In this issue of Blood, Genís and colleagues provide evidence for a dynamic interaction between membrane type 1–matrix metalloproteinase (MT1-MMP) and nitric oxide (NO) in migrating endothelial cells.
The small polyvalent molecule, nitric oxide, generated by 3 nitric oxide synthases (NOSs), acts as a vasoactive agent, signaling molecule, neurotransmitter, and free radical in mammalian systems. NO participates in many biological functions including gene expression, immune responses, cell growth and survival, and matrix remodeling. In the cardio-vascular system, NO plays numerous roles in the endothelial cell, affecting survival, apoptosis, proliferation, migration, and differentiation as well as adhesion-molecule and tissue-factor expression.
One emerging area of investigation has been the regulation of protease activities via NO levels. Several recent reports have demonstrated distinct NO-dependent pathways that modulate MMP-2, MMP-9 and TIMP-1 levels, which in turn have the potential of affecting a wide range of cellular behaviors. Genís and colleagues have now added MT1-MMP to the group of matrix metalloproteinases that are regulated by NO. Specifically they have demon-strated (1) that eNOS is active at motility-associated structures in endothelial cells and colocalizes with MT1-MMP; (2) that NO upregulates MT1-MMP activity and membrane clustering on migratory endothelial cells; (3) that NO is required for MT1-MMP homeostasis and function in migrating endothelial cells; and (4) that NO-induced endothelial-cell migration and tube formation requires MT1-MMP expression and activity. These findings provide new insights into our understanding of the complex roles that dysregulation of NO plays in eliciting inhibition of the development of the vitelline circulation (vasculogenesis and angiogenesis) and cardiac-cushion development (epithelial-to-mesenchymal transition) as well as in the normal development of other organs systems and during the postinjury repair process (angiogenesis).1
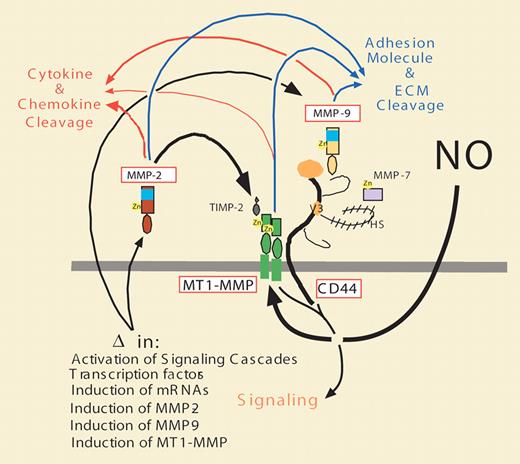
The modulation of MT1-MMP expression, localization, and activity in endothelial cells by NO is also likely to have significant effects on other far-reaching signaling pathways. Specifically, in regulating MT1-MMP expression, localization, and activity, NO may have profound effects on local chemokine and cytokine activation, inhibition, and release from cell surfaces and extracellular matrix components by affecting MT1-MMP localization and activity. This regulation may also influence proteolysis of cell-surface adhesion molecules. Differential clustering of MT1-MMP may also affect tethering and activation of MMP-2, and association with and proteolysis of CD44. This, in turn, may affect tethering of MMP-7 and MMP-9, as well as subsequent signaling via the cytoplasmic domains of MT1-MMP and CD44 (see figure).
Schematic illustrating the tethering, association, and signaling properties of MT1-MMP on the cell surface. Following regulation by NO, MT1-MMP expression (MMP-14), localization, and activities are modulated and effect the expression levels, activities, and functions of CD44, MMP-2, MMP-9, and possibly other MMPs (MMP-7), as well as the cleavage of adhesion molecules, ECM components (blue arrows), and chemokines and cytokines (red arrows), thus affecting a wide range of epithelial, lymphocytic, and endothelial cell behaviors.
Schematic illustrating the tethering, association, and signaling properties of MT1-MMP on the cell surface. Following regulation by NO, MT1-MMP expression (MMP-14), localization, and activities are modulated and effect the expression levels, activities, and functions of CD44, MMP-2, MMP-9, and possibly other MMPs (MMP-7), as well as the cleavage of adhesion molecules, ECM components (blue arrows), and chemokines and cytokines (red arrows), thus affecting a wide range of epithelial, lymphocytic, and endothelial cell behaviors.
Along with these advances in NO biology driven by tissue culture and animal studies, there have been several recent clinical studies using inhaled nitric oxide (iNO) in preterm infants with persistent pulmonary hypertension, respiratory failure, and severe intraventricular hemorrhage or periventricular leukomalacia.2-4 In each of these studies, the premature infants treated with iNO exhibited reduced overall risk of brain injury, decreased risk of cerebral palsy, and improved neurodevelopmental outcomes at 2 years of age. While the underlying mechanisms responsible for these improvements are uncertain at this time, it is tempting to speculate that NO modulation of protease behavior in endothelial cells (and other cell types) is partially responsible. In light of our rapidly increasing understanding of NO as a pleomorphic modulator of a broad range of signaling pathways and continuously emerging targeting technologies, future therapies for a variety of diseases may include “just saying NO.”
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal