Abstract
Neutrophils are professional phagocytes that migrate early, in high number, to the infection sites. Our study has analyzed how neutrophils cross-present antigens and influence CD8+ T-cell responses. By using highly purified neutrophils from peritoneal exudates and bone marrow, we have shown that neutrophils cross-present ovalbumin to a CD8+ T-cell hybridoma and to naive CD8+ T cells from OT1 transgenic mice. Cross-presentation by neutrophils was TAP and proteasome dependent and was as efficient as in macrophages. Moreover, it actually occurred earlier than in professional antigen-presenting cells. Peritoneal exudate neutrophils from mice injected intraperitoneally with ovalbumin also cross-presented ovalbumin, proving that neutrophils take up and present exogenous antigens into major histocompatibility complex I (MHC I) molecules in vivo. We then evaluated the in vivo influence of antigen cross-presentation by neutrophils on CD8+ T-cell response using β2-microglobulin-deficient mice transferred with OT1 CD8+ T cells and injected with ovalbumin-pulsed neutrophils. Four days after neutrophil injection, OT1 cells proliferated and expressed effector functions (IFN-γ production and cytolysis). They also responded efficiently to a rechallenge with ovalbumin-pulsed dendritic cells in CFA. These data are the first demonstration that neutrophils cross-prime CD8+ T cells in vivo and suggest that they may constitute, together with professional antigen-presenting cells, an attractive target to induce cytotoxic T cells in vaccines.
Introduction
Although it was originally thought that peptides presented into major histocompatibility complex I (MHC I) molecules to CD8+ T cells derived exclusively from endogenous proteins, it is now admitted that, under certain circumstances, professional antigen-presenting cells (APCs; dendritic cells [DCs] and macrophages) can present some exogenous antigens in the MHC I molecules.1,2 This process, called antigen cross-presentation, can occur by 2 different mechanisms. One is dependent on the transporter associated with antigen processing (TAP) that includes proteasome-dependent and -independent pathways, and the other one is TAP independent.3
Due to their ability (1) to migrate from peripheral tissues to the lymph nodes, where they encounter recirculating naive T cells, and (2) to express high levels of costimulatory molecules, DCs are usually considered as the only APC able to prime naive T cells.4,5 This property has recently been extended to murine macrophages.6 Activated DCs can present some exogenous proteins to naive CD8+ T cells and induce their differentiation into effector cytotoxic T cells (CTLs). This process, referred to as cross-priming, allows the in vivo generation of protective cytotoxic responses against microorganisms that do not infect DCs or that infect DCs but inhibit their properties.7 Cross-priming by DCs has opened new perspectives in vaccines based on CTL induction.7,8 In the absence of licensing, DCs stimulate an abortive response that leads to cross-tolerance rather than immunity. In this context, upon ingestion of apoptotic cells, bone marrow–derived APCs present self-derived peptides in MHC I molecules to CD8+ T cells and participate in the maintenance of peripheral tolerance.9 Murine liver sinusoidal endothelial cells also cross-present antigens and may contribute, in some circumstances, to maintain tolerance against soluble antigens in vivo.10 The origin of the antigen, the nature of the cross-presenting cell, the presence of CD4+ T-cell help,11 and the local microenvironment control the outcome of cross-presentation (cross-priming versus cross-tolerance).3
Innate immune cells participate to the first line of defense against invading microorganisms. Among them, polymorphonuclear neutrophils rapidly migrate at the infection site (1 to 4 hours after microorganism entry) and accumulate locally more than any other cell type. Their migration and subsequent activation are controlled by chemokines, such as CXCL8, CCL3, or CXCL2.12,13 Microorganism-derived Toll-like receptor (TLR) agonists also activate neutrophils.14 Neutrophils exhibit potent phagocytic properties similar to those of macrophages15 and have a unique arsenal of microbicidal mediators that are rapidly released upon contact with pathogens. Microorganisms are either phagocytosed by neutrophils and destroyed via oxygen-dependent and -independent mechanisms or sequestered in extracellular traps.16
Although historically regarded as strict innate cells characterized by the ability to kill, accumulating data suggest that neutrophils may influence adaptive immunity by acting either indirectly (via APCs) or directly on T cells.17,18 (1) Activated neutrophils modulate DC maturation18-21 and trafficking.22,23 (2) Infected neutrophils may serve as substrates for antigen cross-presentation by DCs.24 (3) Activated neutrophils acquire the expression of MHC II molecules, costimulatory molecules (such as CD80 in mouse and CD86 and CD80 in human),25,26 and the DC maturation marker CD8327 and present in vitro antigens into MHC II molecules to memory CD4+ T cells.28-30 (4) Murine neutrophils phagocytose and process exogenous bacteria in vitro via an alternate MHC I–processing pathway.31 (5) Lastly, in some circumstances, neutrophils migrate from the periphery to the lymph nodes, where they may encounter recirculating T cells.18,32
In this study, we evaluated whether neutrophils may cross-present antigens and participate in vivo in the control of CD8+ T-cell responses to exogenous antigens. We provide evidence that murine and human neutrophils cross-present exogenous antigens in vitro. Using an in vivo model, in which professional APCs do not express functional MHC I molecules, we show that injection of antigen-pulsed neutrophils induces naive CD8+ T-cell differentiation into cytotoxic T cells.
Patients, materials, and methods
Animals
C57BL/6 mice and ovalbumin (Ova)-specific T-cell receptor (TCR) transgenic mice OT1 were from Charles River Laboratories (L'Arbresle, France). B6;129S-TAP1tm1Arp (TAP−/−) mice and β2-microglobulin–deficient mice (B6.129P2-β2mtm1Unc) were from Institut Curie (Paris, France). Experiments were conducted according to institutional guidelines.
Ovalbumin
Ova (Affiland, Liège, Belgium) was used after dialysis33 and endotoxin decontamination (Profos, Regensburg, Germany). Ova was conjugated to iron oxide beads (Biomag; Perseptive Diagnostics, Cambridge, MA) and the amount of Ova bound to the beads was calculated as described by the manufacturer. The same concentrations of Ova, either free or bound to beads, were used, in parallel, in the experiments.
Murine BMN and PEN purification
Single-cell bone marrow preparations were placed on top of a Percoll (Sigma, St Louis, MO) step gradient (52%, 65%, and 75% Percoll in PBS). An enriched neutrophil preparation was recovered at the interface between 65% and 75% Percoll. Then, bone marrow neutrophils (BMNs) were purified by positive selection based on Ly-6G expression (using PE-labeled anti–Ly-6G monoclonal antibody [mAb]; BD Pharmingen, Le pont de Claix, France) and anti-PE mAb magnetic microbeads (Miltenyi Biotech, Bergish Gladbach, Germany). Cell purity, determined by fluorescence-activated cell sorter (FACS) staining with anti–Ly-6G and antineutrophil (clone 7/4) mAbs, was greater than 99%. BMNs were incubated overnight in RPMI medium supplemented with 2 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, 10 mM Hepes, and 0.1 mM nonessential amino acids (cRPMI; all from Life Technologies, Cergy Pontoise, France) supplemented with 50 μM β-mercaptoethanol (Sigma) and 10% FCS (Cambrex, Saint-Beauzire, France) and containing 5 ng/mL of GM-CSF (R&D Systems, Abingdon, United Kingdom).
Peritoneal exudate cells, obtained 6 hours after intraperitoneal injection with 1 mL 3% thioglycollate (Biomérieux, Marcy l'étoile, France), were depleted in MHC II+ cells using anti–MHC II mAb-coated magnetic beads (Miltenyi Biotech) before sedimentation on a discontinuous Percoll gradient, followed by a positive magnetic selection of Ly-6G+ cells. Peritoneal exudate neutrophil (PEN) purity, determined by FACS, was routinely greater than 99%.
Human neutrophil purification
After Ficoll-Paque centrifugation of peripheral blood from healthy subjects, neutrophils were separated from erythrocytes by 3% dextran (Amersham Biosciences, Uppsala, Sweden) density gradient sedimentation. Purity, determined by FACS analysis on forward scatter/side scatter parameters and using specific mAbs (see “Flow-cytometry analysis”) was greater than 99%. Human blood samples were obtained with written informed consent in accordance with the Declaration of Helsinki and Angers University Hospital ethical committee requirements.
Murine DC and macrophage generation
Murine bone marrow–derived DCs were prepared as described.34 DCs were cultured for 5 days in 10% FCS cRPMI with 5 ng/mL GM-CSF. At day 5, nonadherent immature DCs (that express CD11c and intermediate levels of I-Ab) were used in in vitro and in vivo experiments. Macrophages were isolated from mice peritoneal exudates 5 days after thioglycollate injection. DC and macrophage populations contained more than 95% of CD11c+ and F4/80+ cells, respectively.
Murine naive CD8+ T-cell purification
Naive CD8+ T cells from OT1 mice were isolated from spleen and lymph nodes using the CD8+ T-cell isolation kit (Miltenyi Biotech), followed by 2 additional purification steps: depletion of DCs by CD11c+ microbeads, and then positive selection of naive CD62Lhigh cells using CD62L+ microbeads (Miltenyi Biotech). Cell purity, determined by FACS staining for CD3, CD8, CD44, and CD62L, was greater than 99%.
Flow-cytometry analysis
Murine cells were phenotyped using FITC-labeled antineutrophils (rat IgG2a; clone 7/4; Serotec, Oxford, United Kingdom), anti–MHC I H2-Kb, anti–MHC II I/Ab, anti-CD3, anti-CD11b, anti-CD11c, anti-CD62L, anti-CD44, anti-CD80, and anti-CD86 mAbs, PE-labeled anti–Ly-6G, anti-CD8α (all from BD Pharmingen, San Diego, CA), and anti-F4/80 Abs (Dako, Glostrup, Denmark). FITC- and PE-labeled isotype controls were from Serotec and BD Pharmingen. In some experiments, murine neutrophils were analyzed with the 25-D1.16 anti–H2-Kb-SIINFEKL mAb (kindly provided by Prof Claude Perreault, University of Montreal, Montreal, QC). In others, murine neutrophils were incubated for 20 minutes at 37°C with Texas Red–labeled Ova (Invitrogen, Carlsbad, CA) before FACS analysis. Human neutrophil purity was assessed using FITC-labeled anti-CD13, anti-CD3, anti–BDCA1–3, and anti-CD14 mAbs (BD Pharmingen). Isotype control mAbs were from BD Pharmingen. Cells were analyzed using a FACScalibur cytofluorometer (BD Biosciences, Erembodegem, Belgium).
In vitro and ex vivo antigen cross-presentation assays
Murine PENs and BMNs from wild-type and TAP−/− mice, DCs, macrophages, and LB27.4 cells (ATCC, Manassas, VA), used at 2 × 105 cells/well in 96-well plates (or at the indicated number of cells/well), were pulsed for different time periods with different concentrations of Ova. In some experiments, cells were also incubated with 10 μM lactacystin (Sigma). After washing, cells were fixed with 0.01% glutaraldehyde (Sigma) for 3 minutes at room temperature, washed, and incubated with 1 × 105 ovalbumin-specific CD8+ B3Z T-hybridoma cells or naive OT1 CD8+ T cells for 18 hours and 24 hours, respectively, in 1% FCS cRPMI. B3Z cell activation was measured using the fluorogenic substrate methyl-umbelliferyl-D-galactoside (Sigma).35 Results are expressed as stimulation index (SI) defined as follows: SI = (A − B)/B, where A and B are the levels of fluorescence obtained in response to cells pulsed with or without Ova, respectively. OT1 T-cell stimulation was assessed by measuring IL-2 production by enzyme-linked immunosorbent assay (ELISA; BD Pharmingen). In ex vivo experiments, C57BL/6 mice were intraperitoneally injected with thioglycollate + 200 μg ovalbumin. PENs were purified and cultured with OT1 CD8+ cells for 24 hours in 96-well plates. Cross-presentation by human neutrophils was analyzed using the CD8+ T-cell clone NS3–1 specific for the HLA-A2 binding peptide 1406-1415 of the HCV NS3 c33c subtype 1a protein.36 Human neutrophils (HLA-A2+) pulsed with different concentrations of HCV NS3 c33c protein subtype 1a (amino acids 1192-1459; Biodesign, Saco, ME) or with Ova (used as negative control) were incubated for 6 hours in 1% FCS cRPMI, washed, and cultured with the NS3 TCC (at a 1:1.5 T/neutrophil cell ratio). IFN-γ was quantified by ELISA in the 24-hour supernatants (Mabtech AB, Stockholm, Sweden).
In vivo antigen cross-presentation
PENs and BMNs (2 × 106) from wild-type mice pulsed with 0.4 mM Ova (for 4 h and 2 h, respectively) in 1% FCS cRPMI were washed and intravenously or subcutaneously transferred into β2-microglobulin−/− mice. Naive OT1 CD8+ T cells (3 × 106) were stained with 5 μM fluorescent dye CFDA-SE (Molecular Probes, Carlsbad, CA) before intravenous injection into β2-microglobulin−/− mice at day 1. At day 4, spleen and lymph node cells were incubated with PE-labeled anti-CD8α+ mAb and analyzed by flow cytometry for CFDA-SE expression.
In vivo assay for immune tolerance
OT1 CD8+ T cells (3 × 106) were intravenously injected into β2-microglobulin−/− mice. After 24 hours, mice were intravenously injected with PBS, 200 μg Ova, and 3 × 106 unpulsed neutrophils (BMNs and PENs), or with 3 × 106 BMNs, PENs, and LB27.4, or with DCs previously pulsed with 0.4 mM Ova. At day 13, mice were boosted with CFA (Sigma) plus 2 × 106 DCs pulsed with or without Ova. Three days later, spleen and lymph node cells were restimulated in vitro and IL-2 and IFN-γ were quantified by ELISA (BD Pharmingen).
In vivo cytolysis assay
In vivo CTL assay was performed as described.37 Briefly, a 1:1 mixture of SIINFEKL peptide-pulsed and unpulsed syngeneic splenocytes (3 × 106 each) labeled with 10 (CFSEhigh) and 1 μM (CFSElow) CFDA-SE, respectively, were intravenously injected into β2-microglobulin−/− mice that were previously intravenously or subcutaneously injected with 200 μg Ova; 3 × 106 unpulsed neutrophils (BMNs and PENs); 3 × 106 BMNs, PENs, or LB27.4; or DCs previously pulsed with 0.4 mM Ova. Three hours later, lymph node and spleen cells were evaluated by flow cytometry for CFDA-SE expression. Specific killing was evaluated by the reduction of the CFSEhigh population without any reduction of the CFSElow population relative to control mice according to the following formula: 100 − [(number of peptide-pulsed cells in primed mouse/number of unpulsed cells in primed mouse)/(number of peptide-pulsed cells in unprimed mouse/number of unpulsed cells in unprimed mouse) × 100].6
Statistical analysis
Data are shown as mean (± SD) and analyzed either by the Mann-Whitney test or the analysis of variance (ANOVA) test. P value less than .05 was taken as the level of significance.
Results
Murine neutrophils cross-present soluble and particulate antigens
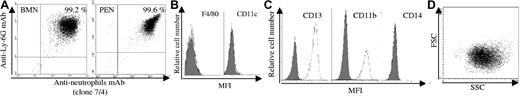
In this study, we analyzed whether murine neutrophils isolated from peritoneal exudates (PENs) or from bone marrow (BMNs) may cross-present soluble Ova. In order to exclude the involvement of contaminating APCs in in vitro and in vivo experiments, we used highly purified populations of neutrophils obtained by 2- (for BMNs) or 3-step (for PENs) purification protocols. Phenotypic analysis showed that neutrophil populations consisted of more than 99% of Ly-6Ghigh–positive and clone 7/4-positive cells (Figure 1A) as well as of CD11c-negative and F4/80-negative cells (Figure 1B). Giemsa staining also showed that more than 99% of cells exhibited a neutrophil morphology (intracellular granules and segmented nucleus; data not shown).
Purity of human and murine neutrophils. (A) Analysis by FACS of BMN (left panel) and PEN (right panel) purity using PE-labeled anti–Ly-6G mAb and FITC-labeled antineutrophil mAb (7/4). (B) Analysis of BMN purity using FITC–anti-CD11c and PE–anti-F4/80 mAbs (white histograms; gray histograms correspond to isotype control mAbs); similar data were obtained for PENs. (C,D) Analysis by FACS of human neutrophil purity using FITC–anti-CD13, anti-CD11b, and anti-CD14 mAbs (white histograms; gray histograms correspond to isotype control mAbs (C) and using the FSC/SSC parameters (D).
Purity of human and murine neutrophils. (A) Analysis by FACS of BMN (left panel) and PEN (right panel) purity using PE-labeled anti–Ly-6G mAb and FITC-labeled antineutrophil mAb (7/4). (B) Analysis of BMN purity using FITC–anti-CD11c and PE–anti-F4/80 mAbs (white histograms; gray histograms correspond to isotype control mAbs); similar data were obtained for PENs. (C,D) Analysis by FACS of human neutrophil purity using FITC–anti-CD13, anti-CD11b, and anti-CD14 mAbs (white histograms; gray histograms correspond to isotype control mAbs (C) and using the FSC/SSC parameters (D).
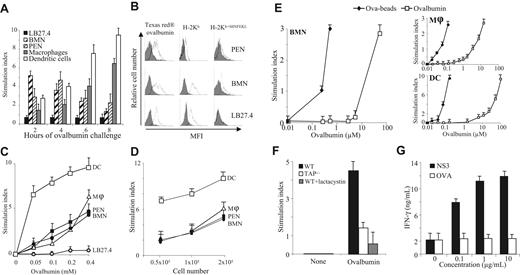
Neutrophils were pulsed with 0.4 mM Ova for 2 to 8 hours and used to stimulate the Ova-specific CD8+ T-cell hybridoma B3Z. Results showed that PENs and BMNs efficiently cross-presented Ova to B3Z cells, with a maximum observed when BMNs were pulsed for 2 hours (SI = 5.4 ± 0.5; mean ± SD, n = 5) and PENs were pulsed for 4 hours (SI = 4.9 ± 0.8; Figure 2A). In the following experiments, BMNs and PENs were pulsed for 2 and 4 hours, respectively. Cross-presentation by thioglycolate-elicited macrophages and bone marrow–derived DCs, used as positive controls, was maximal when pulsed for 8 hours with Ova (SI = 6.4 ± 0.5 and 9.3 ± 0.7, respectively; Figure 2A). The LB27.4 cell line, used as a negative control,2 did not cross-present Ova, regardless of the time point analyzed (Figure 2A). Antigen uptake is required to endow a cell with cross-presentation capacity. Supporting our observations, PENs and BMNs efficiently internalized Ova (Figure 2B left panels), expressed cell-surface MHC I molecules (Figure 2B middle panels), and presented SIINFEKL in Kb molecules after Ova pulse (Figure 2B right panels). In contrast, although LB27.4 cells expressed MHC I molecules and endocytosed Ova, they did not express Kb-SIINFEKL after Ova pulse, showing that antigen uptake is not sufficient for antigen cross-presentation (Figure 2B).
Antigen cross-presentation by neutrophils. (A) LB27.4 cells, BMNs, PENs, macrophages, and DCs pulsed for 2, 4, 6, or 8 hours with 0.4 mM Ova were incubated with B3Z cells. B3Z stimulation was measured by quantifying the release of β-galactosidase. Results are expressed in SI as mean (± SD) of 5 experiments. (B; Left panel) PENs, BMNs, and LB27–4 were incubated (white histogram) or not (gray histogram) for 20 minutes at 37°C with 0.4 mM Texas Red–Ova. (Middle panel) Cells were incubated with anti–H-2Kb mAb (white histogram) or isotype control mAb (gray histogram). (Right panel) Cells were pulsed with 0.4 mM Ova for 2, 4, and 8 hours, respectively, at 37°C before detection by FACS of SIINFEKL in MHC I molecules, using the 25-D1.16 mAb (white histogram) or isotype control mAb (gray histogram). Results are representative of 1 of 3 experiments. (C) DCs (□), macrophages (▵), PENs (■), BMNs (●), and LB27.4 (○) pulsed with the indicated concentrations of Ova for 2 hours (BMNs), 4 hours (PENs), or 8 hours (macrophages, DCs, and LB27.4) were incubated with B3Z cells. (D) Different numbers of DCs (□), macrophages (▵), PENs (■), and BMNs (●) pulsed with 0.4 mM Ova for 2 hours (BMNs), 4 hours (PENs), or 8 hours (macrophages and DCs) were incubated with B3Z cells. (E) BMNs (left panel), macrophages (top right panel), and DCs (bottom right panel) pulsed with the indicated concentrations of Ova, either soluble (□) or coated to beads (♦), were incubated with B3Z cells. (F) BMNs from wild-type mice either untreated (■) or treated with lactacystin (▩) and BMNs from TAP−/− mice (□) pulsed with 0.2 mM Ova for 2 hours were incubated with B3Z cells. (A,C-E) Results are expressed in SI as mean (± SD) of 5 experiments. (G) HLA-A2+ human neutrophils pulsed with or without 0.1 to 10 μg/mL HCV1a-NS3 protein (■) or with Ova (□), for 4 hours, were cultured with a human CD8+ T-cell clone specific for the HCV-NS3 peptide 1406-1415. IFN-γ production was quantified by ELISA in the 16-hour supernatants. Results are expressed in ng/mL as mean (± SD) of triplicate values and are representative of the data obtained with the neutrophils of 1 of 3 subjects.
Antigen cross-presentation by neutrophils. (A) LB27.4 cells, BMNs, PENs, macrophages, and DCs pulsed for 2, 4, 6, or 8 hours with 0.4 mM Ova were incubated with B3Z cells. B3Z stimulation was measured by quantifying the release of β-galactosidase. Results are expressed in SI as mean (± SD) of 5 experiments. (B; Left panel) PENs, BMNs, and LB27–4 were incubated (white histogram) or not (gray histogram) for 20 minutes at 37°C with 0.4 mM Texas Red–Ova. (Middle panel) Cells were incubated with anti–H-2Kb mAb (white histogram) or isotype control mAb (gray histogram). (Right panel) Cells were pulsed with 0.4 mM Ova for 2, 4, and 8 hours, respectively, at 37°C before detection by FACS of SIINFEKL in MHC I molecules, using the 25-D1.16 mAb (white histogram) or isotype control mAb (gray histogram). Results are representative of 1 of 3 experiments. (C) DCs (□), macrophages (▵), PENs (■), BMNs (●), and LB27.4 (○) pulsed with the indicated concentrations of Ova for 2 hours (BMNs), 4 hours (PENs), or 8 hours (macrophages, DCs, and LB27.4) were incubated with B3Z cells. (D) Different numbers of DCs (□), macrophages (▵), PENs (■), and BMNs (●) pulsed with 0.4 mM Ova for 2 hours (BMNs), 4 hours (PENs), or 8 hours (macrophages and DCs) were incubated with B3Z cells. (E) BMNs (left panel), macrophages (top right panel), and DCs (bottom right panel) pulsed with the indicated concentrations of Ova, either soluble (□) or coated to beads (♦), were incubated with B3Z cells. (F) BMNs from wild-type mice either untreated (■) or treated with lactacystin (▩) and BMNs from TAP−/− mice (□) pulsed with 0.2 mM Ova for 2 hours were incubated with B3Z cells. (A,C-E) Results are expressed in SI as mean (± SD) of 5 experiments. (G) HLA-A2+ human neutrophils pulsed with or without 0.1 to 10 μg/mL HCV1a-NS3 protein (■) or with Ova (□), for 4 hours, were cultured with a human CD8+ T-cell clone specific for the HCV-NS3 peptide 1406-1415. IFN-γ production was quantified by ELISA in the 16-hour supernatants. Results are expressed in ng/mL as mean (± SD) of triplicate values and are representative of the data obtained with the neutrophils of 1 of 3 subjects.
In additional experiments, neutrophils and professional myeloid APCs (all used at 2 × 105/mL) were pulsed with increasing concentrations of Ova and used to stimulate B3Z cells. PENs and BMNs cross-presented Ova in a dose-dependent manner (Figure 2C); PENs and BMNs were as potent as macrophages (excepted for the highest concentration of Ova tested [0.4 mM]), while less efficient than DCs (at any concentration of Ova tested; Figure 2C). Experiments performed with graded doses of myeloid cells reinforce this observation and also evidence a direct relationship between the number of neutrophils and the amplitude of the CD8+ T-cell response (Figure 2D). Particulate antigens have been reported to be more efficiently cross-presented than soluble antigens by professional APCs.2,38 We then tested the ability of neutrophils to cross-present Ova coupled to iron beads (Ova-beads). Cross-presentation by BMNs required 100 lower concentrations of Ova when coupled to iron beads compared with soluble Ova (Figure 2E). PENs and macrophages were as potent as BMNs in cross-presenting Ova beads (Figure 2E top right panel). In contrast, DCs were more efficient than macrophages and neutrophils in cross-presenting soluble Ova and Ova beads (Figure 2C,E bottom right panel). Together, these data demonstrate that neutrophils cross-present soluble and particulate antigens in vitro and that antigen cross-presentation by neutrophils occurs earlier than in myeloid APCs and is as efficient as in macrophages.
Antigen cross-presentation by murine neutrophils is proteasome and TAP dependent
One of the major antigen cross-presentation pathway depends on proteasomes and TAP. To investigate the mechanism(s) involved in antigen cross-presentation by neutrophils, we first used neutrophils purified from TAP−/− mice. Cross-presentation by neutrophils from TAP−/− mice was reduced (SI = 1.2 ± 0.4, corresponding to a decrease of 65% ± 10%; mean ± SD, n = 3) compared with neutrophils from wild-type mice (SI = 4.3 ± 0.4; Figure 2F). Moreover, incubation of wild-type BMNs with lactacystin reduced Ova cross-presentation (SI = 0.5 ± 0.8; decrease of 85% ± 15%; Figure 2F). Similar data were observed using PENs (data not shown). These data show that neutrophils process and cross-present soluble Ova via a proteasome- and TAP-dependent mechanism.
Human neutrophils cross-present soluble antigens
We then evaluated whether human peripheral blood neutrophils may also cross-present soluble antigens using the NS3–1 CD8+ T-cell clone (NS3 TCC), which is specific for the HLA-A2–restricted peptide NS31406-1415 of the HCV-NS3 c33c protein.36 Activation of the NS3–1 TCC was assessed by measuring IFN-γ secretion.39 We excluded the presence of contaminating myeloid APCs in human neutrophils by FACS analysis: greater than 99% of the cells were CD13+, CD11b+ (Figure 1C) and presented forward and side scatter (FSC/SSC) parameters characteristic of neutrophils (Figure 1D). No CD14+ (Figure 1C) or BDCA1–3+ cells (data not shown) were detected. Human neutrophils were pulsed with NS3 or Ova (as a negative control) and used to stimulate the NS3–1 TCC. Results showed that human neutrophils pulsed with NS3, but not with Ova, stimulated the NS3–1 TCC in a manner that was dependent on the concentration of NS3 (Figure 2G). These data show that human neutrophils cross-present exogenous antigen.
Murine neutrophils cross-prime naive T cells in vitro
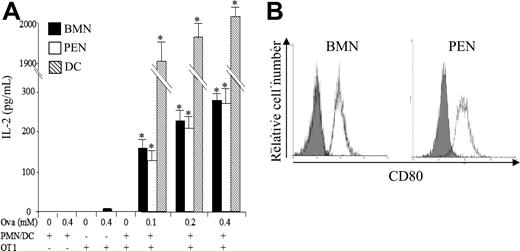
We then investigated whether neutrophils may prime naive CD8+ T cells in vitro. Murine neutrophils were pulsed with Ova and used to stimulate highly purified naive CD8+ T cells from OT1 transgenic mice (purity > 99%; data not shown). OT1 CD8+ T-cell stimulation was assessed by quantifying IL-2 production. Results showed that both BMNs and PENs cross-primed OT1 CD8+ T cells in vitro (IL-2 = 0.28 ± 0.02 and 0.27 ± 0.04 ng/mL, respectively, with 0.4 mM Ova; mean ± SD, n = 3; Figure 3A). Supporting these data, BMNs and PENs expressed CD80 (mean fluorescence intensity [MFI] = 50 ± 4 and 52 ± 7, respectively; mean ± SD, n = 3)25,26 (Figure 3B), and BMNs also weakly expressed CD8625 (MFI = 12 ± 4; data not shown). Neutrophils were less potent than DCs (IL-2 = 2 ± 0.02 ng/mL with 0.4 mM Ova; Figure 3A). Together, these data demonstrate that neutrophils are able to cross-present soluble antigens to naive CD8+ T cells in vitro.
Neutrophils cross-prime naive CD8+ T cells in vitro. (A) BMNs, PENs, and DCs were pulsed with or without the indicated concentrations of Ova. After 2 hours (BMNs, ■), 4 hours (PENs, □), or 8 hours (DCs, ▧), cells were washed and cultured with highly purified naive OT1 CD8+ T cells at a stimulator-responder cell ratio of 2:1. After 24 hours, IL-2 was quantified in the supernatants by ELISA. Results are expressed in pg/mL as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for DCs and PMNs (*P < .03) when compared with controls. (B) Analysis by FACS of CD80 expression by BMNs and PENs using FITC-labeled anti-CD80 mAb (white histograms); gray histograms correspond to isotype control mAbs.
Neutrophils cross-prime naive CD8+ T cells in vitro. (A) BMNs, PENs, and DCs were pulsed with or without the indicated concentrations of Ova. After 2 hours (BMNs, ■), 4 hours (PENs, □), or 8 hours (DCs, ▧), cells were washed and cultured with highly purified naive OT1 CD8+ T cells at a stimulator-responder cell ratio of 2:1. After 24 hours, IL-2 was quantified in the supernatants by ELISA. Results are expressed in pg/mL as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for DCs and PMNs (*P < .03) when compared with controls. (B) Analysis by FACS of CD80 expression by BMNs and PENs using FITC-labeled anti-CD80 mAb (white histograms); gray histograms correspond to isotype control mAbs.
Murine neutrophils cross-present antigen to naive CD8+ T cells in vivo
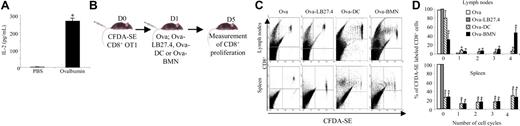
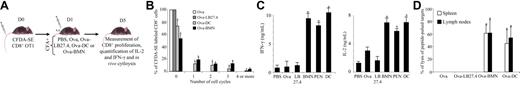
To determine whether cross-presentation by neutrophils also occurs in vivo, we analyzed the cross-presentation capacity of neutrophils isolated from the peritoneal exudates of mice previously intraperitoneally injected with Ova. Highly purified neutrophils from Ova-injected mice, but not from PBS-injected mice, stimulated OT1 CD8+ T cells in vitro (IL-2 = 0.27 ± 0.02 ng/mL; mean ± SD, n = 3; Figure 4A), demonstrating that neutrophils efficiently take up and process Ova into MHC I–bound peptides in vivo.
Neutrophils induce ovalbumin-specific CD8+ T-cell proliferation ex vivo and in vivo. (A) Neutrophils present Ova as MHC I–bound peptides in vivo. PENs purified from mice 6 hours after a peritoneal injection of 200 μg Ova or PBS were used to stimulate OT1 CD8+ T cells. After 24 hours, IL-2 was quantified in the supernatants by ELISA. Results are expressed in pg/mL as mean ± SD of 3 independent experiments. Mann-Whitney test was performed. The data were statistically significant for PMNs (*P < .03) when compared with PBS. (B-D) Ova-pulsed neutrophils induce naive CD8+ T-cell proliferation in lymph nodes and spleen (C). β2-Microglobulin−/− mice were injected with CFDA-SE naive OT1 CD8+ T cells followed 1 day later by injection of Ova or Ova-pulsed LB27.4, Ova-pulsed DCs, or Ova-pulsed BMNs. Four days later, lymphocytes were isolated from spleen and lymph nodes and CD8+ T-cell proliferation was analyzed. Data are representative of 1 of 3 separate experiments. (D) Data are presented as the mean percentage of OT1 CD8+ T cells in each cycle (mean ± SD of 3 independent experiments). ANOVA test was performed. The data were statistically significant for DCs and PMNs (*P < .001) when compared with controls.
Neutrophils induce ovalbumin-specific CD8+ T-cell proliferation ex vivo and in vivo. (A) Neutrophils present Ova as MHC I–bound peptides in vivo. PENs purified from mice 6 hours after a peritoneal injection of 200 μg Ova or PBS were used to stimulate OT1 CD8+ T cells. After 24 hours, IL-2 was quantified in the supernatants by ELISA. Results are expressed in pg/mL as mean ± SD of 3 independent experiments. Mann-Whitney test was performed. The data were statistically significant for PMNs (*P < .03) when compared with PBS. (B-D) Ova-pulsed neutrophils induce naive CD8+ T-cell proliferation in lymph nodes and spleen (C). β2-Microglobulin−/− mice were injected with CFDA-SE naive OT1 CD8+ T cells followed 1 day later by injection of Ova or Ova-pulsed LB27.4, Ova-pulsed DCs, or Ova-pulsed BMNs. Four days later, lymphocytes were isolated from spleen and lymph nodes and CD8+ T-cell proliferation was analyzed. Data are representative of 1 of 3 separate experiments. (D) Data are presented as the mean percentage of OT1 CD8+ T cells in each cycle (mean ± SD of 3 independent experiments). ANOVA test was performed. The data were statistically significant for DCs and PMNs (*P < .001) when compared with controls.
We further assessed whether Ova-pulsed neutrophils may also induce naive CD8+ T-cell proliferation in vivo. To respond to this question, we used β2-microglobulin–deficient mice. In these mice, bone marrow–derived APCs lack MHC I molecules6 and cannot cross-present Ova or Ova-pulsed neutrophils.
β2-Microglobulin−/− mice were intravenously injected with CFDA-SE–labeled naive (CD44low, CD62Lhigh) CD8+ T cells from OT1 mice (Figure 4B); OT1 CD8+ T -ell purity, based on CD3 and CD8 expression, was greater than 99.5%, allowing exclusion of a potential contamination by myeloid cells (data not shown). At day 1, mice were intravenously injected with Ova-pulsed BMNs and PENs purified from wild-type (wt) mice. As controls, mice were injected with either PBS, 200 μg Ova, Ova-pulsed LB27.4 (Ova-LB27.4), or Ova-pulsed DCs (Ova-DC) generated from wt mice (Figure 4B). Four days later, a proliferation of OT1 CD8+ T cells was observed in the lymph nodes (Figure 4C,D top panel) and in the spleen (Figure 4C,D bottom panel) of mice injected with Ova-pulsed BMNs, Ova-pulsed PENs (data not shown), or Ova-pulsed DCs. PBS, Ova, and Ova-pulsed LB27.4 failed to induce CD8+ T-cell proliferation in vivo (data not shown and Figure 4C,D), allowing exclusion of (i) Ova or Ova-pulsed neutrophil cross-presentation by APCs from β2-microglobulin−/− mice and (ii) the presence of contaminating APCs in neutrophils or CD8+ T-cell preparations. Finally, unpulsed neutrophils and NS3-pulsed neutrophils did not induce CD8+ T-cell proliferation, showing that Ova-pulsed neutrophil-induced CD8+ T-cell proliferation is antigen specific (data not shown). These data demonstrate that Ova-pulsed neutrophils cross-present antigen to naive T cells in vivo.
Murine neutrophils do not induce cross-tolerance in vivo
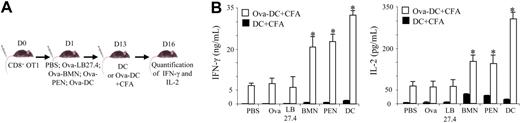
In response to antigen cross-presentation, the in vivo proliferation of naive CD8+ T cells does not systematically lead to effective T-cell cross-priming. After several rounds of cell division, naive CD8+ T cells can be deleted, leading to a specific tolerance.40 We therefore had to determine whether Ova-pulsed neutrophils induce cross-tolerance or cross-priming in vivo. We first tested the cross-tolerance hypothesis. Peripheral tolerance is the inability to respond to a second antigen challenge delivered together with a strong adjuvant.40 β2-Microglobulin−/− mice, adoptively transferred with OT1 CD8+ T cells, were injected with Ova-pulsed BMNs, Ova-pulsed PENs, or, as controls, with PBS, Ova, Ova-pulsed LB27.4, or Ova-pulsed DCs. At day 13, mice were injected with either Ova-pulsed DCs plus CFA or with unpulsed DCs plus CFA (Figure 5A). Three days after DC injection, we analyzed IL-2 and IFN-γ production by in vitro–restimulated splenic CD8+ T cells. Interestingly, CD8+ T cells from mice injected with either Ova-pulsed BMNs or with Ova-pulsed PENs and restimulated by Ova-pulsed DCs plus CFA responded to a similar extent (IFN-γ = 20 ± 3 and 23 ± 2 ng/mL, respectively; IL-2 = 165 ± 18 and 160 ± 22 pg/mL, respectively; mean ± SD, n = 3) and more efficiently than CD8+ T cells from PBS-injected mice (IFN-γ = 7 ± 0.8 ng/mL and IL-2 = 65 ± 15 pg/mL; Figure 5B). CD8+ T-cell stimulation in mice injected with Ova-pulsed DCs was higher than in Ova-pulsed neutrophils (Figure 5B), especially for IL-2 production. Compared with PBS-injected mice, the CD8+ response remained unchanged in both Ova and Ova-pulsed LB27.4-injected mice (Figure 5B). No specific production of IL-2 or IFN-γ by CD8+ T cells from mice injected with unpulsed DCs plus CFA was detected. Altogether, these data show that Ova-pulsed neutrophils do not render mice tolerant to Ova.
Ova-pulsed neutrophils fail to induce cross-tolerance in vivo. (A) β2-Microglobulin−/− mice were injected with 3 × 106 naive OT1 CD8+ T cells. One day later, mice were injected with PBS, Ova, or 3 × 106 Ova-pulsed LB27.4, BMNs, PENs, or DCs. At day 13, mice were injected with Ova-pulsed DCs (□) or unpulsed DCs (■), plus CFA. Three days later, spleen CD8+ T cells were purified and IL-2 and IFN-γ production was analyzed by ELISA 24 hours after in vitro restimulation. (B) IFN-γ and IL-2 production was determined by ELISA at day 16. Results are expressed in ng/mL (IFN-γ) and in pg/mL (IL-2) as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for BMNs, PENs, and DCs (*P < .03) when compared with controls.
Ova-pulsed neutrophils fail to induce cross-tolerance in vivo. (A) β2-Microglobulin−/− mice were injected with 3 × 106 naive OT1 CD8+ T cells. One day later, mice were injected with PBS, Ova, or 3 × 106 Ova-pulsed LB27.4, BMNs, PENs, or DCs. At day 13, mice were injected with Ova-pulsed DCs (□) or unpulsed DCs (■), plus CFA. Three days later, spleen CD8+ T cells were purified and IL-2 and IFN-γ production was analyzed by ELISA 24 hours after in vitro restimulation. (B) IFN-γ and IL-2 production was determined by ELISA at day 16. Results are expressed in ng/mL (IFN-γ) and in pg/mL (IL-2) as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for BMNs, PENs, and DCs (*P < .03) when compared with controls.
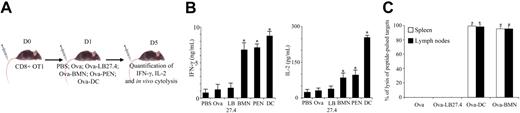
Murine neutrophils cross-prime CD8+ T cells in vivo
We then tested whether Ova-pulsed neutrophils may cross-prime CD8+ T cells in vivo. The initiation of an effective immune response is associated with a priming of T cells that is characterized not only by T-cell proliferation but also by the acquisition of effector functions (IFN-γ production and cytolysis). β2-Microglobulin−/− mice, adoptively transferred with OT1 CD8+ T cells, were injected with Ova-pulsed BMNs, Ova-pulsed PENs, or, as controls, with PBS, Ova, Ova-pulsed LB27.4, or Ova-pulsed DCs. After 4 days, CD8+ T cells were analyzed for IFN-γ and IL-2 production in response to in vitro stimulation (Figure 6A,B) and for the acquisition of cytolytic function determined in vivo (Figure 6C), as previously described.40
Ovalbumin-pulsed neutrophils induce naive CD8+ T-cell differentiation into effector cells. (A) OT1 CD8+ T cells were adoptively transferred into β2-microglobulin−/− mice. One day later, the mice were injected intravenously with PBS, Ova, Ova-pulsed LB27.4, Ova-pulsed BMNs, or Ova-pulsed DCs. Three days later, spleen CD8+ T cells were purified and IL-2 and IFN-γ production was analyzed by ELISA, or mice were injected intravenously with target cells prepared as described in “In vivo cytolysis assay.” After 3 hours, cytolysis was analyzed by FACS. (B) At day 5, IFN-γ and IL-2 production was determined by ELISA 24 hours after in vitro restimulation. Results are expressed in ng/mL (IFN-γ) and in pg/mL (IL-2) as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for DCs and PMNs (*P < 0.03) when compared with controls. (C) In vivo cytolysis assay was performed by injecting 3 × 106 unpulsed CFDA-SElow–labeled splenocytes and 3 × 106 SIINFEKL-pulsed CFDA-SEhigh–labeled splenocytes. Three hours later, CDFA-SE expression was analyzed by FACS in spleen and lymph nodes. Percentage of lysis was calculated as described in “In vivo cytolysis assay.” Results are expressed as mean (± SD) of 3 separate experiments. ANOVA test was performed. The data were statistically significant for DCs and PMNs (*P < .001) when compared with controls.
Ovalbumin-pulsed neutrophils induce naive CD8+ T-cell differentiation into effector cells. (A) OT1 CD8+ T cells were adoptively transferred into β2-microglobulin−/− mice. One day later, the mice were injected intravenously with PBS, Ova, Ova-pulsed LB27.4, Ova-pulsed BMNs, or Ova-pulsed DCs. Three days later, spleen CD8+ T cells were purified and IL-2 and IFN-γ production was analyzed by ELISA, or mice were injected intravenously with target cells prepared as described in “In vivo cytolysis assay.” After 3 hours, cytolysis was analyzed by FACS. (B) At day 5, IFN-γ and IL-2 production was determined by ELISA 24 hours after in vitro restimulation. Results are expressed in ng/mL (IFN-γ) and in pg/mL (IL-2) as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for DCs and PMNs (*P < 0.03) when compared with controls. (C) In vivo cytolysis assay was performed by injecting 3 × 106 unpulsed CFDA-SElow–labeled splenocytes and 3 × 106 SIINFEKL-pulsed CFDA-SEhigh–labeled splenocytes. Three hours later, CDFA-SE expression was analyzed by FACS in spleen and lymph nodes. Percentage of lysis was calculated as described in “In vivo cytolysis assay.” Results are expressed as mean (± SD) of 3 separate experiments. ANOVA test was performed. The data were statistically significant for DCs and PMNs (*P < .001) when compared with controls.
CD8+ T cells from Ova-pulsed BMN-injected mice secreted higher levels of IFN-γ and IL-2 than CD8+ T cells from mice injected with PBS, Ova, or Ova-pulsed LB27.4 (Figure 6B) and acquired the capacity to lyse peptide-pulsed targets (Figure 6C). In parallel, CD8+ T cells from Ova-pulsed PEN-injected mice presented effector functions similar to CD8+ T cells from Ova-pulsed BMN-injected mice (data not shown and Figure 6B). CD8+ T cells from Ova-pulsed DC-injected mice secreted the highest levels of IFN-γ and IL-2 (Figure 6B) and also exhibited cytolytic function (Figure 6C).
No specific cytolysis was observed in mice injected with PBS, Ova, or Ova-pulsed LB27.4 (data not shown and Figure 6C). These data indicate that in our experimental conditions, Ova-pulsed neutrophils have cross-primed naive T cells, as they have induced their differentiation into functional effector cells.
Subcutaneously injected neutrophils cross-prime CD8+ T cells in vivo
We then tested whether Ova-pulsed neutrophils injected via the subcutaneous route may cross-prime CD8+ T cells in vivo. β2-Microglobulin−/− mice, adoptively transferred with OT1 CD8+ T cells, were subcutaneously injected with Ova-pulsed BMNs + CFA, Ova-pulsed PENs + CFA, or, as controls, with PBS + CFA, Ova + CFA, Ova-pulsed LB27.4 + CFA, or Ova-pulsed DCs + CFA (Figure 7A). At day 5, in Ova-pulsed BMN-injected mice (Figure 7B) and Ova-pulsed PEN-injected mice (data not shown), we observed that OT1 CD8+ T cells proliferated in the lymph nodes; secreted higher levels of IFN-γ and IL-2 than CD8+ T cells from mice injected with PBS, Ova, or Ova-pulsed LB27.4 (Figure 7C); and acquired the capacity to lyse Ova-peptide–pulsed targets (Figure 7D and data not shown). Similar results were obtained in Ova-pulsed DC-injected mice (Figure 7B-D). In contrast, CD8+ T cells from mice injected subcutaneously with PBS + CFA, Ova + CFA, or Ova-pulsed LB27.4 + CFA failed to proliferate (Figure 7B) and to acquire cytolytic function (Figure 7D). Lastly, CD8+ T-cell proliferation and activation were not detected in mice injected with polymorphonuclear neutrophils (PMNs) in the absence of CFA and in the spleen of mice injected subcutaneously with unpulsed PMNs + CFA (data not shown). These data indicate that in our experimental conditions, subcutaneously injected Ova-pulsed neutrophils cross-prime naive T cells.
Ovalbumin-pulsed neutrophils injected via subcutaneous route induce naive CD8+ T-cell differentiation into effector cells. (A) CFDA-SE–labeled or unlabeled OT1 CD8+ T cells were adoptively transferred into β2-microglobulin−/− mice. One day later, mice were injected subcutaneously with CFA plus PBS, Ova, Ova-pulsed LB27.4, Ova-pulsed BMNs, or Ova-pulsed DCs and OT1 activation was measured at day 5. (B) OT1 CD8+ T-cell proliferation was expressed as the percentage of CD8+ T cells in each cycle (mean ± SD of 3 separate experiments). ANOVA test was performed. The data were statistically significant for DCs and PMNs (*P < .001) when compared with controls. (C) At day 5, IFN-γ and IL-2 production was determined by ELISA 24 hours after in vitro restimulation. Results are expressed in ng/mL as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for BMNs, PENs, and DCs (*P < .03) when compared with controls. (D) In vivo cytolysis assays were performed by injecting 3 × 106 unpulsed CFDA-SElow–labeled splenocytes and 3 × 106 CFDA-SEhigh–labeled SIINFEKL-pulsed splenocytes. Three hours later, CDFA-SE expression was analyzed by FACS in spleen and lymph nodes. Percentage of lysis was calculated as described in “In vivo cytolysis assay.” Data are presented as a percentage of lysis of peptide-pulsed targets of 3 separate experiments (mean ± SD). The data were statistically significant for DCs and PMNs when compared with controls (ANOVA test, *P < .001).
Ovalbumin-pulsed neutrophils injected via subcutaneous route induce naive CD8+ T-cell differentiation into effector cells. (A) CFDA-SE–labeled or unlabeled OT1 CD8+ T cells were adoptively transferred into β2-microglobulin−/− mice. One day later, mice were injected subcutaneously with CFA plus PBS, Ova, Ova-pulsed LB27.4, Ova-pulsed BMNs, or Ova-pulsed DCs and OT1 activation was measured at day 5. (B) OT1 CD8+ T-cell proliferation was expressed as the percentage of CD8+ T cells in each cycle (mean ± SD of 3 separate experiments). ANOVA test was performed. The data were statistically significant for DCs and PMNs (*P < .001) when compared with controls. (C) At day 5, IFN-γ and IL-2 production was determined by ELISA 24 hours after in vitro restimulation. Results are expressed in ng/mL as mean (± SD) of 3 separate experiments. Mann-Whitney test was performed. The data were statistically significant for BMNs, PENs, and DCs (*P < .03) when compared with controls. (D) In vivo cytolysis assays were performed by injecting 3 × 106 unpulsed CFDA-SElow–labeled splenocytes and 3 × 106 CFDA-SEhigh–labeled SIINFEKL-pulsed splenocytes. Three hours later, CDFA-SE expression was analyzed by FACS in spleen and lymph nodes. Percentage of lysis was calculated as described in “In vivo cytolysis assay.” Data are presented as a percentage of lysis of peptide-pulsed targets of 3 separate experiments (mean ± SD). The data were statistically significant for DCs and PMNs when compared with controls (ANOVA test, *P < .001).
Discussion
This study provides the first evidence for an in vivo role of neutrophils in antigen cross-presentation and naive T-cell cross-priming. Our findings demonstrate that (1) human and murine neutrophils cross-present antigens in vitro, (2) ovalbumin cross-presentation by neutrophils is proteasome and TAP dependent, and (3) antigen-pulsed neutrophils do not induce cross-tolerance but induce naive CD8+ T-cell differentiation into effector cells in vivo. These properties of neutrophils may open new perspectives to boost the efficacy of a vaccine based on CTL induction.
Neutrophils are the first cells to migrate, in high number, at the site of infection. Their main recognized function is to clear invading microorganisms. To date, the ability of neutrophils to present exogenous antigens to CD8+ T cells remains poorly documented. One study reported that neutrophils loaded with CTL peptides stimulate memory T cells in vitro, showing that MHC I molecules on neutrophils are functional.41 Potter and Harding31 observed that neutrophils that have phagocytosed Ova-expressing bacteria, processed Ova in a proteasome-independent manner for presentation to memory CD8+ T cells, thereby demonstrating that the vacuolar pathway associated with antigen cross-presentation is functional in neutrophils.31 Using lactacystin-treated neutrophils and neutrophils from TAP−/− mice, we report that soluble antigen cross-presentation by neutrophils depends on TAP machinery and on proteasomes. This pathway is the most extensively described in antigen cross-presentation.3 The professional APCs, DCs, and macrophages have also been reported to cross-present Ova in a proteasome- and TAP-dependent manner.38 Supporting our findings, neutrophils have been reported to express proteasomes and TAP.42,43 Altogether, these data demonstrate that the 2 main pathways associated with antigen cross-presentation are functional in neutrophils.
Antigen cross-presentation may lead in vivo to immunity or tolerance.3 Upon contact with bacteria or fungi, neutrophils are the main cells that accumulate early and take up the microorganisms. Moreover, activated neutrophil infiltration in an inflammatory site is associated with some autoimmune disorders such as vasculitis.44 It therefore appeared crucial to determine whether neutrophils are involved in cross-tolerance or cross-priming. Our observations that neutrophils cross-present antigens to naive T cells in vitro and that Ova-pulsed neutrophils induce naive OT1 CD8+ T-cell proliferation in vivo were not sufficient to speculate about a role for neutrophils in cross-tolerance versus cross-priming. As examples, endothelial cells cross-present antigen to naive CD8+ T cells in vitro while they induce cross-tolerance in vivo.45 The proliferation of naive OT1 CD8+ T cells induced by targeting ovalbumin to DEC-205 on DCs is followed by a rapid T-cell death and peripheral CD8+ T-cell tolerance.40 We therefore analyzed the consequences of antigen cross-presentation by neutrophils in an in vivo model, where DCs and macrophages were unable to cross-present antigens, in order to exclude a representation of neutrophils or neutrophil-derived fragments by bone marrow–derived APCs. In this model, Ova-pulsed neutrophils cross-prime naive T cells, as they induce their differentiation into functional effector cells. These data are the first demonstration of an in vivo CD8+ T-cell cross-priming by neutrophils.
Neutrophils are terminally differentiated cells with a short lifespan. Recent data suggest that some signals (ie, proinflammatory cytokines or TLR agonists) increase their survival.46,47 In agreement with these observations, our data show that the survival time period of intravenously or subcutaneously injected Ova-pulsed neutrophils is sufficient to allow neutrophils to migrate to the spleen and the lymph nodes to encounter and to stimulate naive CD8+ T cells. Recent observations also demonstrated that neutrophils migrate from the periphery to the draining lymph nodes.18,32 Taken together, these data support a potential role for neutrophils in CD8+ T-cell cross-priming.
Our data, added to observations from others showing that neutrophils can vehicle antigens or microorganisms from the periphery to the draining lymph nodes, suggest that neutrophils could be useful to cross-prime CD8+ T cells against microorganisms that do not infect DCs or down-regulate their properties.
Cross-tolerance versus cross-priming in vivo depends on parameters such as the level of antigen that is cross-presented, the presence of CD4+ T-cell help, and the degree of costimulatory signals expressed on the antigen cross-presenting cells. Firstly, in in vitro experiments, we observed that neutrophils were as potent as macrophages in cross-presenting antigen. Secondly, in our models, APCs from β2-microglobulin−/− mice express functional MHC II molecules and can prime Ova-specific CD4+ T cells. In some circumstances, an efficient in vivo cross-priming requires cognate CD4+ T-cell help. In this 3-cell model, the antigen is presented both into the MHC I and the MHC II by the same APC,48-50 and antigen-stimulated CD4+ T cells contribute to APC activation via CD40L expression. Although the ability of neutrophils to present antigens in MHC II molecules in vivo remains to be demonstrated, stimulated neutrophils express MHC II25 and are activated by CD40L.51 Thirdly, in agreement with recent data showing that neutrophils contain preformed CD80 and CD86 molecules that are rapidly expressed on the cell surface upon stimulation, we reported that murine BMNs and PENs expressed CD80 (and CD86 for BMNs). Together, the ability of neutrophils to efficiently cross-present antigen and to express costimulatory molecules supports their ability to cross-prime CD8+ T cells.
We report here that neutrophils cross-prime CD8+ T cells in vivo. The observations that (1) neutrophils accumulate at the site of infection before DCs and macrophages, and (2) in vitro cross-presentation by neutrophils occurs earlier than in professional APCs, suggest that neutrophils may interact in vivo with naive T CD8+ cells prior to DCs. More precisely, in response to a pathogen, whether neutrophils and DCs that have cross-presented the antigen may sequentially interact with CD8+ T cells and the consequences of these sequential interactions on CD8+ T-cell responses should be evaluated. This point will be difficult to analyze in vivo. In a model where both neutrophils and DCs express functional MHC I molecules, live and apoptotic neutrophils that have taken up the antigen can serve as substrates for cross-presentation by DCs.21,24 Lastly, the observation that neutrophil depletion reduces the CD8+ T-cell response against nonsecreted bacterial antigen24 supports our observation, although the mechanisms used by neutrophils to boost the CD8+ response remained undetermined. In our in vivo model, we excluded neutrophil cross-presentation by APCs and demonstrated that antigen cross-priming by neutrophils in vivo enhanced CD8+ T-cell response to a subsequent restimulation by DCs. These data thereby suggest that neutrophils may act in concert with DCs in vivo to initiate and/or amplify CD8+ T-cell responses to exogenous antigens.
Different observations have suggested a role of activated CD8+ T cells in some autoimmune disease such as Wegener granulomatosis (WG)52,53 and multiple sclerosis (MS).54 These pathologies are also associated with an accumulation of neutrophils at the inflammatory site55 or with an increase of IL-8 production,56 respectively. Moreover, in WG, circulating neutrophils also express MHC II molecules.57 Based on our data, it seems of interest to evaluate whether, in these pathologies, neutrophils may cross-present self-antigens and contribute to the generation and/or maintenance of autoreactive CD8+ T-cell responses. This may lead to define more specific immunotherapies that could be used together with immunosuppressive agents.
In conclusion, these data show that neutrophils cross-present antigens and, in our experimental conditions, cross-prime CD8+ T cells in vivo. These findings suggest that it could be of interest to reanalyze the role of neutrophils in the physiopathology of some autoimmune disorders. Furthermore, this new property of neutrophils could be exploited to boost the efficacy of vaccines based on CTL induction, for example by using preferential adjuvants that favor neutrophil recruitment and migration into the lymph nodes.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr N. Shastri (University of California, Berkeley) for providing the B3Z hybridoma. We thank Lena Manoukian for typing assistance and Patrice Chiron for animal breeding assistance.
This work was supported by INSERM Programme Avenir and the Canceropole Grand-Ouest.
Authorship
Contribution: C.B. performed research, analyzed data, and wrote the paper; Y.D. designed research and wrote the paper; M.S. performed research; A.P. and V.B. provided KO and transgenic mice and the human T-cell clone; H.G. is the director of the laboratory; V.B. and P.G. provided vital reagents and participated in the design of experiments; and P.J. supervised the entire project, designed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pascale Jeannin, INSERM Unit 564, University Hospital of Angers, 4 rue Larrey, 49933 Angers, France; e-mail: pascale.jeannin@univ-angers.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal