To the editor:
Two recent papers in Blood provide the first indications that deregulated Wnt signaling plays a role in the development of acute lymphoblastic leukemias (ALLs).1,2 Activating mutations in the critical Wnt mediator β-catenin lead to the development of thymic lymphomas, the murine counterpart of T-ALL.1 The Wnt signaling pathway plays a key role in the development of various cell types and is subject to strict molecular regulation. The localization and consequently the function of β-catenin is regulated by Ser/Thr and possibly Tyr phosphorylation. Nuclear β-catenin serves as a coactivator for Tcf-mediated transcription of target genes that play important roles in leukemic progression.
Given the importance of the phosphorylation status of β-catenin, we have previously generated an antibody specific for β-catenin dephosphorylated at residues Ser37 and Thr41. Wnt signals specifically increase the levels of dephosphorylated β-catenin as detected with this antibody.3,4 The antibody, named antiactive β-catenin (ABC), is produced by hybridoma clone 8E7. Another antibody claimed to recognize the dephosphorylated form is termed 8E4 (www.upstate.com).
Using 8E4 instead of 8E7, various investigators have studied activation of the Wnt pathway. Gottardi and Gumbiner5 report that dephosphorylated β-catenin readily interacts with both cytoplasmic cadherins and nuclear Tcf. Derksen et al6 and Diks et al7 report very similar patterns of bands in Western blots using 8E4 and a pan–β-catenin antibody, whereas Jamieson et al8 using 8E7 antibody show that Wnt signaling is activated in blast crises of chronic myelogenous leukemia (CML).
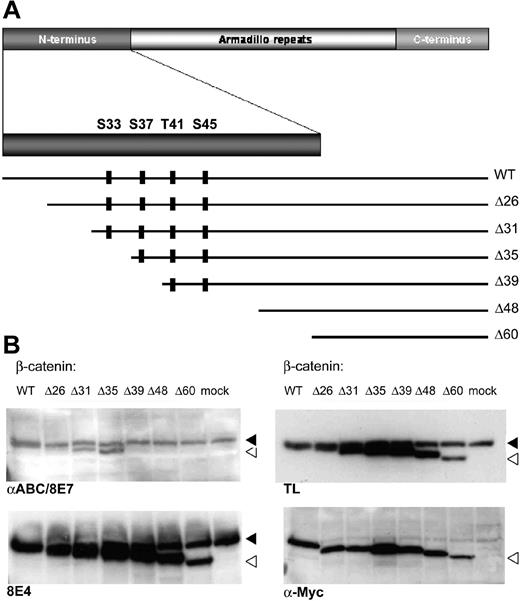
These conclusions critically depend on the specificity of the antibodies. We therefore mapped the antigenic epitope recognized by the 8E4 and 8E7 antibodies using a set of N-terminal deletion clones of β-catenin covering the regulatory region that is the target of the Wnt pathway kinases (Ser33 to Ser45; Figure 1A). Using these constructs, 8E4 was found to recognize all deleted forms (Figure 1B), implying that it does not recognize the regulatory region of β-catenin. These results are at odds with the claims on the company's website (http://www.upstate.com) that this antibody was raised against amino acids 27-37 of human β-catenin. As published previously, the 8E7 epitope mapped directly C-terminal to amino acid 35.3
8E4 recognizes all deleted forms of B-catenin. (A) Schematic overview of the β-catenin molecule, with emphasis on the N-terminus. The 4 well-characterized Ser and Thr amino acids that are regulated by phosphorylation are shown as well as the various deletion constructs used to express myc-tagged truncated forms of β-catenin. (B) The 293 cells were transfected with myc-tagged β-catenin deletion constructs to identify the epitope recognized by 8E7 and 8E4. The endogenous β-catenin band is indicated by a black arrow, the open arrow indicates the transfected, N-terminal deleted form. Similar to the pan–β-catenin antibody (TL), 8E4 recognizes all deletion constructs, whereas 8E7 (αABC) only recognizes deleted β-catenin forms up to Δ35. The following antibodies were used: αABC/8E7 (Upstate Biotech, Lake Placid, NY; catalog no. 05-665), 8E4 (Upstate Biotech; catalog no. 05-601), TL (BD Transduction Laboratories, San Jose, CA; anti–β-catenin; catalog no. 610154), α-Myc (clone 9E10; supernatant of hybridoma used).
8E4 recognizes all deleted forms of B-catenin. (A) Schematic overview of the β-catenin molecule, with emphasis on the N-terminus. The 4 well-characterized Ser and Thr amino acids that are regulated by phosphorylation are shown as well as the various deletion constructs used to express myc-tagged truncated forms of β-catenin. (B) The 293 cells were transfected with myc-tagged β-catenin deletion constructs to identify the epitope recognized by 8E7 and 8E4. The endogenous β-catenin band is indicated by a black arrow, the open arrow indicates the transfected, N-terminal deleted form. Similar to the pan–β-catenin antibody (TL), 8E4 recognizes all deletion constructs, whereas 8E7 (αABC) only recognizes deleted β-catenin forms up to Δ35. The following antibodies were used: αABC/8E7 (Upstate Biotech, Lake Placid, NY; catalog no. 05-665), 8E4 (Upstate Biotech; catalog no. 05-601), TL (BD Transduction Laboratories, San Jose, CA; anti–β-catenin; catalog no. 610154), α-Myc (clone 9E10; supernatant of hybridoma used).
It is unfortunate that these antibodies with such similar names, commercialized by the same company, have strikingly different specificities. Our observations imply that the 8E4 antibody does not possess the advertised specificity for the dephosphorylated regulatory region of β-catenin and should not be used to study activity of the Wnt pathway, for instance, on cytospin preparations of cells suspected for hematologic malignancies.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frank J. T. Staal, Department of Immunology, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 50, 3015GE, Netherlands; e-mail: f.staal@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal