Donor lymphocyte infusions (DLIs) can produce lasting remissions in patients with relapsed chronic myeloid leukemia (CML), but are less effective in non-CML diseases. We hypothesized that lymphodepletion, achieved with cyclophosphamide (Cy) and fludarabine (Flu), would promote in vivo expansion of the infused lymphocytes enhancing their immunologic effects. Fifteen patients with relapsed non-CML disease who received Cy/Flu/DLI were compared with 63 controls who received DLI without chemotherapy. Only the patients receiving Cy/Flu/DLI became lymphopenic at the time of DLI. Compared with controls, patients who received Cy/Flu/DLI developed significantly more grades II to IV (60% vs 24%, P = .01) and grades III to IV acute graft-versus-host disease (GVHD) (47% vs 14%, P = .01) with greater GVHD lethality. In Cy/Flu/DLI patients, T-cell proliferation was elevated at 14 days after DLI. Although these data suggest that chemotherapy-induced lymphodepletion enhances activation of donor lymphocytes, the toxicity needs to be managed before testing whether better disease control can be achieved. This trial was registered at www.clinicaltrials.gov as no. NCT00303693 and www.cancer.gov/clinicaltrials as no. NCT00167180.
Introduction
The success of allogeneic hematopoietic cell transplantation (HCT) to treat leukemia depends upon both lymphohematopoietic reconstitution and also the induction of a graft-versus-leukemia immunologic response. The importance of the lymphocyte antitumor effect was shown by the ability of donor lymphocyte infusion (DLI) to induce durable remissions in patients with chronic myeloid leukemia (CML) relapse after transplantation.1,,,–5 However, for patients with non-CML malignancies, this approach remains inadequate.6,7 One strategy to potentiate the immune effects of donor lymphocytes is to manipulate the host milieu to promote in vivo expansion of donor T cells from the DLI product. Adoptive transfer experiments have shown that lymphodepletion improves in vivo lymphocyte expansion by (1) providing lymphoid space, (2) eliminating host antidonor immune reactivity, (3) decreasing competition for growth factors that promote lymphocyte expansion, or (4) elimination of suppressive regulatory T cells.8,,,,,–14 Based on these observations, we designed a clinical trial to test whether lymphodepleting chemotherapy could enhance the immune effects of DLI
Patients, materials, and methods
Patients were given a lymphodepleting regimen of intravenous cyclophosphamide (Cy) 50 mg/kg once on day −6 and fludarabine (Flu) 25 mg/m2 for 5 consecutive days (−6 to −2), a regimen that included one less dose of Cy than that initially reported by the NIH group.15 DLI consisted of mononuclear cells with a fixed T-cell dose of 108/kg given 48 hours after the last Flu dose. Between 2004 and 2006, 15 patients with non-CML disease (1 acute lymphoblastic leukemia [ALL], 6 acute myeloid leukemia [AML], 2 myelodysplastic syndrome [MDS], 1 non-Hodgkin lymphoma [NHL], 2 chronic lymphoblastic leukemia [CLL], 1 multiple myeloma, 1 juvenile CML, and 1 myelofibrosis) received Cy/Flu/DLI as their first treatment of relapse after HCT. The control group included CML patients who received DLIs containing 108 T cells /kg (n = 28) and non-CML patients (n = 35) who received DLIs with higher T-cell doses (3 daily lymphapheresis products) from 1993 to 2003. The CML and non-CML patients were analyzed as a single control group because there was no difference in their rates of graft-versus-host disease (GVHD). A report on 42 patients from the control cohort has been published previously.7 All patient samples and data were collected after obtaining informed consent in accordance with the Declaration of Helsinki. This study was approved by the University of Minnesota institutional review board. Peripheral blood mononuclear cells were analyzed as indicated with anti-CD3, -CD4, -CD8, and -Ki-67 monoclonal antibodies (all from Becton Dickinson, San Jose, CA). Cumulative incidence was used to estimate acute GVHD, treating non-GVHD death as a competing risk.16 Overall survival was estimated by the Kaplan-Meier method.17 The independent effect of factors was evaluated in a Cox regression model,18 and other comparisons were analyzed using log-rank tests.
Results and discussion
Fifteen patients treated with Cy/Flu/DLI for relapse of hematologic malignancy after HCT were compared with 63 patients who received DLI without chemotherapy. All patients received DLI from their initial donor and there was no significant difference in the percentage of patients who received DLI from sibling donors between the Cy/Flu/DLI (93%) and control (79%) cohorts. Although the proportion of pediatric patients between the cohorts was similar, the age in the control group (median, 40 years; range, 2-60 years) was slightly lower than in the Cy/Flu/DLI group (median, 51 years; range, 4-63 years; P ≤ .01). Control patients were not leukocytopenic (white blood cell count [WBC], 7.8 ± 0.2 × 109/L [7800 ± 200/μL] mean ± SEM) or lymphopenic (1.4 ± 0.3 × 109/L [1400 ± 300/μL]) at the time of DLI and were treated predominantly as outpatients. In striking contrast, the Cy/Flu-treated patients developed severe leukocytopenia (WBC, 0.61 ± 0.14 × 109/L [610 ± 140/μL]; P ≤ .001) and lymphopenia (0 ± 0 × 109/L [0 ± 0/μL]; P < .001) at the time of the DLI and were all treated in the hospital due to absolute neutropenia.
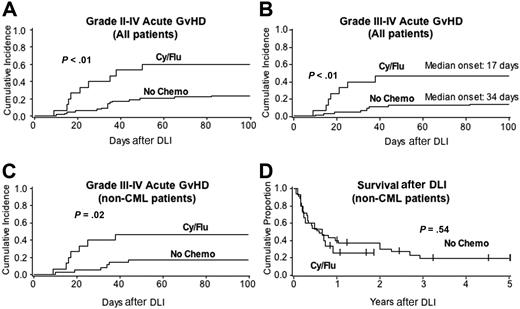
Importantly, patients who received Cy/Flu/DLI developed significantly more grades II to IV (60% vs 24%, P = .01) and grades III to IV (47% vs 14%, P = .01) acute GVHD compared with controls (Figure 1A,B). The interval from DLI to grades III to IV GVHD tended to be shorter in Cy/Flu/DLI patients compared with controls (median = 17 days vs 34 days; P = .19). A multiple regression analysis adjusting for diagnosis, age, weight, sex, time from diagnosis to relapse, and donor type showed a strong significant effect of Cy/Flu lymphodepletion on acute GVHD risk (relative risk, 5.3; 95% CI, 1.8-15.5, P < .01). There was no significant difference in acute GVHD risk based on donor type (sibling vs unrelated). The significant differences in acute GVHD rates remained after excluding CML patients from the control cohort (Figure 1C). Since survival was significantly different between CML and non-CML cohorts treated with DLI alone,7 CML patients were excluded from the survival analysis. Although there were no significant differences in survival between non-CML patients treated with Cy/Flu/DLI and DLI alone (Figure 1D), the cause of death differed significantly between cohorts. Patients receiving DLI without chemotherapy died primarily due to disease persistence or recurrence and only 5% of deaths were attributed to GVHD. In contrast, 5 (45%) of 11 deaths in the Cy/Flu/DLI cohort were directly related to GVHD (P < .01), which triggered the stopping rules for this study.
Lymphodepleting chemotherapy prior to DLI induces greater immune activation than does DLI alone and is manifested as more severe acute GVHD. The Cy/Flu/DLI cohort (n = 15) consisted of 14 matched sibling donors (93%) and one 5 of 6 mismatched HLA-A, -B, -DR (high-resolution typing) unrelated donor. The control cohort receiving DLI without chemotherapy (n = 63) consisted of 49 matched sibling donors (79%, P = NS), 1 single antigen mismatched sibling donor, and 13 unrelated donors (10 6/6 matched and 3 mismatched). GVHD (A-C) and overall survival (D) are shown for patients who received Cy/Flu/DLI compared with those receiving DLI alone for all patients (A-B) or for those patients with non-CML diseases only (n = 35, C-D).
Lymphodepleting chemotherapy prior to DLI induces greater immune activation than does DLI alone and is manifested as more severe acute GVHD. The Cy/Flu/DLI cohort (n = 15) consisted of 14 matched sibling donors (93%) and one 5 of 6 mismatched HLA-A, -B, -DR (high-resolution typing) unrelated donor. The control cohort receiving DLI without chemotherapy (n = 63) consisted of 49 matched sibling donors (79%, P = NS), 1 single antigen mismatched sibling donor, and 13 unrelated donors (10 6/6 matched and 3 mismatched). GVHD (A-C) and overall survival (D) are shown for patients who received Cy/Flu/DLI compared with those receiving DLI alone for all patients (A-B) or for those patients with non-CML diseases only (n = 35, C-D).
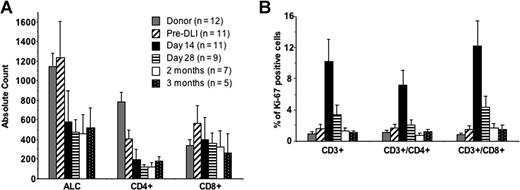
Patient absolute lymphocyte counts were significantly lower after DLI compared with their pretherapy counts or to donor counts (Figure 2). Absolute CD4 counts, which were lower than those of donors and recipients before therapy, decreased further after therapy and had not recovered at 3 months. The CD4 lymphopenia is most likely attributable to relapse, chemotherapy before DLI, and the known immunosuppressive effects of GVHD and its therapy. Absolute CD8+ counts did not change substantially after Cy/Flu/DLI when measured on day 14 through 3 months after treatment, consistent with the effect of lymphopenia on CD8+ T-cell homeostatic expansion. NK cells did not increase at any time point (data not shown). T-cell proliferation, as measured by expression of the Ki67 marker (a nuclear protein associated with proliferation), increased significantly in recipients 14 days after DLI (10.3% ± 2.8%) compared with the pre-Cy/Flu baseline values (1.6% ± 0.6%, P = .012), and was returning to baseline levels by 28 days after DLI (3.5% ± 1.2%, P = .19). Increased in vivo expansion of both CD4 and CD8 lymphocytes was measured at day 14 after DLI (Figure 2).
T lymphocytes expand in vivo in patients who receive Cy/Flu/DLI. Peripheral blood mononuclear cells were collected from the DLI donor and from patients prior to initiating chemotherapy (pre-DLI) and 14 days, 28 days, 2 months, and 3 months after Cy/Flu/DLI therapy. (A) The absolute lymphocyte counts (ALCs) from the clinical complete blood count were used to calculate absolute CD4+ and CD8+ counts at all time points. (B) Cells were stained for the intracellular Ki-67 antigen as a marker for proliferating T cells. Results are shown as the percentage Ki-67-positive cells gated on CD3+, CD3+/CD4+, and CD3+/CD8+ cells. Error bars indicate SEM.
T lymphocytes expand in vivo in patients who receive Cy/Flu/DLI. Peripheral blood mononuclear cells were collected from the DLI donor and from patients prior to initiating chemotherapy (pre-DLI) and 14 days, 28 days, 2 months, and 3 months after Cy/Flu/DLI therapy. (A) The absolute lymphocyte counts (ALCs) from the clinical complete blood count were used to calculate absolute CD4+ and CD8+ counts at all time points. (B) Cells were stained for the intracellular Ki-67 antigen as a marker for proliferating T cells. Results are shown as the percentage Ki-67-positive cells gated on CD3+, CD3+/CD4+, and CD3+/CD8+ cells. Error bars indicate SEM.
In human studies, Dudley et al were the first to show that a Cy/Flu-based regimen could alter the host milieu allowing the safe in vivo expansion of melanoma-specific cytotoxic T lymphocytes.15 We have shown that this same regimen can promote in vivo expansion of allogeneic NK cells, which is associated with a surge in IL-1519,20 and, likely, IL-7 as well.21,–23 The peak in Ki-67–positive cells, used as a surrogate marker of in vivo proliferation, was temporally associated with the early onset of severe acute GVHD. Despite aggressive therapy, 4 of the 7 patients who developed grades III to IV GVHD died from complications of GVHD and the trial was stopped due to safety concerns.
In conclusion, we show “proof of concept” that DLI following lymphodepleting chemotherapy leads to in vivo lymphocyte expansion with increased immune activation as evidenced by higher rates of severe acute GVHD than those seen in patients who receive DLI alone. The ability of lymphodepletion to enhance the immune effects of DLI is promising if alloreactivity does indeed correlate with remission induction. To study this further, subsequent patients are being treated with a lower T-cell dose (5 × 107 T cells /kg), and preliminary results show less GVHD mortality (1 of 7). Alternate strategies using DLI of select cell populations or of cells engineered to carry suicide genes may be necessary to safely balance the toxic versus beneficial effects of the enhanced immune activity in DLI with lymphodepleting chemotherapy to treat patients with aggressive hematologic malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health Grants P01-CA-111412, P01-CA-65493, and R01 CA72669 to the University of Minnesota.
National Institutes of Health
Authorship
Contribution: J.S.M. and B.R.B. designed the study, analyzed data, and wrote the paper; D.J.W., L.J.B., A.S., J.E.W., M.R.V., and S.C. analyzed data and wrote the paper; R.W. and S.K.F. were responsible for laboratory correlates; R.N. performed study coordination and data analysis; T.D. was responsible for biostatistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, Professor of Medicine, University of Minnesota Cancer Center, MMC 806, Division of Hematology, Oncology, and Transplantation, Harvard St at East River Rd, Minneapolis, MN 55455; e-mail:mille011@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal