for acute medical illness, nursing home confinement, trauma, cancer, neurologic disease, previous superficial vein thrombosis, and central venous catheters.2-4 All studies show that markers of chronic systemic inflammation increase with age, but unlike the case of atherothrombotic disease, there is no clear association between chronic inflammation and VTE.10,20 A link with acute inflammation, however, has been reported.26 Body weight also increases with age,27 and obesity is increasingly recognized as an important VTE risk factor.28-30 Underlying acquired biochemical and genetic thrombophilias, such as antiphospholipid syndrome and factor V Leiden, though known to increase risk in the elderly, have little predictive value for individual patients.12 A reasonable model to understand VTE risk3 is that an event occurs in a patient at a point in time when the “thrombosis threshold” is exceeded by the sum total of acquired and genetic risk factors in the context of one or more specific triggering factors that can be balanced by appropriate use of prophylaxis.
VTE is not a benign disease. Population-based studies have reported 30-day case fatality rates for DVT of 5% and for PE of 33%; 25% of patients with PE present as sudden death.31 In the LITE cohort, the 28-day case-fatality rate was 11% after a first clinically recognized VTE.23,31 Predictors of death after a first VTE include age; obesity; tobacco use; heart failure; chronic lung, kidney, or neurologic disease; and cancer. Of patients who survive, 30% develop recurrence and 30% to 50% develop postthrombotic syndrome (PTS) within 10 years.32-34 Risk for recurrence increases with age (∼ 15%-20% increase per decade) and is higher in patients with cancer, obesity, neurologic disease, and certain thrombophilic conditions. Patients with more than one VTE are at higher risk for another, as are patients whose first event is PE rather than DVT. Recent research has identified potential biomarkers, such as D-dimer,35-37 that might predict increased risk of recurrence, but these data have not yet been validated in the elderly who are known to have higher D-dimer levels in the absence of history of VTE.11,20
Little information is available regarding quality of life (QOL) after VTE. A recent small study revealed that VTE patients scored lower on a standard QOL survey, especially if they had PTS.38 Another suggested that the QOL impairment for some patients was temporary, but the results were not reported in the context of aging.39 For patients with marginal physical capacity prior to an event, the incremental decline resulting from DVT would probably have a greater impact, but this remains to be tested.
Much remains to be learned about the epidemiology of VTE in the elderly. VTE is not usually assessed in large National Institutes of Health (NIH)–sponsored population cohort studies; it may be possible to build information by adding such assessments to ongoing studies, and NIH should be encouraged to support such efforts. Administrative databases (such as Medicare) include large numbers of subjects, but limited information and validation. There may be opportunities to make administrative databases more effective by capturing information on physical and cognitive functional status. The Centers for Disease Control Thrombosis and Hemostasis Research and Prevention Network may also be a venue for this work.
Diagnosis, treatment, and prevention of VTE in the elderly
Combining an assessment of pretest probability (PTP) using established clinical prediction rules with a D-dimer assay is used to evaluate patients with suspected VTE.40 Low PTP and normal D-dimer levels effectively exclude the diagnosis. Patients with high PTP or elevated D-dimer are usually evaluated by compression ultrasound and/or computed tomography (CT) angiography to detect DVT or PE. A problem applying this decision model to the elderly is that D-dimer levels increase with age11,20 so the cutoff of less than 500 ng/mL may be less useful. Use of age-adjusted cutoff levels might be an effective strategy, but the impact on sensitivity is unknown.
Once DVT is diagnosed, anticoagulant therapy is used unless there is a contraindication. In such cases, retrievable or permanent inferior vena cava filters can be inserted depending on whether the contraindication is temporary or permanent. Studies of long-term efficacy of filters or their late complications in the elderly have not been done. For extensive iliofemoral DVT or evidence of compartment compression syndrome, thrombolytic therapy can be considered; this can be accomplished via localized catheter-based delivery systems, some of which include mechanical clot destruction and removal.41 Again, little data on thrombolytic therapy in the elderly are available.
After initial anticoagulation for at least 5 days with unfractionated or low-molecular-weight heparin overlapping with the oral vitamin K antagonist warfarin, oral anticoagulation (OAC) is continued for various durations. Special challenges of OAC in the elderly include lower and more variable warfarin dose requirements and increased risk of major hemorrhage.42 Complicating factors include the difficulty some elderly patients encounter in getting to a laboratory for monitoring INR at regular intervals and in following often complicated dosing schedules. The elderly have increased risk of bleeding, including intracranial hemorrhage, but the underlying mechanisms are unknown and could relate to genetic predisposition and/or loss of vascular wall integrity. In the brain, the latter may be influenced by age-related amyloid deposition and decline in brain mass, which may render vessels more susceptible to physical damage. The risks of OAC in older people are compounded by common coexisting need for other medications, many of which interact with warfarin. To the extent that drugs are added and withdrawn, it is difficult to maintain therapeutic levels of anticoagulation. Elderly patients also are more likely to have inadequate dietary habits, and studies suggest that low vitamin K intake contributes to fluctuating levels of anticoagulation.43 Whatever the cause, fluctuating INRs require more frequent office visits, placing additional strain on the patient and family. Thus, adherence to prescribed medications is likely to be lower, compounding the difficulty in achieving safe and effective management. A recent study of self-management found that elderly patients undergoing self-monitoring were in the appropriate therapeutic range more often than those followed by routine methods.44
Following DVT, a chronic symptom complex of pain, swelling, and skin abnormalities known as PTS often occurs.45-48 Causes may relate to incomplete clot resolution and/or valve damage. Such symptoms cause significantly greater morbidity in the elderly and may seriously impact functional independence. Some QOL studies show that patients who undergo successful thrombolysis do better than patients who receive anticoagulants alone or in whom thrombolysis failed,41,46 but no randomized trial data are available on the effectiveness of thrombolysis in preventing PTS. Risk factors for development of PTS are poorly characterized, and it is not known whether it is more frequent or resolves more slowly in older adults. To the extent that aging involves dysregulation of inflammatory pathways, older individuals may be at increased risk of PTS if inflammation plays a pathogenic role.
Effective strategies have been developed to prevent VTE in high-risk hospitalized patients.49 These include mechanical lower extremity compression devices and/or anticoagulant drugs such as unfractionated or low-molecular-weight heparin or fondaparinux. Most regimens call for lower doses than are typically used to treat established VTE and use fixed administration schedules without monitoring blood tests. The efficacy of these approaches depends on a successful balance of treating those at highest risk of VTE while avoiding those most likely to suffer a bleeding complication.50 There are limited data on the patterns of use of prophylaxis in the elderly, although anecdotal evidence suggests that because of fear of bleeding risk prophylaxis is underutilized on both surgical and medical services. The potential benefit of thromboprophylaxis in nursing home patients has also not been studied.
Clinical research on VTE in the elderly
Better oral anticoagulant drugs are needed for long-term use by elderly patients. This unmet need may not be adequately addressed by current clinical trials that frequently exclude the elderly because of the existence of comorbidities, general frailty, and/or difficulty in accrual. Informed consent may be challenging for elderly patients with impaired cognitive function, and enrolling elderly patients in clinical trials of new anticoagulants for prophylaxis is complicated by FDA mandates for venography as an end point. This invasive procedure is more likely to produce morbidity in elderly patients and therefore limits enthusiasm for enrollment. It is thus not clear if results from existing clinical trials of VTE treatment and prevention are applicable to elderly patients.
Most clinical trials for anticoagulation therapies use thrombosis and bleeding as primary end points. In the elderly, equally important are the impact on functional status and maintenance of autonomy. This is particularly true in studies of PTS. Given the difficulties in enrolling elderly patients in pivotal phase 3 trials of new anticoagulant agents, postmarketing studies that include elderly patients would be helpful, especially if they include assessments of QOL and functional status.
A major unmet need in clinical thrombosis research is development of predictive biomarkers or genetic markers that could identify low- or high-risk patients for thrombosis, embolization, PTS, and bleeding on OAC. Such markers could be valuable in determining which hospitalized patients would gain benefit from VTE prophylaxis and who might benefit from thrombolysis or prolongation of OAC after a first VTE. For the arterial side of the circulation, noninvasive measures of endothelial function are available (eg, brachial artery vascoreactivity),51 but no measures of endothelial health on the venous side exist.
Conclusions
There is a strong need to understand the aging/thrombosis interface. Laboratory scientists should be encouraged to examine mechanisms that increase VTE risk with age and to explore the pathophysiology of embolization and PTS. Clinical investigators should focus on developing safe and effective treatment strategies, including optimal approaches for the use of warfarin, defining the role for thrombolytics, and establishing indications for vena cava filters. Furthermore, as new anticoagulants are developed, they should be tested in older patients to accurately define safe and effective dosing. Epidemiologic studies are needed to clarify and identify specific risk factors for VTE and its complications in the elderly and to determine the effectiveness of thromboprophylaxis in hospitalized elderly patients. The importance of VTE with regard to the critical geriatric issues of function, independence, and QOL could be approached by a large, multicenter inception cohort study with appropriate outcome measures.
Acknowledgments
This paper summarizes findings of a workshop organized and sponsored by the American Society of Hematology (ASH) in May 2006 to examine the problem of VTE in the elderly. The authors wish to acknowledge the contributions of all participants in the workshop and thank the staff of ASH, especially Stephanie Kart, for their outstanding help in organizing the workshop.
Authorship
Contribution: R.L.S. chaired the ASH workshop and wrote the paper; K.A.B., M.C., W.B.E., C.T.E., and R.P.T. chaired sessions during the workshop and contributed to the writing and editing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roy L. Silverstein, Chairman, Department of Cell Biology, Lerner Research Institute NC10, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: silverr2@ccf.org.