Abstract
Nitric oxide (NO) is essential for vascular homeostasis and is also a critical modulator of angiogenesis; however, the molecular mechanisms of NO action during angiogenesis remain elusive. We have investigated the potential relationship between NO and membrane type 1–matrix metalloproteinase (MT1-MMP) during endothelial migration and capillary tube formation. Endothelial NO synthase (eNOS) colocalizes with MT1-MMP at motility-associated structures in migratory human endothelial cells (ECs); moreover, NO is produced at these structures and is released into the medium during EC migration. We have therefore addressed 2 questions: (1) the putative regulation of MT1-MMP by NO in migratory ECs; and (2) the requirement for MT1-MMP in NO-induced EC migration and tube formation. NO upregulates MT1-MMP membrane clustering on migratory human ECs, and this is accompanied by increased degradation of type I collagen substrate. MT1-MMP membrane expression and localization are impaired in lung ECs from eNOS-deficient mice, and these cells also show impaired migration and tube formation in vitro. Inhibition of MT1-MMP with a neutralizing antibody impairs NOinduced tube formation by human ECs, and NO-induced endothelial migration and tube formation are impaired in lung ECs from mice deficient in MT1-MMP. MT1-MMP thus appears to be a key molecular effector of NO during the EC migration and angiogenic processes, and is a potential therapeutic target for NO-associated vascular disorders.
Introduction
Vascular homeostasis is achieved through the fine-tuned regulation of the integrity of the endothelial cell (EC) monolayer by vasoactive modulators. One of these critical factors is nitric oxide (NO), which was first identified as the endothelial-derived relaxing factor responsible for the regulation of vascular tone.1 Indeed, proper amounts of NO are essential for correct endothelial functioning. Besides, endothelial dysfunction is characterized by impaired NO production by ECs, and is critically involved in the initiation and development of several cardiovascular diseases.2 NO is an essential mediator of physiologic vascular remodeling in the adult and is also implicated in cardiovascular pathology.3
Endothelial cell quiescence is disrupted in certain pathophysiologic conditions. A variety of stimuli promote vasodilation and blood vessel permeability mainly by increasing the production of NO and vascular endothelial growth factor (VEGF), respectively. This can subsequently result in initiation of the angiogenic cascade, giving rise to the production of new capillaries from preexisting ones.4 Angiogenesis is critical for many physiologic and pathologic processes such as wound healing and tissue remodeling, and recent findings suggest that this process may be important in the development of cardiovascular disorders like atherosclerosis.5 There is also considerable evidence that effective angiogenesis requires the synthesis of NO by ECs. VEGF upregulates the expression and activity of endothelial NO synthase (eNOS/NOS3),6,7 and endothelium-derived NO appears to play an important role in each step of angiogenesis, in particular endothelial migration and tube formation.8-10 Wound healing and angiogenesis are impaired in eNOS-deficient mice, showing that eNOS activity is required for proper EC migration, proliferation, and differentiation; moreover, ECs from eNOS-deficient animals show impaired capillary formation in the Matrigel plug system.11 However, to date, the molecular effectors of NO action regulating proteolysis are incompletely defined.
During angiogenesis, ECs undergo a transition from a quiescent to a polarized migratory phenotype. Migratory ECs subsequently employ proteolytic enzymes to remodel the basement membrane of the existing capillary. Among these proteases, matrix metalloproteinases (MMPs) are functionally prominent. MMPs are Zn-dependent endopeptidases that are capable not only of degrading extracellular matrix (ECM) components but also of modulating the bioactivity of transmembrane receptors and soluble factors.12 In our previous work we showed that membrane type 1–matrix metalloproteinase (MT1-MMP), membrane-anchored MMP, plays an important role during angiogenesis. MT1-MMP expression and activity are up-regulated during EC migration, and its activity is required for migration, invasion, and capillary formation in vitro and in vivo.13-15 MT1-MMP activity appears to be fine-tuned in ECs through modulation of its subcellular localization, internalization, and dimerization. The subcellular localization of MT1-MMP depends on its interaction with integrins and on its trafficking via caveolae; both of these processes are dependent on the ECM composition.16,17
Since eNOS and MT1-MMP are both important for EC migration and capillary tube formation and are both regulated by caveolae,18,19 we have investigated the possibility that NO might regulate MT1-MMP function during the angiogenic response, and that MT1-MMP might therefore be a downstream effector of NO-induced angiogenesis. By using human ECs and lung ECs from mice deficient in either eNOS or MT1-MMP, we demonstrate that NO promotes focal clustering and activation of MT1-MMP at the surface of migrating ECs and that this protease is essential for proper NO-induced endothelial migration and capillary tube formation.
Materials and methods
Approval was obtained from the Instituto de Salud Carlos III Institutional Review Board for these studies.
Antibodies and reagents
The following monoclonal antibodies (mAbs) were as previously described13 : anti–VE-cadherin TEA1/31, anti-CD31 TP1/15, and anti–MT1-MMP LEM-2/15 and LEM-2/63. Anti–caveolin-1 mAb (clone 2234) and anti-eNOS mAb were from BD Transduction Laboratories (Lexington, KY); anti–ICAM-2 (CD102) and anti-CD16 antibodies were from Pharmingen (San Jose, CA); and anti-phospho eNOS (Ser 1177) antibody was from Cell Signaling (Danvers, MA). Antitubulin mAb, type IV gelatin, and bradykinin were from Sigma-Aldrich (St Louis, MO). Type I collagen (COL I) was from ICN Biomedicals (Costa Mesa, CA). The chemokines CCL2 ([C-C motif] ligand 2; monocyte chemotactic protein-1) and CXCL12 ([C-X-C motif] ligand 12; stromal cell–derived factor-1) were from R&D Systems (Minneapolis, MN). DEA-NONOate and DETA-NONOate were from Alexis Biochemicals (Carlsbad, CA). SNAP, L-NAME, DAF-FM–diacetate and DQ-Collagen type I (DQ-COL I) were from Invitrogen (Carlsbad, CA).
Cells and cell cultures
Human umbilical vein endothelial cells (HUVECs) were obtained and cultured as previously described.13 ECs were grown on 1% gelatin (GEL). Mice of the C57BL/6 strain deficient in eNOS or MT1-MMP (Mmp14) have been previously characterized.11,20 Mouse lung endothelial cells (MLECs) were purified from wild-type and eNOS or MT1-MMP null mice as previously described.15 ECs were incubated in serum-free medium for 16 hours before experiments. ECs were stimulated to migrate by disrupting the monolayer with a scraper (wound-healing) or by seeding at subconfluence.
Immunofluorescence microscopy
Subconfluent ECs on coverslips coated with GEL were incubated with the anti–MT1-MMP mAb and labeled with an Alexa 488–conjugated secondary mAb. For eNOS staining, cells were permeabilized for 2 minutes with 0.1% Triton X-100, and then incubated with anti-eNOS monoclonal or rabbit Ab and labeled with an Alexa Cy3–conjugated secondary Ab. Samples were mounted with Mowiol (Calbiochem, Merck KgaA, Darmstadt, Germany) or ProLong Gold (Invitrogen). Samples were examined under an Axiovert 200 M SP LSM5 fluorescence microscope from Zeiss (Jena, Germany) equipped with 40×/1 NA or 63×/1.4 NA oil-immersion objectives or a confocal microscope Biorad Radiance 2100 (Zeiss) with a 40×/1.3 NA or 60×/1.4 NA oil-immersion objective. Images were acquired with an AxiocamHRm digital camera and Axiovision Rel 4.2 software (Zeiss). Alternatively, a Leica DMI6000 microscope (Leica Microsystems GmbH, Wetzlar, Germany) equipped with a 40×/1.25 NA or 63×/1.4 NA oil immersion objective was used; pictures were acquired with a Leica DFC350FX digital camera and LAS AF software (Leica). Linear processing of images was performed with Adobe Photoshop CS software (Adobe, San Jose, CA). To quantify MT1-MMP clustering on migrating ECs, HUVECs were treated over a time course with NO donors in 6 independent experiments; in each experiment, 2 blinded observers examined at least 15 control and treated cells for each time point. For quantitative comparison of MT1-MMP distribution between wild-type and eNOS-deficient MLECs, 3 blinded observers examined at least 80 cells of either genotype in 3 independent experiments to quantitate cells displaying concentrated MT1-MMP at lamellipodia/filopodia versus a more diffuse, punctate pattern.
In situ detection and measurement of NO production
For in situ visualization of NO, we used a specific membrane-permeable NO probe, DAF-FM–diacetate. MLECs or HUVECs were incubated with 5 μM DAF-FM in Hanks balanced salt solution (HBSS) supplemented with 1% fetal bovine serum (FBS) for 30 minutes. ECs were then washed 3 times with phosphate-buffered saline (PBS) and observed under a Leica TCS SP2-AOBS confocal microscope equipped with a 40×/1.25 NA or 63×/1.4 NA objective. For real-time assays, HUVECs were analyzed by time-lapse confocal microscopy. Fluorescent and phase-contrast images were recorded at 20-second intervals over 6 minutes. For the NO measurement assays, HUVECs were pretreated with L-NAME (500 μM) and stimulated to migrate by wound healing. NO production was determined from the accumulation of nitrites (NO2-) in the cell culture medium after 15 to 30 minutes; nitrites were reduced with acidified NaI and the NO product detected with a NOA 280 NO analyzer (Sievers Instruments, Boulder, CO). Peaks were integrated with Origin software (OriginLab, Northampton, MA).
Flow cytometry analysis
Migrating (subconfluent or wound-healing) HUVECs were incubated with 100 μM DEA-NONOate or DETA-NONOate for 3, 6, or 16 hours and processed as previously described.13 Samples were analyzed in a FACSCanto (Becton Dickinson Labware, Lincoln Park, NJ) or Cytomics-FC500 (Beckman-Coulter, Fullerton, CA) flow cytometer.
Western blot assays
ECs were lysed and analyzed as previously described.16
Triton X-114 extraction
Subconfluent HUVECs were treated with 100 μM DEA-NONOate or DETA-NONOate for different times, and cell fractionation was performed by Triton X-114 extraction. In brief, cells were lysed in 1.5% Triton X-114 at 4°C, and after 5 minutes of centrifugation at 9000g, supernatants were heated at 37°C for 2 minutes. Hydrophobic (caveolin-1 positive) and hydrophilic (tubulin positive) phases were collected after 5 minutes of centrifugation at 9000g. Samples were assayed for Western blot as described.16
In situ detection of MT1-MMP activity
Since MT1-MMP is a major collagenase in ECs,21 COL I degradation was used to analyze MT1-MMP activity in these cells. First, we used a fluorescein-conjugated substrate (DQ-COL I), which emits fluorescence upon cleavage, to visualize MT1-MMP proteolytic activity at the cell surface. HUVECs were seeded onto 10 μg/mL COL I or DQ-COL I and were treated with 1 μM bradykinin or 100 μM DETA-NONOate from 1 to 6 hours. After fixing, samples were visualized under a fluorescence microscope. An additional assay for measuring COL I degradation was used in MLECs from different genetic mouse strains. These cells were seeded on 1 mg/mL rat tail COL I (Roche Pharma, Basel, Switzerland) and stimulated with 1 μM bradykinin for 24 hours. After fixing, COL I was immunostained with an anti–COL I mAb (Sigma-Aldrich) and samples were visualized under an Axiovert 200 M SP LSM5 fluorescence microscope (Zeiss). Dark areas around ECs corresponding to COL I degradation were quantitated with Laserpix software (Bio-Rad, Hercules, CA). In each experiment, 10 cells were counted.
Cell migration and in vitro angiogenesis assays
Cell migration and in vitro angiogenesis assays were performed as described previously.13 Briefly, for migration assays 40 × 103 MLECs were seeded on GEL-coated Transwell chambers and stimulated to migrate with bradykinin or NO donors in the upper chamber or toward the chemokines CCL2 or CXCL12 in the lower chamber. ECs were counted after 16 hours of migration by fixing and staining with 0.2% violet crystal, 2% ethanol. For in vitro angiogenesis assays, 10 × 103 ECs were seeded on Matrigel (BD Matrigel Basement Membrane Matrix; BD Biosciences, San Jose, CA) with or without treatments, and cords were counted after 6 hours. ECs were pretreated for 15 minutes before seeding when anti–MT1-MMP Ab or control Abs were used. All experiments were run in triplicate.
Statistical analysis
Experimental and control samples were compared for statistical significance by using the Student t test.
Results
eNOS and MT1-MMP colocalize at membrane protrusions of migrating ECs where eNOS is found to be active
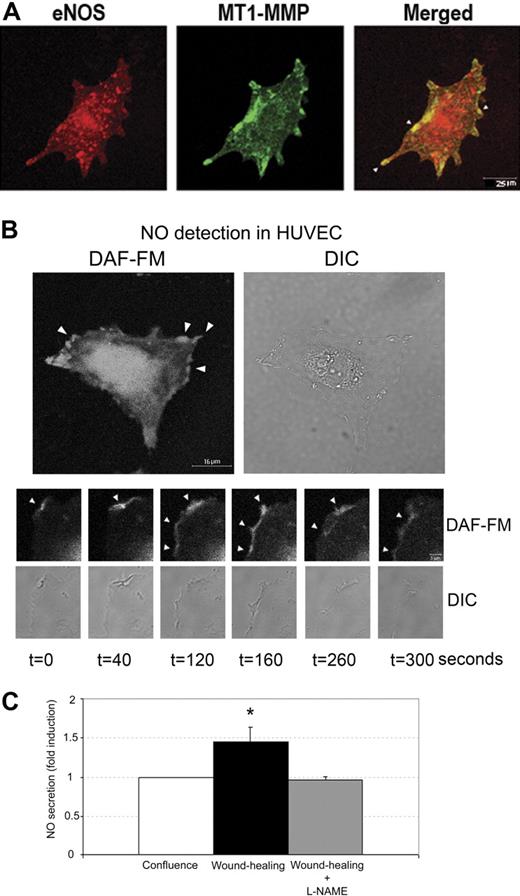
Cell migration is a critical step during early angiogenesis and is tightly regulated by a variety of receptors, ECM components, and soluble factors, including NO.22-24 MT1-MMP also plays an essential role in endothelial migration induced by distinct angiogenic factors in vivo and in vitro.13,14,20 To investigate the potential relationship between NO and MT1-MMP during angiogenesis, we first studied the localization and activity of eNOS and MT1-MMP in human ECs. Our previous work established that MT1-MMP, together with integrin αvβ3 and caveolin-1, is enriched at lamellipodia and filopodia in migratory ECs.13,16,17 We therefore examined eNOS expression in the cytosolic projections of migrating human ECs from subconfluent cultures by immunofluorescence. Both eNOS and MT1-MMP were detected at the lamellipodia and filopodia of migrating ECs. Confocal microscopy analysis revealed colocalization of these proteins at these structures (Figure 1A). Given that EC migration upregulates MT1-MMP activity, we wondered whether migration might also stimulate eNOS activity at these sites. We investigated local NO production during endothelial migration by using the probe DAF-FM, which reacts with NO to form a fluorescent product; the specificity of this approach was confirmed by the irresponsiveness of eNOS-deficient ECs (Figure S1, available on the Blood web site; see the Supplemental Materials link at the top of the online article). These experiments demonstrated that NO is produced locally at the lamellipodia and filopodia of migrating human ECs. This localized production was highly dynamic with foci of NO production arising and disappearing in a matter of seconds or minutes throughout the observation period (Figure 1B and Video S1). We next examined whether this local NO production was associated with release of NO to the extracellular medium. ECs induced to migrate in the wound-healing assay released significantly more NO during the first 15 to 30 minutes after the induction of migration compared with confluent ECs (Figure 1C). This increased NO release was blocked by pretreatment of ECs for 1 hour with the NOS inhibitor L-NAME, providing further evidence for the activation of eNOS during EC migration (Figure 1C). The colocalization of eNOS and MT1-MMP at motility-associated structures, together with the activation of eNOS at these structures and subsequent NO production by migrating ECs, prompted us to investigate (1) the possible regulation of MT1-MMP by NO in migrating ECs, and (2) the requirement of MT1-MMP functional activity during NO-induced EC migration and tube formation. These 2 questions were addressed by using both human ECs and murine lung ECs (MLECs) obtained from mice deficient in eNOS or MT1-MMP.
eNOS is active at motility-associated structures of human endothelial cells, where it colocalizes with MT1-MMP. (A) Subcellular location of eNOS (red) and MT1-MMP (green) was analyzed by immunofluorescence double-staining of subconfluent HUVECs. The merged image reveals colocalization (yellow); arrows indicate colocalization at membrane protrusions. (B) Migrating HUVECs were preloaded for 30 minutes with the NO probe DAF-FM, and NO production was clearly visible at cellular protrusions (top). The lower figure shows representative real-time capture (n = 4) of DAF-FM fluorescence at the lamellipodia of a migrating EC. The location of NO production was recorded over 6 minutes at lamellipodia in migrating ECs at 20-second intervals; t = 0 indicates the beginning of recording. Note the appearance and disappearance of NO production at lamellipodia during the recorded period (bottom, arrowheads). (C) HUVECs were left untreated or treated with L-NAME for 1 hour. Migration was initiated by disrupting monolayers (“In situ detection and measurement of NO production”). NO production was measured as the accumulation of NO2- released into the culture medium over 30 minutes by resting (confluent) or migrating (wound-healing) HUVECs. The chart shows the arithmetic mean plus or minus the standard deviation (SD) of the fold induction (FI) over nitrite release from 3 independent experiments run in duplicate. The absolute value corresponding to FI = 1 was 307 nM. *P < .04.
eNOS is active at motility-associated structures of human endothelial cells, where it colocalizes with MT1-MMP. (A) Subcellular location of eNOS (red) and MT1-MMP (green) was analyzed by immunofluorescence double-staining of subconfluent HUVECs. The merged image reveals colocalization (yellow); arrows indicate colocalization at membrane protrusions. (B) Migrating HUVECs were preloaded for 30 minutes with the NO probe DAF-FM, and NO production was clearly visible at cellular protrusions (top). The lower figure shows representative real-time capture (n = 4) of DAF-FM fluorescence at the lamellipodia of a migrating EC. The location of NO production was recorded over 6 minutes at lamellipodia in migrating ECs at 20-second intervals; t = 0 indicates the beginning of recording. Note the appearance and disappearance of NO production at lamellipodia during the recorded period (bottom, arrowheads). (C) HUVECs were left untreated or treated with L-NAME for 1 hour. Migration was initiated by disrupting monolayers (“In situ detection and measurement of NO production”). NO production was measured as the accumulation of NO2- released into the culture medium over 30 minutes by resting (confluent) or migrating (wound-healing) HUVECs. The chart shows the arithmetic mean plus or minus the standard deviation (SD) of the fold induction (FI) over nitrite release from 3 independent experiments run in duplicate. The absolute value corresponding to FI = 1 was 307 nM. *P < .04.
Nitric oxide promotes MT1-MMP membrane clustering and COL I degradation by migrating human ECs
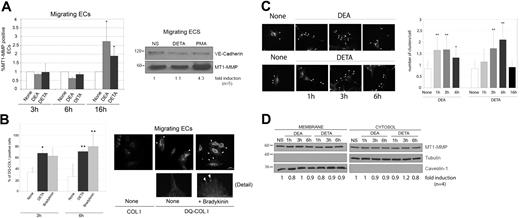
We first investigated the possible effect of NO on MT1-MMP expression at the surface of migrating ECs. Migratory HUVECs were treated with the NO donors DEA-NONOate or DETA-NONOate,25 and MT1-MMP membrane expression was detected by flow cytometry. NO donors induced a significant up-regulation of the percentage of cells expressing MT1-MMP at the cell surface, but this was seen only after 16 hours (Figure 2A); long after the endogenous NO release observed in migrating cells (Figure 1C). Western blot of whole-cell lysates indicated that NO-augmented expression of MT1-MMP is not related to de novo protein synthesis (Figure 2A). To further explore the regulation of MT1-MMP by NO we next examined the effect of NO donors on MT1-MMP activity. No modulation of MT1-MMP activity by NO donors was observed by fibrinogen or gelatin zymography of whole-cell lysates of migratory human ECs (data not shown); however, zymography will not detect possible focal protease activation at sites of MT1-MMP and eNOS colocalization. Given that MT1-MMP is the main collagenase in ECs, we therefore seeded migratory subconfluent ECs onto the fluorescein-conjugated substrate DQ-COL I and stimulated with DETA-NONOate or the physiologic eNOS activator bradykinin. Treatment with either agent significantly increased the percentage of cells displaying DQ-COL I degradation at the cell membrane (Figure 2B left); DQ-COL I degradation was more evident at membrane lamellipodia and filopodia (Figure 2B right; Figure S2). This increased MT1-MMP activity was detected after treatment for 3 and 6 hours; after 16 hours, DQ-COL I degradation returned to the level detected in unstimulated cells (data not shown). Only nonspecific signals were detected in subconfluent ECs grown on nonfluorescent COL I or in confluent ECs on DQ-COL I (Figure 2B right, and data not shown).
NO regulates MT1-MMP activity and membrane clustering in migrating ECs. (A) Migrating HUVECs were treated with 100 μM DEA-NONOate (DEA) or DETA-NONOate (DETA) for 3, 6, or 16 hours, and membrane expression of MT1-MMP was detected by flow cytometry. The chart shows the mean plus or minus SD of the fold induction of the percent of MT1-MMP–positive cells from 3 independent experiments (left). None indicates nonstimulated controls. Total cellular expression of MT1-MMP was analyzed by Western blot in migratory HUVECs treated for 16 hours with 100 μM DETA-NONOate (DETA) or 20 ng/mL PMA (right). VE-cadherin is shown as loading control. The fold induction relative to nonstimulated (NS) cells was estimated from densitometric analysis of 5 independent experiments. (B) NO increases collagenolytic activity at the cell membrane of migratory ECs. HUVECs were seeded at subconfluence onto 10 μg/mL COL I or DQ-COL I and left untreated (None) or treated with 100 μM DETA-NONOate or 1 μM bradykinin for 3 or 6 hours. DQ-COL I cleavage was visualized by its fluorescent product. Data are the means plus or minus SD of the percent of cells showing DQ-COL I fluorescence (an average of 40 cells were counted per condition); *P < .05; **P < .02 (left). DQ-COL I cleavage at cell protrusions was increased in bradykinin-treated ECs (right; arrowheads). (C) NO increases membrane clustering of MT1-MMP. The subcellular location of MT1-MMP was detected by immunostaining of subconfluent HUVECs treated with 100 μM DEA-NONOate (DEA) and DETA-NONOate (DETA) for different periods (left). Two blinded observers independently quantitated the number of clusters per cell. Data show the means plus or minus SD of at least 80 cells per condition from 6 independent experiments. *P < .04; **P ≤ .02 (right). (D) The distribution of MT1-MMP between caveolar membrane and cytosolic fractions was assayed by cell membrane isolation with Triton X-114 from HUVECs treated with DEA-NONOate or DETA-NONOate as in panel C. One representative experiment of 4 performed is shown. The fold induction relative to nonstimulated (NS) cells was estimated from densitometric analysis.
NO regulates MT1-MMP activity and membrane clustering in migrating ECs. (A) Migrating HUVECs were treated with 100 μM DEA-NONOate (DEA) or DETA-NONOate (DETA) for 3, 6, or 16 hours, and membrane expression of MT1-MMP was detected by flow cytometry. The chart shows the mean plus or minus SD of the fold induction of the percent of MT1-MMP–positive cells from 3 independent experiments (left). None indicates nonstimulated controls. Total cellular expression of MT1-MMP was analyzed by Western blot in migratory HUVECs treated for 16 hours with 100 μM DETA-NONOate (DETA) or 20 ng/mL PMA (right). VE-cadherin is shown as loading control. The fold induction relative to nonstimulated (NS) cells was estimated from densitometric analysis of 5 independent experiments. (B) NO increases collagenolytic activity at the cell membrane of migratory ECs. HUVECs were seeded at subconfluence onto 10 μg/mL COL I or DQ-COL I and left untreated (None) or treated with 100 μM DETA-NONOate or 1 μM bradykinin for 3 or 6 hours. DQ-COL I cleavage was visualized by its fluorescent product. Data are the means plus or minus SD of the percent of cells showing DQ-COL I fluorescence (an average of 40 cells were counted per condition); *P < .05; **P < .02 (left). DQ-COL I cleavage at cell protrusions was increased in bradykinin-treated ECs (right; arrowheads). (C) NO increases membrane clustering of MT1-MMP. The subcellular location of MT1-MMP was detected by immunostaining of subconfluent HUVECs treated with 100 μM DEA-NONOate (DEA) and DETA-NONOate (DETA) for different periods (left). Two blinded observers independently quantitated the number of clusters per cell. Data show the means plus or minus SD of at least 80 cells per condition from 6 independent experiments. *P < .04; **P ≤ .02 (right). (D) The distribution of MT1-MMP between caveolar membrane and cytosolic fractions was assayed by cell membrane isolation with Triton X-114 from HUVECs treated with DEA-NONOate or DETA-NONOate as in panel C. One representative experiment of 4 performed is shown. The fold induction relative to nonstimulated (NS) cells was estimated from densitometric analysis.
The identification of focal activation of MT1-MMP by NO donors at times when its surface expression appeared to be unaltered suggests that NO might regulate the distribution of MT1-MMP within the membrane. Immunofluorescence showed that MT1-MMP clustering was modulated by exposure to NO donors: DEA-NONOate and DETA-NONOate both increased the proportion of MT1-MMP located in discrete membrane-domain clusters on migrating ECs, with significantly increased clustering detected after 1, 3, and 6 hours of exposure to DEA-NONOate and after 3 and 6 hours of exposure to DETA-NONOate (Figure 2C). The different timing of these responses reflects the rapid and slow release of NO from DEA-NONOate and DETA-NONOate, respectively. After exposure to DETA-NONOate for 16 hours, clustering returned to the level of nontreated cells. To further define the mechanism of NO-mediated MT1-MMP membrane compartmentation, we examined the distribution of MT1-MMP by cell fractionation. The ratio of caveolar membrane MT1-MMP to cytosolic MT1-MMP was not altered by exposure to NO donors for 1, 3, 6, or 16 hours (Figure 2D and data not shown). These data thus indicate that short- to medium-term exposure to NO induces MT1-MMP clustering and activity at motility-associated structures and suggest that NO might directly or indirectly regulate MT1-MMP activity at specific sites on the cell surface during EC migration.
Nitric oxide is necessary for proper MT1-MMP membrane expression, localization, activity, and function in migratory ECs
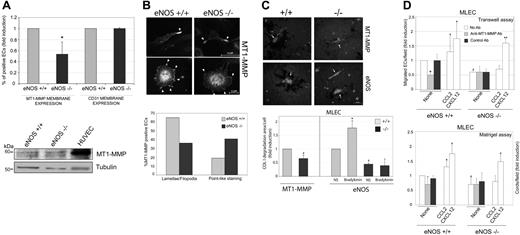
To examine the role of endogenously produced NO in the regulation of MT1-MMP homeostasis, we analyzed the expression and localization of MT1-MMP in MLECs from wild-type and eNOS-deficient mice. Surface expression of MT1-MMP, but not CD31, was decreased in MLECs from eNOS-deficient mice by an average rate of 50% (Figure 3A, top). However, there were no apparent differences in total MT1-MMP expression (Figure 3A bottom), consistent with the effect of NO on human ECs (Figure 2A). Immunofluorescence analysis showed that MT1-MMP was present at membrane protrusions of migrating MLECs regardless of the expression of eNOS; however, the pattern of MT1-MMP staining at these protrusions differed between wild-type and eNOS-deficient MLECs (Figure 3B top, and Figure S3): compared with wild-type cells, the percentage of eNOS-null MLECs showing concentrated MT1-MMP staining at lamellipodia or filopodia was decreased and the percentage showing pointlike MT1-MMP staining was increased (Figure 3B bottom). In addition, image analysis showed that MT1-MMP–positive clusters spanned a wider area at the cell membrane in wild-type MLECs compared with eNOS-deficient cells (Figure S3). These data are consistent with the modulation of MT1-MMP clustering by NO donors observed in HUVECs (Figure 2C).
NO is required for proper MT1-MMP homeostasis and function in migratory ECs. (A) Subconfluent MLECs from wild-type and eNOS-null mice were analyzed for MT1-MMP membrane expression by flow cytometry. The means plus or minus SD of the percent of MT1-MMP–positive cells from 5 independent experiments are represented. *P < .01 (versus eNOS+/+) (top). The Western blot shows MT1-MMP expression in total cell lysates from wild-type and eNOS-deficient MLECs (bottom). MT1-MMP expression in HUVECs is shown for comparison. (B) MT1-MMP subcellular localization was visualized by immunofluorescence staining in MLECs from wild-type and eNOS-deficient mice; arrowheads indicate MT1-MMP staining at membrane protrusions (top). The chart shows quantification of the percentage of cells showing MT1-MMP clusters at lamellipodia/filopodia versus pointlike staining (n = 80 cells counted from 3 independent experiments) (bottom). (C) MT1-MMP activity was detected as the area of COL I degradation (dark) by wild-type and eNOS-deficient and MT1-MMP–deficient MLECs (top). Arrowheads indicate individual endothelial cells. One representative experiment is shown. The chart shows quantification of COL I degradation areas from wt (+/+) and MT1-MMP– and eNOS-deficient cultures (-/-). The effect of stimulation with the eNOS activator bradykinin on COL I degradation is shown. Data are the means plus or minus SD (n = 5 for MT1-MMP and n = 4 for eNOS; bottom). Absolute value corresponding to FI = 1 is 94.5 μm2. #P < .01 (versus +/+); *P < .01 (versus NS). (D) Top panel: Migration of wild-type and eNOS-null MLECs was analyzed in Transwell chambers after exposure of cells for 16 hours to the neutralizing anti–MT1-MMP mAb LEM-2/63 or control anti-CD31 mAb. Treatments with CCL2 and CXCL12 (10 nM) were included as controls of MT1-MMP–dependent and MT1-MMP–independent migration, respectively. Data are the means plus or minus SD of the FI above untreated wild-type cells from 3 independent experiments. The absolute value corresponding to FI = 1 was 18 migrated cells/field. *P = .05; **P < .02 (versus None); +P < .02 (versus No Ab); #P < .01 (versus eNOS+/+). Bottom panel: Cord formation by wild-type and eNOS-null MLECs was analyzed by seeding onto Matrigel in the presence of the anti–MT1-MMP or control anti-CD31 mAbs. Treatments with CCL2 and CXCL12 (10 nM) were included as controls of MT1-MMP dependence and independence, respectively. Cord formation was quantified after 6 hours. Data are the means plus or minus SD of the FI above tube formation by untreated wild-type cells from 5 (eNOS+/+) and 4 (eNOS-/-) independent experiments. The absolute value corresponding to FI = 1 was 48.21 cords/field. *P < .04 (versus None); +P < .01 (versus No Ab). eNOS-/- MLECs were significantly less efficient at forming cords than eNOS+/+ MLECs; #P < .04 (versus eNOS+/+None). All experiments were run in triplicate.
NO is required for proper MT1-MMP homeostasis and function in migratory ECs. (A) Subconfluent MLECs from wild-type and eNOS-null mice were analyzed for MT1-MMP membrane expression by flow cytometry. The means plus or minus SD of the percent of MT1-MMP–positive cells from 5 independent experiments are represented. *P < .01 (versus eNOS+/+) (top). The Western blot shows MT1-MMP expression in total cell lysates from wild-type and eNOS-deficient MLECs (bottom). MT1-MMP expression in HUVECs is shown for comparison. (B) MT1-MMP subcellular localization was visualized by immunofluorescence staining in MLECs from wild-type and eNOS-deficient mice; arrowheads indicate MT1-MMP staining at membrane protrusions (top). The chart shows quantification of the percentage of cells showing MT1-MMP clusters at lamellipodia/filopodia versus pointlike staining (n = 80 cells counted from 3 independent experiments) (bottom). (C) MT1-MMP activity was detected as the area of COL I degradation (dark) by wild-type and eNOS-deficient and MT1-MMP–deficient MLECs (top). Arrowheads indicate individual endothelial cells. One representative experiment is shown. The chart shows quantification of COL I degradation areas from wt (+/+) and MT1-MMP– and eNOS-deficient cultures (-/-). The effect of stimulation with the eNOS activator bradykinin on COL I degradation is shown. Data are the means plus or minus SD (n = 5 for MT1-MMP and n = 4 for eNOS; bottom). Absolute value corresponding to FI = 1 is 94.5 μm2. #P < .01 (versus +/+); *P < .01 (versus NS). (D) Top panel: Migration of wild-type and eNOS-null MLECs was analyzed in Transwell chambers after exposure of cells for 16 hours to the neutralizing anti–MT1-MMP mAb LEM-2/63 or control anti-CD31 mAb. Treatments with CCL2 and CXCL12 (10 nM) were included as controls of MT1-MMP–dependent and MT1-MMP–independent migration, respectively. Data are the means plus or minus SD of the FI above untreated wild-type cells from 3 independent experiments. The absolute value corresponding to FI = 1 was 18 migrated cells/field. *P = .05; **P < .02 (versus None); +P < .02 (versus No Ab); #P < .01 (versus eNOS+/+). Bottom panel: Cord formation by wild-type and eNOS-null MLECs was analyzed by seeding onto Matrigel in the presence of the anti–MT1-MMP or control anti-CD31 mAbs. Treatments with CCL2 and CXCL12 (10 nM) were included as controls of MT1-MMP dependence and independence, respectively. Cord formation was quantified after 6 hours. Data are the means plus or minus SD of the FI above tube formation by untreated wild-type cells from 5 (eNOS+/+) and 4 (eNOS-/-) independent experiments. The absolute value corresponding to FI = 1 was 48.21 cords/field. *P < .04 (versus None); +P < .01 (versus No Ab). eNOS-/- MLECs were significantly less efficient at forming cords than eNOS+/+ MLECs; #P < .04 (versus eNOS+/+None). All experiments were run in triplicate.
Collagenolytic activity in subconfluent eNOS-deficient MLECs was significantly reduced compared with wild-type cells; moreover, the collagenolytic activity of eNOS-deficient MLECs was similar to the level of activity in MLECs from MT1-MMP–deficient mice (Figure 3C). In addition, bradykinin increased COL I degradation by wild-type but not by eNOS-deficient cells (Figure 3C). These results indicate that MT1-MMP activity is decreased in the absence of endogenous NO production via eNOS, consistent with the regulation of MT1-MMP activity by NO observed in HUVECs (Figure 2B).
MT1-MMP activity is known to be important for endothelial migration and the formation of capillary tubes.13,14 To dissect the influence of endogenous NO on MT1-MMP function we therefore compared the effect of inhibiting MT1-MMP on the ability of wild-type and eNOS-deficient MLECs to migrate and form capillary cords. Consistent with our own and others' earlier reports,11,26 eNOS-deficient MLECs migrated and formed cords less efficiently than their wild-type counterparts (Figure 3D). These defects seemed to be directly related to the absence of NO production because NO donors increase cell migration and tube formation in eNOS-deficient MLECs (data not shown). In wild-type MLECs, exposure to the inhibitory anti–MT1-MMP mAb LEM-2/6313 inhibited migration by 50%; in contrast, this mAb had no effect on constitutive migration by eNOS-deficient MLECs (Figure 3D top). Similarly, mAb LEM-2/63 inhibited cord formation by 30% in wild-type MLECs but had no effect on eNOS-deficient cells (Figure 3D bottom). In addition, CCL2 (an MT1-MMP–dependent stimulus) did not induce migration or cord formation in eNOS-deficient MLECs, whereas the action of CXCL12 (an MT1-MMP–independent stimulus) was unaffected.14 These data suggest that proper function of MT1-MMP during endothelial migration and tube formation requires the presence of eNOS and the associated production of NO by ECs.
NO-induced endothelial migration and capillary tube formation require MT1-MMP expression and activity
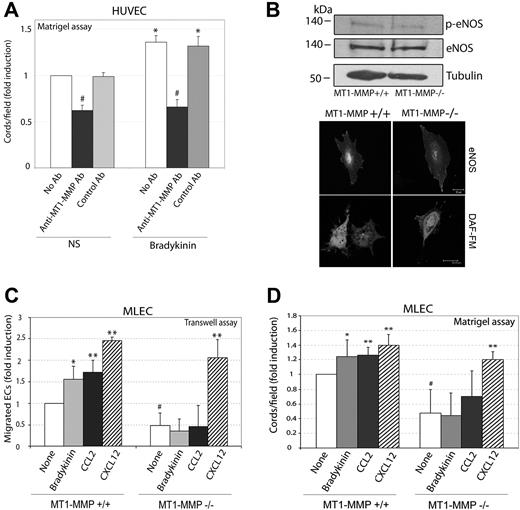
Having established that NO regulates MT1-MMP in migratory ECs, we addressed whether this protease is in fact required for NO induction of EC migration and/or tube formation. Bradykinin-induced tube formation by human ECs was specifically diminished by the inhibitory anti–MT1-MMP mAb LEM-2/15,13 indicating that MT1-MMP is required for NO-induced capillary tube formation by human ECs (Figure 4A). To confirm this, we examined migration and capillary tube formation in ECs from MT1-MMP–deficient mice. No major differences were observed in the amounts of eNOS or phospho-eNOS between wild-type and MT1-MMP–deficient MLECs (Figure 4B top). Moreover, comparison of eNOS subcellular localization showed a similar pattern of staining in both cell types, and NO production detected by DAF-FM staining was also comparable (Figure 4B bottom, and Figure S4). These data thus indicate that eNOS expression, localization, and activity are similar in wild-type and MT1-MMP–deficient MLECs. To test the effect of endogenous NO, we treated MLECs with the eNOS activator bradykinin. Consistent with our earlier work,14,15 ECs deficient in MT1-MMP migrated and formed cords less efficiently than their wild-type counterparts (Figure 4C,D). Bradykinin significantly increased migration and capillary tube formation by wild-type MLECs, but had no effect on MT1-MMP–deficient MLECs (Figure 4C,D). Similar effects were observed with the MT1-MMP–dependent stimulus CCL2 (Figure 4C,D), whereas migration and tube formation induced by the MT1-MMP–independent inducer CXCL12 was not affected. The integrity of other bradykinin-activated signaling pathways in MT1-MMP–null cells was demonstrated by the phosphorylation of ERK upon bradykinin stimulation (Figure S5).
NO-induced migration and cord formation requires MT1-MMP expression and activity. (A) HUVECs were treated with 1 μM bradykinin in the presence or absence of anti–MT1-MMP (mAb LEM-2/15) or control anti-CD31 and were seeded onto Matrigel. Cord formation was quantified after 6 hours. Data are the means plus or minus SD of the FI above tube formation by untreated cells from 3 experiments. The absolute value corresponding to FI = 1 was 59.5 cords/field. *P < .01 (versus NS); #P ≤ .01 (versus No Ab). All experiments were run in triplicate. (B) Top panel: Western blot of eNOS expression (phosphorylated and total) in MLECs from wild-type (+/+) and MT1-MMP null mice (−/−). Bottom panel: eNOS localization was visualized by immunofluorescence and NO production by DAF-FM labeling in wild-type and MT1-MMP–null MLECs. (C) MLECs from wild-type and MT1-MMP–null mice were induced to migrate on Transwell chambers during 16 hours by addition of 1 μM bradykinin in the upper chamber, or toward 10 nM CCL2 or CXCL12 in the lower chamber. Data are the means plus or minus SD of the fold induction (FI) from 4 independent experiments. The absolute value corresponding to FI = 1 was 15.25 migrated cells/field. *P < .03; **P < .02 (versus None); #P < .02 (versus MT1-MMP+/+ None). (D) Wild-type and MT1-MMP–null MLECs were treated as indicated with bradykinin (1 μM) or with CCL2 or CXCL12 (10 nM) and seeded onto Matrigel. Cord formation was quantified after 6 hours. Data are the means plus or minus SD of the FI above tube formation by untreated cells (None), of several independent determinations (n = 5 for None and bradykinin, n = 4 for CCL2, and n = 3 for CXCL12). The absolute value corresponding to FI = 1 was 50.7 cords/field. **P < .03; **P < .01; (versus None); # P < .01 (versus MT1-MMP+/+ None).
NO-induced migration and cord formation requires MT1-MMP expression and activity. (A) HUVECs were treated with 1 μM bradykinin in the presence or absence of anti–MT1-MMP (mAb LEM-2/15) or control anti-CD31 and were seeded onto Matrigel. Cord formation was quantified after 6 hours. Data are the means plus or minus SD of the FI above tube formation by untreated cells from 3 experiments. The absolute value corresponding to FI = 1 was 59.5 cords/field. *P < .01 (versus NS); #P ≤ .01 (versus No Ab). All experiments were run in triplicate. (B) Top panel: Western blot of eNOS expression (phosphorylated and total) in MLECs from wild-type (+/+) and MT1-MMP null mice (−/−). Bottom panel: eNOS localization was visualized by immunofluorescence and NO production by DAF-FM labeling in wild-type and MT1-MMP–null MLECs. (C) MLECs from wild-type and MT1-MMP–null mice were induced to migrate on Transwell chambers during 16 hours by addition of 1 μM bradykinin in the upper chamber, or toward 10 nM CCL2 or CXCL12 in the lower chamber. Data are the means plus or minus SD of the fold induction (FI) from 4 independent experiments. The absolute value corresponding to FI = 1 was 15.25 migrated cells/field. *P < .03; **P < .02 (versus None); #P < .02 (versus MT1-MMP+/+ None). (D) Wild-type and MT1-MMP–null MLECs were treated as indicated with bradykinin (1 μM) or with CCL2 or CXCL12 (10 nM) and seeded onto Matrigel. Cord formation was quantified after 6 hours. Data are the means plus or minus SD of the FI above tube formation by untreated cells (None), of several independent determinations (n = 5 for None and bradykinin, n = 4 for CCL2, and n = 3 for CXCL12). The absolute value corresponding to FI = 1 was 50.7 cords/field. **P < .03; **P < .01; (versus None); # P < .01 (versus MT1-MMP+/+ None).
Discussion
In this report we identify a functional link between the bioactive molecule NO and the protease MT1-MMP during endothelial cell migration and cord formation. We show that eNOS and MT1-MMP colocalize and are both activated at motility-associated membrane protrusions. Analysis of the molecular mechanisms involved in this interplay reveals that NO increases MT1-MMP membrane clustering and activity in migrating ECs. The presence of eNOS, the enzyme responsible for NO production in ECs, is required for proper MT1-MMP expression and distribution at the cell surface, collagenolytic activity, and function in migratory ECs. Finally, MT1-MMP is required for NO-induced endothelial migration and formation of capillary tubes in vitro.
The transition of quiescent endothelial cells to a migratory state can be triggered by a variety of stimuli. Both eNOS and MT1-MMP are implicated in endothelial activation and both are therefore potential targets of signaling pathways that initiate endothelial migration and the angiogenic response. Our identification of eNOS at the lamellipodia and filopodia of migratory human ECs is similar to recently published findings27 and suggests a possible interplay between eNOS and MT1-MMP.13 Indeed, confocal analysis demonstrated a degree of colocalization of eNOS and MT1-MMP at these structures. We have previously identified a molecular complex composed of MT1-MMP, caveolin-1, and αvβ3 integrin present at migration-associated protrusions of ECs17 ; our present results thus suggest eNOS as a possible additional molecular partner that might regulate this complex during the migratory response. Moreover, we have also found that eNOS compartmentation in ECs can be regulated by growth on different ECM substrates (data not shown) in a similar way to MT1-MMP.14 It thus appears that the compartmentation of eNOS and MT1-MMP can be modulated by related molecular pathways. The identity of these pathways remains obscure but might involve caveolae-mediated internalization and/or regulated palmitoylation.17,28-30
The detection of NO production at EC membrane protrusions and the release of NO by migratory ECs provide the first demonstration of the activation of eNOS during EC migration. This activation may act as a positive feedback mechanism in angiogenesis and other pathophysiologic contexts. An especially notable feature of the NO production detected at membrane protrusions was that it was highly dynamic, with concentrated bursts detected by DAF-FM fluorescence arising and disappearing over a period of seconds to minutes. Several signaling pathways could potentially underlie this dynamic up-regulation of eNOS activity at defined sites of the cell membrane of migrating ECs. For example, calcium waves are induced at the leading edge of migratory ECs.31 Another possible mechanism involves the small GTPase Rac, which plays roles in EC migration and the formation of membrane protrusions32 and acts as an upstream regulator of eNOS in VEGF-induced increases in EC permeability.33 The activity of eNOS can also be up-regulated by phosphorylation at Ser 1177 by Akt, and the Akt pathway is required for endothelial migration and angiogenesis.34,35
In our previous work we characterized the activation and action of MT1-MMP in EC migration.13 Here, we report that MT1-MMP is a direct or indirect downstream effector of NO in migratory ECs, via a mechanism involving focal clustering of MT1-MMP. MT1-MMP clustering at the cell surface of human ECs appears to be directly related to the amount of NO available, since the timing of clustering induced by DEA-NONOate and DETA-NONOate correlate with the rapid and delayed release of NO by these agents. In addition, NO donors or bradykinin increased MT1-MMP collagenolytic activity at EC membrane protrusions at times similar to those at which clustering is induced, suggesting that the 2 events are related. In contrast to the regulation of MT1-MMP expression by migration,13 NO does not affect the total amount of MT1-MMP protein detected by Western blot; similarly, the flow cytometry and cell fractionation do not show general or sustained effects on MT1-MMP levels at the membrane in the initial period after exposure to NO, when MT1-MMP clustering and increased activity are detected. Considering the dynamic nature of NO production at membrane protrusions (Figure 1B), it may be that the MT1-MMP clustering is similarly dynamic and fluctuating. Nonetheless, the increased proportion of cells expressing MT1-MMP after 16 hours of exposure to NO donors might indicate a more general regulation of MT1-MMP in the longer term response of ECs to NO activators, consistent with the reduced membrane expression of MT1-MMP observed in eNOS-deficient MLECs. But over the short term it is important to consider that MT1-MMP clustering and colocalization with eNOS is highly focused, and only affects a subset of the total cellular MT1-MMP content. It is therefore possible that MT1-MMP clustering and eNOS colocalization involve localized cycling between the cytosol and cell membrane that is not detectable by whole-cell assays such as flow cytometry and crude cell fractionation. Nitric oxide has recently been proposed to play an important role in protein trafficking,36 and our data will reinforce the view of NO as a critical regulator of protein compartmentation at local sites within cells. Whether NO regulates MT1-MMP mobilization to the membrane, its internalization, or both, and through which kind of vesicles and/or microdomains, will require further investigation.
A role for MT1-MMP as an effector of NO during EC migration is strongly supported by the experiments in eNOS-deficient MLECs, which demonstrate that endogenous NO is required for MT1-MMP homeostasis in ECs: (1) MT1-MMP localization at membrane protrusions is altered in the absence of eNOS, strongly supporting a role for NO in regulating MT1-MMP compartmentation; (2) COL I degradation is reduced in the absence of NO; and (3) MT1-MMP function in endothelial migration and tube formation is impaired in ECs deficient in eNOS and therefore NO production. It is noteworthy that MT1-MMP regulation by NO in ECs seems to take place in migratory conditions associated to distinct pathophysiologic processes but not in the quiescent endothelial monolayer. Since MT1-MMP is not universally required for all angiogenic responses,14,37 we also addressed whether MT1-MMP might be an active downstream effector during NO-induced angiogenic responses. Experiments in MLECs from MT1-MMP–deficient mice and in human ECs treated with a neutralizing anti–MT1-MMP mAb confirmed that MT1-MMP is required for proper NO-induced endothelial migration and capillary tube formation in standard in vitro assays. The role played by MT1-MMP during NO-induced EC migration and angiogenesis might involve direct action on the ECM or an indirect action involving the modulation of the bioactivity of distinct substrates.
In summary, we have identified a functional interplay between NO and MT1-MMP during endothelial migration. NO regulates proper MT1-MMP expression, compartmentation, activity, and function in ECs, and MT1-MMP is required for efficient NO-induced capillary tube formation. This cooperation between NO and MT1-MMP might act as an amplification loop during angiogenesis. NO represents one of the most important defenses against cardiovascular disease; endothelial dysfunction, which involves altered production of NO, is involved in the development of important pathologies such as atherosclerosis.2,38 The recent identification of MT1-MMP as a factor involved in neointima formation39 further highlights the potential of this protease as a therapeutic target for the treatment of atherosclerotic disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank S. Bartlett for editing and D. Megías and Dr M. Montoya for assistance with confocal microscopy.
This work was supported by National Institutes of Health grant AR47074 (S.A.), Comunidad Autónoma de Madrid grants CAM 08.4/0023/2003 (C.Z.) and GR/SAL/0309/2004 (A.G.A.), Spanish Ministerio de Sanidad y Consumo grant CP03/00 025 (A.M.R.), and Spanish Ministerio de Educación y Ciencia grants SAF2005-06025 (C.Z.), SAF2006-02410 (S.L.), and SAF2005-02228 (A.G.A.). P.G. is a postdoctoral researcher from the “Juan de la Cierva” program (MCyT) and C.Z. is a research investigator from the “Ramón y Cajal” program (MCyT).
National Institutes of Health
Authorship
Contribution: L.G. performed research and analyzed data; P.G., A.S.T., and B.G.G. performed research; A.M.-R. analyzed data; C.Z., S.L., K.T., and S.S.A. provided mice; A.G.A. designed and supervised the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alicia G. Arroyo, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Melchor Fernández Almagro 3, 28029 Madrid, Spain; e-mail: agarroyo@cnic.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal